Abstract
Endotoxin tolerance (ET) is a reduced responsiveness of innate immune cells like macrophages/monocytes to an endotoxin challenge following a previous encounter with the endotoxin. Although ET in peripheral systems has been well studied, little is known about ET in the brain. The present study showed that brain immune cells, microglia, being different from peripheral macrophages, displayed non-cell autonomous mechanisms in ET formation. Specifically, neurons and astroglia were indispensable for microglial ET. Macrophage colony-stimulating factor (M-CSF) secreted from these non-immune cells was essential for governing microglial ET. Neutralization of M-CSF deprived the neuron-glia conditioned medium of its ability to enable microglia to form ET when microglia encountered two lipopolysaccharide (LPS) treatments. Recombinant M-CSF protein rendered enriched microglia refractory to the second LPS challenge leading to microglial ET. Activation of microglial M-CSF receptor (M-CSFR; also known as CSF1R) and the downstream ERK1/2 signals was responsible for M-CSF-mediated microglial ET. Endotoxin-tolerant microglia in neuron-glia cultures displayed M2-like polarized phenotypes, as shown by upregulation of M2 marker Arg-1, elevated production of anti-inflammatory cytokine interleukin 10, and decreased secretion of pro-inflammatory mediators (tumor necrosis factor α, nitric oxide, prostaglandin E2 and interleukin 1β). Endotoxin-tolerant microglia protected neurons against LPS-elicited inflammatory insults, as shown by reduced neuronal damages in LPS pre-treatment group compared with the group without LPS pre-treatment. Moreover, while neurons and astroglia became injured during chronic neuroinflammation, microglia failed to form ET. Thus, this study identified a distinct non-cell autonomous mechanism of microglial ET. Interactions of M-CSF secreted by neurons and astroglia with microglial M-CSFR programed microglial ET. Loss of microglial ET could be an important pathogenetic mechanism of inflammation-associated neuronal damages.
Keywords: Microglia, Endotoxin tolerance, Neurons, Astroglia, M-CSF, CSF1R, ERK1/2, M2-like microglia, Neuroprotection
1. Introduction
In response to a potent immune challenge, activated innate immune cells (e.g. monocytes and macrophages) can produce various inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), IL-6, eicosanoids, nitric oxide (NO), and reactive free radicals. When in excess, these inflammatory mediators can cause serious systemic disorders with a high mortality rate (Gordon and Taylor, 2005). Pre-exposure to endotoxin can induce transient unresponsive or reduced sensitivity to a subsequent endotoxin challenge, as shown by decreases in production of inflammatory mediators, febrile reaction, and lethality rate (Mendez et al., 1999). This phenomenon is termed endotoxin tolerance (ET). Although the incidence of ET in peripheral immune systems has been observed both in vitro and in vivo (Lopez-Collazo and del Fresno, 2013), whether the central immune system exhibits similar immune tolerance has not been well investigated. Furthermore, the regulatory mechanism of ET in peripheral immune cells such as macrophages has been well established at multiple levels from negative signal transduction, transcriptional network to post-translational modifications (Biswas and Lopez-Collazo, 2009; Hoppstadter et al., 2015; Lopez-Collazo and del Fresno, 2013). However, molecular mechanisms by which microglia program ET are still unclear. In addition, the physiological and pathological role of microglial ET in brain health and diseases warrants further investigation.
ET can be modeled by two consecutive lipopolysaccharide (LPS) challenges with an interval of hours to days. Historically, a large body of knowledge on the development of ET in peripheral innate immune cells, especially monocytes and macrophages, has been obtained through the study of responses of enriched cell culture systems or experimental animals to repeated LPS stimulation. Such experimental designs did not allow for a readily examination of the temporal and possibly causative relationship between non-immune cells and ET-development immune cells. As a result, the contribution of non-immune cells to ET formation still remains an open question.
Ample evidence has demonstrated important functions of neurons and astroglia in keeping microglia in a quiescent state and reducing their activation upon immune challenge. Indeed, the low turnover rate and limited replenishment mechanism of microglia (the sole type of immune cells in the brain) demand tight spatial and temporal regulation for maintaining immune homeostasis as well as structural and functional integrity of the central nervous system (CNS). Up to now, how neurons and astroglia affect the response of microglia to repeat endotoxin exposure has not been investigated.
In this study, using various re-constituted primary cultures, we investigated roles of brain non-immune cells in microglial ET formation and further studied mechanism of microglial ET. We show for the first time that neurons and astroglia regulate microglial ET development through the release of macrophage colony-stimulating factor (M-CSF) and consequent activation of its receptor (M-CSFR; also known as CSF1R) and downstream ERK1/2 signals. This study identified a novel molecular mechanism for ET development in central immune system.
2. Materials and Methods
2.1 Animals
All the animals were treated humanely and with regard for alleviation of suffering following the National Institutes of Health Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996). All procedures were approved by the NIEHS Animal Care and Use Committee.
2.2 Recombinant proteins, protein kinase inhibitors, and other reagents
LPS (E. coli O111:B4, Cat# 437627, protein contaminants ≤2.0%, nucleic acid contaminants ≤2.5%), SP600125, and Bay 11-7821 were obtained from EMD Millipore Corporation (Darmstadt, Germany), Abcam Inc. (Cambridge, MA), and TOCRIS bioscience (Bristol, UK), respectively. U0126 and SB203580 were purchased from Cell Signaling Technology, Inc. (Danvers, MA). The following reagents were purchased from R&D Systems (Minneapolis, MN): anti-M-CSF antibody, recombinant mouse TNF-α, M-CSF, interleukin 34 (IL-34), and IL-1β. Cycloheximide, cytosine arabinoside (Ara-C), and L-leucine methyl ester (LME) were from Sigma-Aldrich (Saint Louis, MO).
2.3 Preparation of primary neuron-glia, mixed-glia, neuron-microglia, neuron-enriched, microglia-enriched, and astrocyte-enriched cultures
To investigate roles of different brain cells in the regulation of microglial ET, we prepared enriched cultures of single cell type and co-cultures/reconstituted cultures of multiple cell types. As seen below, although the percentage of microglia in different cultures seems quite different, the number of microglial cells in different cultures is similar. This makes TNF-α measurement and evaluation of microglial ET formation in the different experiment settings comparable. In addition, to reduce any possible influence of serum components on cell response to various treatments, we lowered serum concentration in the treatment media of various cultures to one fifth of their respective culture media, except where indicated otherwise.
2.3.1
Mesencephalic neuron–glia cultures were prepared from the mesencephalon of embryos at gestation day 14 ± 0.5 C57BL/6J mice as previously reported (Gao et al., 2002). Briefly, mesencephalic tissues were dissected and dissociated with a mild mechanical trituration. Cells were seeded to 24-well (5 × 105 cells/well) culture plates pre-coated with poly-D-lysine (20 μg/ml) and maintained in 0.5 ml/well of MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10% heat-inactivated horse serum (HS), 1 g/L glucose, 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.1mM nonessential amino acids. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air and were replenished with 0.5 ml/well fresh medium 3 days later. Seven-day after seeding, cultures were treated with vehicle or desired reagents in MEM treatment medium containing 2% FBS, 2% HS, 2 mM L-glutamine, and 1 mM sodium pyruvate. Immunocytochemical analysis indicated that, at the time of treatment, the neuron–glia cultures were made up of about 11% microglia (~5.5 × 104 microglia/well), 50% astrocytes, and 39% neurons that were immunoreactive (IR) to the antibody against ionized calcium binding adaptor molecule 1 (Iba1), glial fibrillary acidic protein (GFAP), and neuron-specific nuclear protein (NeuN), respectively.
2.3.2
Neuron-enriched cultures were prepared by using cytosine arabinoside (Ara-C; 5 μM) to suppress the proliferation of glial cells 2 days after seeding of disassociated mesencephalic cells as described above (Gao et al., 2011). At 4 days after the initial plating the cultures consisted of 1% GFAP-IR astrocytes, <0.1% OX-42-IR microglia, and ~99% NeuN-IR neurons.
2.3.3
Primary mixed-glia cultures were prepared from whole brains of postnatal day 1 pups from C57BL/6J mice (Chu et al., 2015). Disassociated brain cells were seeded onto 24-well (2.5 × 105 cells/well) and 6-well (1 × 106 cells/well) culture plates and maintained in 1 ml/well DMEM/F-12 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. The medium was changed every 3 days. After reaching confluence at 11–12 days after plating, the cultures were treated with vehicle or desired reagents in DMEM/F-12 treatment medium containing 2% FBS, 2 mM L-glutamine, and 1 mM sodium pyruvate and 0.1 mM nonessential amino acids. At the time of treatment, the cultures contained about 80% GFAP-IR astrocytes and 20% Iba1-IR microglia (~6 × 104 and ~2.5 × 105 microglia per well of 24-well plate and 6-well plate, respectively).
2.3.4
Astroglia-enriched cultures were prepared from mouse mixed-glia cells that were treated with 1.5 mM LME 2 day after cell seeding (Chu et al., 2015). After incubation with LME for 3 days, these cells were replaced with fresh medium without LME. Two days later, the cultures, which contain ~0.1% Iba1-IR microglia and 99.9% GFAP-IR astroglia, were used for treatment.
2.3.5
Microglia-enriched cultures were prepared from the whole brains of 1-day-old C57BL/6J mouse pups as previously reported (Chen et al., 2013). Briefly, brain tissues, devoid of meninges and blood vessels, were dissociated by a mild mechanical trituration. The isolated cells (5 × 107 cells) were seeded in 150 cm2 culture flasks in DMEM/F12 culture medium containing 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air, and the medium was changed 4 days later. On reaching confluence (12–14 days), the microglia were separated from astroglia by shaking the flask for 30 minutes at 180 rpm and plated into 24-well plates (6 × 104/well). The enriched microglia were 98% pure.
2.3.6
Neuron-astroglia cultures were prepared from mouse neuron-glia cells. When neuron-glia cultures reached confluence 5 days after the initial seeding, microglia were depleted by 0.5 mM LME for 3 days. The cultures, which contain less than 0.1% Iba1-IR microglia, 44% NeuN-IR neurons, and 56% GFAP-IR astroglia, were changed to fresh MEM treatment medium and used for preparation of reconstituted cultures as described below.
2.3.7
Neuron-microglia cultures were reconstructed by adding 5 × 104 microglia into neuron-enriched cultures (Gao et al., 2011) and neuron-astroglia cultures in 24-well plates. Two different types of reconstituted cultures containing microglia and neurons were prepared by plating highly enriched microglia directly on top of the neuron layer prepared in regular 24-well culture plates or into transwells (HTS 24-well multiwell insert systems, 1.0 μm pore size, polyethylene terephthalate membrane) that were placed above the existing neuron-astroglia cultures. After 24 hours, the reconstituted cultures were ready for treatment.
2.4 Preparation of peritoneal macrophages
Peritoneal macrophages from C57BL/6J mice were induced and harvested, as previously described. Briefly, C57BL/6J mice were administered with 2 ml 3% sterile thioglycollate by peritoneal injection. After 4 days, peritoneal macrophages were collected by lavage in 5 ml ice-cold RPMI 1640 medium, washed twice, and pre-incubated in serum-free RPMI 1640 medium for 1 hour. The culture was then washed twice to remove non-adherent cells. Adherent macrophages were cultured in RPMI 1640 medium containing 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
2.5 Immunocytochemistry and immunofluorescence
Immunostaining was performed as previously described (Gao et al., 2011) with antibodies for NeuN (1:1000; EMD Millipore Corporation, Billerica, MA, USA), microtubule-associated protein 2 (MAP-2; 1:400; EMD Millipore Corporation, Billerica, MA, USA), Iba1 (1:5000; Wako Chemicals, Richmond, VA, USA) and glial fibrillary acidic protein (GFAP; 1:10,000; Wako Chemicals, Richmond, VA, USA), respectively. Briefly, after blocking, formaldehyde-fixed cells were incubated overnight at 4°C with primary antibodies diluted in antibody diluent. The bound primary antibody was visualized by incubation with an appropriate biotinylated secondary antibody, followed by the Vectastain ABC reagents and color development with 3,3′-diaminobenzidine. Images were recorded with a CCD camera and the MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). For the quantification of protein expression levels in immunostaining images in each treatment group, Nikon NIS-Element BR software (Nikon Instruments Inc., Melville, NY, USA) was used to automatically circle the areas of positive staining cells under the consistent intensity criteria and to measure the value of mean intensity in circled positive-staining areas. Protein expression levels in each of staining images are calculated by positive-staining area multiply mean intensity. Data were expressed as percentage of the corresponding control. For visual enumeration of the immunostained microglia in cultures, 10 representative areas per well of the 24-well plate were counted under a microscope (20X power). Cell counting and quantification of immunstaining density were performed by 2–3 individuals. Immunofluorescence was performed as previously described (Zhou et al., 2013). Enriched microglia with or without M-CSF treatment for 24 hours were co-stained with anti-CSF1R (1:100) and anti-CD11b antibodies (1:100; Abcam, Cambridge, MA). Two second antibodies (Alexa Fluor® 488 and 596) were obtained from Abcam Inc. (Cambridge, MA). After mounting the sections onto glass slides with the Prolong Antifade reagents, fluorescent images were obtained with a Zeiss LSM 710 NLO laser scanning confocal microscope fitted with an Argon ion laser and a HeNe laser and recorded with the Zeiss LSM710 software. For each experiment, two to six wells per treatment condition were used and results from five independent experiments were obtained.
2.6 Gel electrophoresis and Western blot
Western blot analysis was performed using cell extract from either neuron-glia or enriched microglia cultures under multiple treatments (Gao et al., 2011). The whole-cell lysates from cultured cells were homogenized in radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1:100 protease inhibitor mixture), sonicated, and boiled for 10 min. Protein concentrations were determined using the bicinchoninic acid assay (Pierce). Protein samples were resolved on 4–12% SDS-PAGE, and immunoblot analysis was performed using the following antibodies: anti-M-CSFR (Abcam, Cambridge, MA, USA), anti-ERK1/2 (Abcam, Cambridge, MA, USA), anti-pERK1/2 (Abcam, Cambridge, MA, USA), anti-MAP-2 (EMD Millipore Corporation, Billerica, MA, USA) and anti-NeuN (EMD Millipore Corporation, Billerica, MA, USA) antibodies. An antibody against GAPDH (Abcam, Cambridge, MA) was used as loading control to monitor loading errors. Densitometric analysis of immunoblots was performed using the AlphaImager 2200 digital imaging system (Digital Imaging System, CA, USA).
2.7 Quantitative real time-PCR
Total cellular RNA of cells was isolated using RNeasy Mini kit (QIAGEN, Valencia, CA), and first-strand cDNA was synthesized using MuLV reverse transcriptase (Applied Biosystems, Foster City, CA) according to the manufacturer’s instruction. After reverse transcription reaction, quantitative real-time PCR analysis was performed to amplify cDNA by using SYBR-Green Master mix (Applied Biosystems, Foster City, CA). PCR condition was as follows: hold at 95 °C for 10 minutes and start 40 cycles at 95 °C for 15 seconds and 60 °C for 1 minute. Data were normalized to GADPH expression. The primers were designed with Vector NTI software (Invitrogen). The sequences of the primers were the following: mouse TNF-α forward primer 5′ GAC CCT CAC ACT CAG ATC ATC TTC T 3′; mouse TNF-α reverse primer 5′ CCT CCA CTT GGT GGT TTG CT 3′; mouse GAPDH forward primer 5′ TTCAACGGCACAGTCAAGGC 3′; mouse GAPDH reverse primer 5′ GACTCCACGACATACTCAGCACC 3′.
2.8 Nitrite measurement and ELISA of M-CSF, TNF-α, IL-1β, IL-10, and prostaglandin E2 (PGE2)
The production of NO was determined by measuring the accumulated levels of nitrite in the supernatant with the Griess reagent (detection limit: 0.5 μM). Cytokine production and PGE2 release in the culture medium were measured with the commercial ELISA kits from R&D Systems (Minneapolis, MN).
2.9 Statistical analysis
Data were expressed as mean ± SEM. Statistical significance was assessed by ANOVA followed by Bonferroni’s t test using GraphPad Prism software (GraphPad Software Inc., CA). A value of p < 0.05 was considered statistically significant.
3. Results
3.1 Pre-treatment of neuron-glia cultures with LPS programs microglial ET
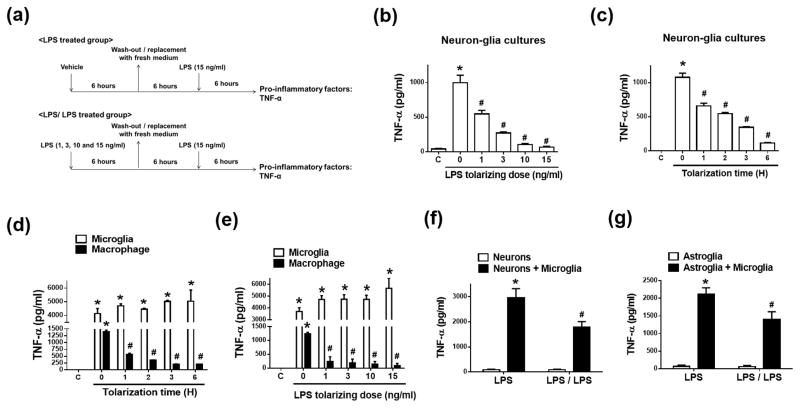
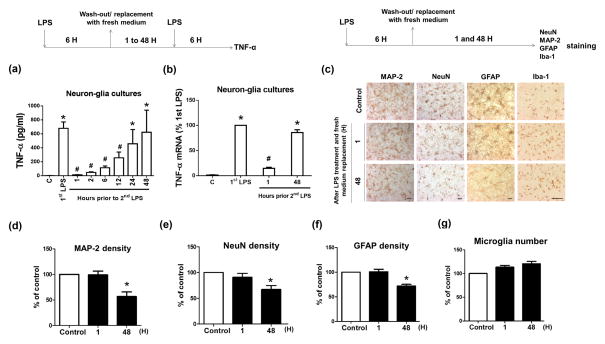
To investigate roles of different brain cells in the regulation of microglial ET, we prepared enriched cultures of single cell type and co-cultures of multiple cell types. Importantly, although the percentage of microglia in different cultures seems quite different, the number of microglial cells in these cultures is similar as shown in Materials and Methods. This makes our results on microglial ET formation in the different experiment settings comparable. Microglia coexist and constantly interact with neurons and astroglia in the brain, thus co-cultures better mimic the physiological microenvironment in the brain. We observed about 40% reduction in cell viability in microglia-enriched cultures 3 or 4 days after seeding. Microglia stay healthier and survive longer in co-culture with neurons and astroglia than in enriched cultures (Gebicke-Haerter et al., 1989; Zhang and Fedoroff, 1996). We pre-treated primary neuron-glia cultures with vehicle or 1–15 ng/ml LPS in the treatment medium for 1 to 6 hours. We then switched these cultures to fresh treatment media and incubated for additional 6 hours before challenging the cultures with 15 ng/ml LPS (Fig. 1a–c). Adopted from published literature, a reduced production of TNF-α after the 2nd LPS challenge was used as the key readout for ET formation in the present study. Because mRNA of LPS-induced pro-inflammatory factors was undetectable 6 hours after LPS was removed (data not shown), TNF-α production after the 2nd LPS treatment should be newly synthesized as a result of the 2nd LPS stimulation. LPS pre-treatment significantly diminished TNF-α secretion in a time- and dose-dependent manner in neuron-glia cultures exposed to the subsequent LPS challenge (Fig. 1b, c). For example, pre-treatment with either 15 ng/ml LPS for 1 hour or 1 ng/ml LPS for 6 hours significantly reduced TNF-α production. LPS-induced TNF-α production decreased by about 90% by pre-treatment with 15 ng/ml LPS for 6 hours (Fig. 1b, c). Such an optimized condition for ET induction was used for subsequent experiments. Collectively, microglia became tolerant to the repeated LPS stimulation in neuron-glia cultures.
Figure 1. Neurons and astroglia are required for microglial endotoxin tolerance.
(a, b) Neuon-glia cultures from C57BL/6J mice were pre-incubated with vehicle or different doses of LPS for 6 hours and then washed and incubated with fresh medium for 6 hours. LPS (15 ng/ml) was re-added into these cultures. TNF-a secretion was detected 6 hours later by ELISA assay. (c) Neuon-glia cultures were pre-treated with vehicle or 15 ng/ml LPS for 1 to 6 hours. After wash and incubation with fresh medium for 6 hours, these cultures were treated with 15 ng/ml LPS. Supernatant levels of TNF-α of these cultures were detected 6 hours later by ELISA assay. (d, e) Pre-treated with either 15 ng/ml LPS for 1 to 6 hours (d) or with different doses of LPS for 6 hours (e), brain microglia and peritoneal macrophages from C57BL/6J mice were washed and incubated with fresh medium for 6 hours. After that, LPS (15 ng/ml) was re-added into these cultures. TNF-α production in the supernatant of these cells was detected at 6 hours following LPS re-addition. Neuron-enriched (f), neuron-microglia (f), astroglia-enriched (g), and astroglia-microglia (g) cultures grown in 24-well plates were pre-treated with vehicle or 15 ng/ml LPS for 6 hours and then replaced with fresh medium and incubated for 6 hours. LPS (15 ng/ml) was re-added into these cells. Six hours later TNF-α production in the supernatant of these cells was detected by ELISA assay. LPS group indicated the cells were treated with LPS once; LPS/LPS group indicated the cells were pre-treated with LPS and then re-treated with LPS after the wash of the cultures and 6 hours of recovery. Asterisk, p<0.05, compared with corresponding vehicle-treated control cultures. Number sign, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment.
3.2 Neurons and astroglia are required for the induction of microglial ET
It is well known that microglia tightly interact with non-immune cells in the brain. We next investigated whether neurons and astroglia modulated microglial ET using multiple co-cultures or reconstituted brain cell cultures. Although the percentage of microglia in different cultures grown in 24-well plates is quite different, the number of microglia in these cultures is similar (5~6 × 104/well) as indicated in Materials and Methods. Microglia-enriched cultures contain ~98% microglia and 2% astroglia, neuron-microglia cultures contain 90% neurons and 10% microglia, mixed-glia cultures contain 80% astroglia and 20% microglia, neuron-enriched cultures contain 99% neurons, and astroglia-enriched cultures contain 99.5% astroglia. Interestingly, pre-treatment of microglia-enriched cultures with various doses of LPS for different periods of time failed to reduce TNF-α production induced by the subsequent LPS treatment (Fig. 1d, e). These results indicated that microglia were unable to form ET in the absence of neurons and astroglia. In contrast to brain microglia, peritoneal macrophages become refractory to the subsequent LPS treatment after LPS pre-treatment, as indicated by less TNF-α production in LPS pre-treatment group than the group without LPS pre-treatment (Fig. 1d, e). Here, our finding of ET in cultured peritoneal macrophages is consistent with previous studies (Kraatz et al., 1998; Zingarelli et al., 2008). Despite obvious differences in the turnover rate and antigen presentation, microglia share many similarities with macrophages (e.g. phagocytosis, cytotoxicity, scavenging, and cellular signaling) and are considered as “brain macrophages” (Gao and Hong, 2008; Gehrmann, 1996; Schmid et al., 2009). However, we found that microglia themselves, in the absence of neurons/astroglia, lacked the capacity to form ET when exposed to two consecutive LPS stimulations. This suggests a distinct regulatory mechanism of microglial ET by non-immune brain cells.
We further distinguish possible roles of neurons and astroglia in microglial ET. The results showed that pre-pretreatment of either neuron-microglia or mixed-glia cultures with LPS led to ~40% reduction in LPS-induced TNF-α production (LPS/LPS group) compared to the cells without LPS pre-treatment (LPS group) (Fig. 1f, g). Neuron-enriched and astroglia-enriched cultures had no TNF-α production in responses to LPS (Fig. 1f, g). Our results suggested that neurons and astroglia both participated in microglial ET. Taken together, we found that the presence of neurons and astroglia was necessary for programing microglia to form ET.
3.3 M-CSF secreted by neurons and astroglia modulates microglial ET
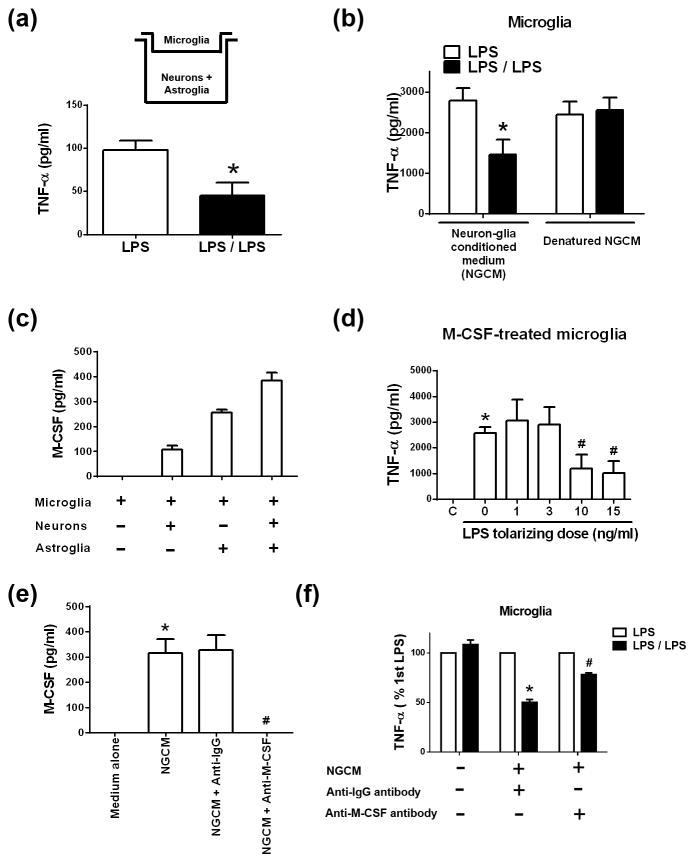
We next examined whether physical contact with neurons and astroglia was required for microglial ET using the transwell culture system (Fig. 2a). Although microglia in the upper inserts have no direct cell-cell contacts with neurons or astroglia grown in the lower compartment of the culture plate, soluble factors are permeable between the upper and the lower compartment of the plate. In this transwell culture system, LPS pre-treatment decreased TNF-α production from microglia triggered by the subsequent LPS treatment by 50% (Fig. 2a). These results indicated that the physical separation did not totally deprive neurons and astroglia of their ability to program microglia to form ET. Thus, it must be secreted soluble factor(s) from neurons and/or astroglia that modulated microglial ET. To more rigorously evaluate this possibility, we incubated microglia-enriched cultures with neuron-glia conditioned medium (NGCM) for 24 hours. In the presence of NGCM, microglia-enriched cultures exhibited refractory to the repeated LPS stimulation, as shown by half amount of TNF-α production in microglia with LPS pre-treatment (LPS/LPS group) compared with microglia without LPS pre-treatment (LPS group) (Fig. 2b). Denatured NGCM by high-temperature failed to affect ET formation in enriched microglia (Fig. 2b), suggesting heat-sensitive soluble factor(s) from neurons and/or astroglia modulated microglial ET.
Figure 2. M-CSF secreted by neurons and astroglia governs microglial endotoxin tolerance.
(a) Microglia were added into transwell inserts while neurons and astroglia grew confluent in the lower compartment of the 24-well plate as indicated. These cells were pre-treated for 6 hours with vehicle (LPS group) or 15 ng/ml LPS (LPS/LPS group) followed with wash and incubation with fresh medium. After that, LPS (15 ng/ml) was re-added into these cultures. TNF-α production in the supernatant of these cells was detected by ELISA at 6 hours following LPS re-addition. Asterisk, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. (b) Incubated for 12 hours in regular medium, neuron-glia conditioned medium (NGCM), or heat-denatured NGCM, microglia-enriched cultures were treated with 15 ng/ml LPS once (LPS group) or twice with a 12-hour interval and wash of the cultures at the middle of this interval (LPS/LPS group). TNF-α production in the supernatant of these cells was detected by ELISA at 6 hours after the last LPS treatment. Asterisk, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. (c) ELISA assay detected M-CSF production in the supernatant of neuron-microglia cultures, mixed-glia cultures containing microglia and astroglia, and neuron-glia cultures containing microglia, neurons and astroglia, but not microglia-enriched cultures. (d) Microglia were incubated with vehicle or 500 pg/ml M-CSF for 24 hours. Pre-treatment of the M-CSF-stimulated microglia with LPS at indicated doses for 6 hours was followed by incubation with fresh media for additional 6 hours. LPS was re-added into these cultures. Six hours later TNF-α production in the supernatant of these cells was detected by ELISA. Asterisk, p<0.05, compared with corresponding vehicle-treated control cultures. Number sign, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. (e) ELISA detected M-CSF in NGCM and NGCM neutralized with anti-IgG or anti-M-CSF antibody. Asterisk, p<0.05, compared with regular medium group. Number sign, p<0.05, compared with NGCM group. (f) Microglia were incubated with regular microglial medium or NGCM that was pre-incubated with either anti-IgG or anti-M-CSF neutralizing antibody for 12 hours. These cells were treated with 15 ng/ml LPS once (LPS group) or twice (LPS/LPS group) as described in (b). Six hours later, TNF-α secretion was detected by ELISA. Asterisk, p<0.05, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. Number sign, p<0.05, compared with LPS/LPS-treated cultures with anti-IgG antibody treatment group.
We then investigated what soluble factor(s) derived from neurons and/or astroglia modulated microglial ET. Among multiple factors secreted in our culture systems, M-CSF was detected in the supernatant of cell cultures containing neurons and astroglia, including neuron-glia cultures, mixed-glia cultures, and neuron-microglia cultures, but not in microglia-enriched cultures (Fig. 2c). Given the origin of M-CSF from neurons/astroglia and the requirement of neurons or astroglia for microglial ET, we studied whether M-CSF regulated microglial ET. Pre-incubation of microglia-enriched cultures with M-CSF recombinant protein for 24 hours endowed microglia with capacity of ET (Fig. 2d). We pre-treated M-CSF-derived microglia with LPS at doses of 1, 3, 10 and 15 ng/ml before stimulating the cells with 15 ng/ml LPS. The results showed that pre-treatment with LPS at 10 and 15 ng/ml significantly reduced LPS-induced TNF-α production in enriched microglia (Fig. 2d). These results indicated that M-CSF-treated microglia were able to form ET when they were exposed to two consecutive LPS treatments. To assess whether M-CSF is a key determinant of microglial ET in the neuron-glia cultures, we removed M-CSF from the NGCM by using M-CSF neutralizing antibody. While anti-IgG antibody did not affect the level of M-CSF in the NGCM, M-CSF was undetectable in neutralized NGCM by anti-M-CSF antibody (Fig. 2e). Pre-incubated with anti-IgG-neutralized NGCM, enriched microglia remained refractory to the second LPS challenge, as shown by reduced TNF-α production by 50% (Fig. 2f). In contrast, removal of M-CSF in the NGCM by neutralization reversed the reduced responsiveness of enriched microglia to the second LPS stimulation following a previous LPS pre-treatment (Fig. 2f). Therefore, M-CSF secreted by neurons and astroglia plays an important role in modulating microglial ET. Other common inflammatory factors detected in our neuron-glia culture systems, including TNF-α, IL-1β, PGE2, and NADPH oxidase-derived superoxide free radical, did not significantly affect microglial ET formation (Supplemental Fig. 1). Thus, M-CSF appears an important determining factor for non-cell autonomous regulation of microglial ET formation.
3.4 M-CSF receptor and its downstream ERK1/2 signals are responsible for M-CSF-mediated microglial ET
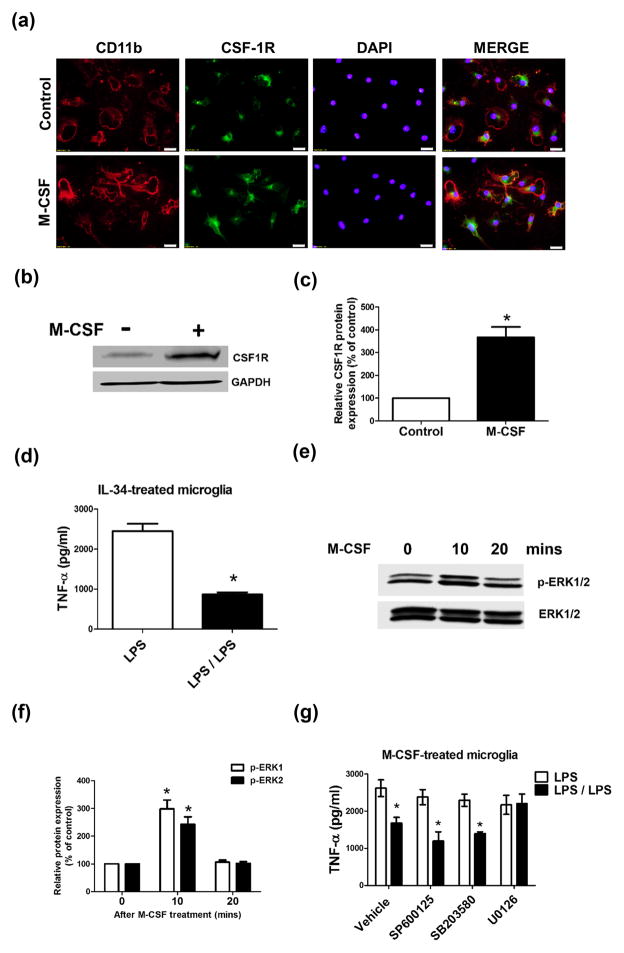
To search for potential signaling pathways mediating the action of M-CSF on microglial ET, we first determined the expression of M-CSFR (also known as CSF1R) on microglia. Immunofluorescence and Western blotting detected a low level of expression of M-CSFR in microglia-enriched cultures under resting condition (Fig. 3a–c). After M-CSF treatment, the expression of M-CSFR in enriched microglia dramatically increased (Fig. 3a–c), which is consistent with a previous publication showing M-CSF-elicited increase in M-CSFR expression in adult human microglia (Smith et al., 2013). M-CSFR was mainly expressed in the membrane that was indicated by immunostaining of CD11b (a surface integrin receptor) and to a lesser degree in the cytoplasm of primary microglia (Fig. 3a). The observed cytoplasmic staining of M-CSFR may partially result from possible receptor internalization after M-CSF binding. M-CSF-induced increase in microglial M-CSFR expression, combined with the demonstrated paracrine of M-CSF, strongly suggests a non-cell-autonomous requirement for M-CSFR activation in microglial ET. Anti-IgG antibody did not detect obvious immune-staining, indicating the specificity of anti-M-CSFR antibody (data not shown). Furthermore, interleukin 34 (IL-34), another ligand for M-CSFR, was used to verify the role of M-CSFR activation in modulating microglial ET. Similar to M-CSF, IL-34 treatment led to ET formation in enriched microglia (Fig. 3d). These results pointed out a critical role of the activation of M-CSFR in the regulation of microglial ET.
Figure 3. M-CSF mediates microglial endotoxin tolerance through M-CSF receptor and its downstream ERK1/2 signaling pathways.
(a, b) Microglia-enriched cultures were treated with vehicle or 500 pg/ml M-CSF for 24 hours. Anti-M-CSFR (CSF1R) antibody was used to detect M-CSFR expression by immunofluorescence (a) and western blot (b). GAPDH was used as loading control (b). (c) The density of the CSF1R signals was quantified and normalized to the corresponding control. The results are expressed as a percentage of the vehicle-treated control group (mean ± SEM) from three to four experiments performed in duplicate and are analyzed using Student’s t-test. Asterisk, P < 0.05 compared with vehicle-treated control. (d) Microglia-enriched cultures were treated with 500 pg/ml IL-34 for 24 hours and then were treated with 15 ng/ml LPS once (LPS group) or twice with a 12-hour interval and wash of the cultures at the middle of this interval (LPS/LPS group). TNFα secretion from these cells was detected at 6 hours later by ELISA. Asterisk, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. (e) Microglia-enriched cells were treated with vehicle or 500 pg/ml M-CSF. Phospho-ERK1/2 (p-ERK1/2) and ERK1/2 were detected at 10 and 20 minutes after M-CSF treatment by western blot. (f) The density of the p-ERK1/2 signals was quantified and normalized to the corresponding controls. The experiment has been performed three times. Results are shown as the mean ± SEM. Asterisk, p < 0.05, compared with corresponding vehicle-treated control cultures. (g) Microglia-enriched cultures were pre-treated with vehicle, SP600125 (5 μM; JNK inhibitor), SB203580 (5 μM; p38 inhibitor), and U0126 (5 μM; MEK1/2 inhibitor) for 1 hour, and then treated with 500 pg/ml M-CSF. Twenty four hours later, these cells were treated with 15 ng/ml LPS once (LPS group) or twice (LPS/LPS group) as described in (b). TNF-α production in the supernatant of these cells was measured at 6 hours later by ELISA. Asterisk, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment.
Binding of M-CSFR by M-CSF has been reported to activate the MAPK pathway (Gobert Gosse et al., 2005; Huang et al., 2013). Treatment of microglia by M-CSF significantly increased the phosphorylation of ERK1/2 (Fig. 3e, f). Importantly, inhibition of ERK1/2 activation by U0126 (a selective inhibitor of the MAPKK MEK1/2) prevented ET formation in M-CSF-treated microglia (Fig. 3g). By contrast, JNK inhibitor SP6000125 and p38 inhibitor SB203580 failed to affect ET of M-CSF-treated microglia (Fig. 3g). Thus, activation of M-CSFR and downstream ERK1/2 signals by M-CSF secreted from neurons and astroglia modulates microglial ET.
3.5 Tolerant microglia appear M2-like polarized phenotype and are neuroprotective
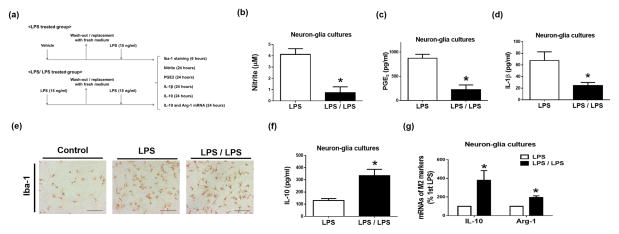
Tolerant microglia released much less pro-inflammatory mediators, including TNF-α (Fig. 1–3), NO, PGE2, IL-1β in neuron-glia cultures in response to the second LPS treatment compared with the first LPS treatment (Fig. 4a–d). However, immune-staining of microglial marker Iba-1 showed similar morphological alterations of activation in LPS and LPS/LPS groups (Fig. 4e). Interestingly, tolerant microglia expressed higher levels of mRNA and protein of anti-inflammatory cytokine IL-10 than microglia exposed to LPS once (Fig. 4f, g). Increased mRNA expression of Arg-1, a M2 marker, after the second LPS treatments of neuron-glia cultures suggested M2 phenotype of tolerant microglia (Fig. 4g).
Figure 4. Tolerant microglia display M2-like polarized phenotype.
(a–g) Neuron-glia cultures were pre-treated with vehicle (LPS group) or 15 ng/ml LPS (LPS/LPS group) for 6 hours and then replaced with fresh treatment medium. Additional 6 hours later, LPS (15 ng/ml) was added into the medium again. At 24 hours after LPS re-stimulation, supernatant levels of nitrite (b) was measured with the Griess reagent, pro-inflammatory factors PGE2 (c) and IL-1β (d) and anti-inflammatory cytokine IL-10 (f) were detected by ELISA at different time points indicated in (a). Levels of Iba-1 protein (e) and mRNA of M2 markers IL-10 and Arg-1 (g) were measured by immunocytochemistry (e) and real-time PCR (g), respectively. Asterisk, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment.
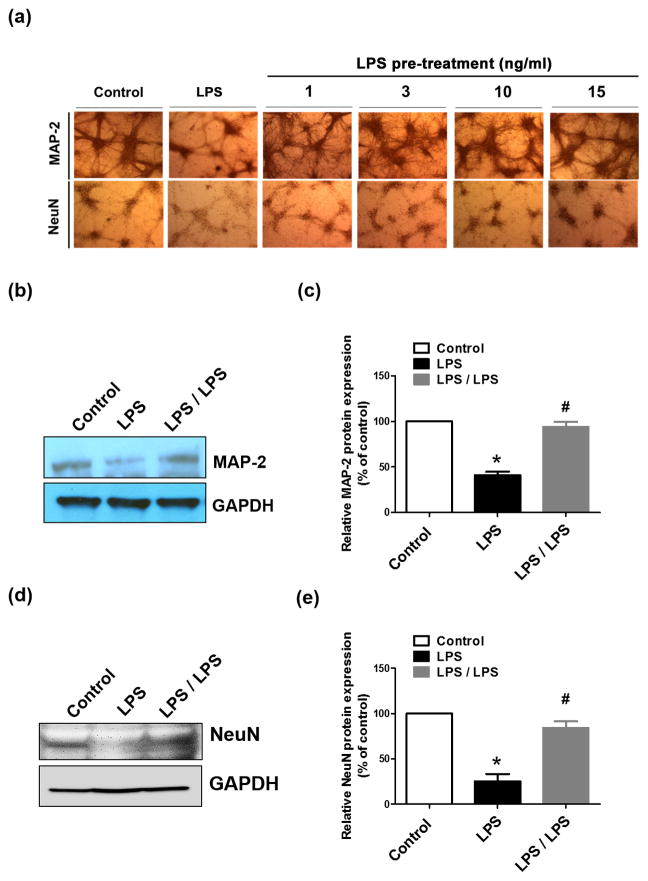
ET is an important mechanism for host protection against over-exuberant inflammation and consequent tissue destruction and pathological manifestation, such as sepsis, acute coronary syndrome, and autoimmune diseases (Lopez-Collazo and del Fresno, 2013). We next found that tolerant microglia prevented neuroinflammation-elicited neuronal damages (Fig. 5). LPS treatment of neuron-glia cultures for 48 hours led to damages to neurons as shown by decreased density of MAP-2-immunoreactive cells and their dendrites and NeuN-immunoreactive cells, (Fig. 5a) as well as reduced expression of MAP-2 and NeuN (Fig. 5b–e). However, LPS pre-treatment for 6 hours followed by wash and a recovery period of 6 hours prevented neuron-glia cultures from neuronal injures elicited by the second LPS exposure for 48 hours (Fig. 5a–e). Thus, microglial ET can play a role in neuroprotection.
Figure 5. Tolerant microglia protect neurons from repeated inflammatory insult.
Neuron-glia cultures were treated with vehicle or 15 ng/ml LPS for 6 hours and then replaced with fresh medium. Additional 6 hours later, LPS (15 ng/ml) was added into the medium again. Expression of MAP-2 (a–c) and NeuN (a, d, e) in these cultures was measured 48 hours later by immunocytochemistry (a) and western blots (b, d). GAPDH served as an internal control. (c, e) The density of the MAP-2 and NeuN immunostaining was quantified and normalized to corresponding controls. The results are expressed as a percentage of the vehicle-treated control group (mean ± SEM) from three to four experiments performed in duplicate. Asterisk, p<0.05, compared with corresponding vehicle-treated control cultures. Number sign, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment.
3.6 Recovery of microglial ET exacerbates inflammation-mediated neuronal injuries
It has been reported that ET of monocytes and macrophages is not permanent and it recovered days after ET induction (Fahmi and Chaby, 1994; Wysocka et al., 2005). It is important to test whether brain microglia recover from ET. We determined ET induction after two consecutive LPS treatments with different time intervals. Within 12 hours after the wash of the cultures (namely 18 hours after LPS pre-treatment), LPS-pre-treated neuron-glia cultures produced less TNF-α when encountered new LPS stimulation than the cultures without LPS pre-treatment (Fig. 6a), indicating the existence of ET. By contrast, at 24 and 48 hours after the wash (namely 30 and 54 hours after LPS pre-treatment, respectively), neuron-glia cultures with or without LPS pre-treatment produced similar amount of TNF-α when stimulated with LPS, indicating no ET formation of microglia when two LPS challenges were separated by longer than 30 hours in our culture system (Fig. 6a). Consistently, measurement of TNF-α mRNA revealed recovery of microglia from refractory state in neuron-glia cultures at 48 hours after the wash of the cultures (namely 54 hours after initial LPS treatment) (Fig. 6b). Thus, microglia in neuron-glia cultures recovered from ET state within 2 days after tolerance induction and could respond to subsequent endotoxin stimulation. Furthermore, expression of MAP-2, NeuN, and GFAP was significantly decreased in neuron-glia cultures at 48 after the wash of the cultures following 6-hour LPS treatment (Fig. 6c–f). Iba-1 staining and counting of microglial cells revealed about 10% increases (insignificant) between the wash of the culture after 6-hour LPS treatment and 48-hour incubation with fresh medium (Fig. 6c, g). Such an insignificant increase excludes the possibility that increased microglial proliferation is responsible for the recovery of ET (Fig. 6a, b). Thus, after LPS challenge, microglia become tolerant at the early stage of neuroinflammation when neurons and astroglia remain healthy, and tolerant microglia recover from refractory state at later stage (Fig. 6a, b). Altogether, neuroinflammation mediates injuries to neurons and astroglia, which in turn damage the capacity of microglial ET. Loss of such tolerance deprives microglia of an essential immune-homeostatic response to repeat exposure to endotoxin thereby losing protection against excessive inflammation, which exacerbates inflammation-mediated neuronal injuries (Fig. 7). Loss of ET is an important pathogenetic mechanism of inflammation-associated neuronal damages. This could be an important mechanism of non-immune cells modulates brain immune homeostasis.
Figure 6. Microglia loss their ability to form ET when surrounding neurons/astroglia were damaged.
(a, b) Neuron-glia cultures were pre-treated with 15 ng/ml LPS for 6 hours. After washed and incubated with fresh media for indicated time, the cultures were treated with LPS and the expression of TNF-α at the level of protein (a) and mRNA (b) was detected 6 hours later. Asterisk, p<0.05, compared with corresponding vehicle-treated control cultures. Number sign, p<0.05, compared with corresponding LPS-treated cultures without LPS pre-treatment. (c–g) Neuron-glia cultures were treated with vehicle or 15 ng/ml LPS for 6 hours and then replaced with fresh medium. Additional 1 or 48 hours later, the expression of MAP-2, NeuN, GFAP and Iba-1 in those cells was measured by immunocytochemistry. (d–f) The density of MAP-2, NeuN and GFAP immunostaining shown in (c) was measured and quantified. (g) The number of Iba-1-immunoreactive microglia of each well was counted under the microscope. Data were shown as the percentage of vehicle-treated control and expressed as the means ± SEM from 5 independent experiments in triplicate. Asterisk, p<0.05 and Number sign, p<0.01, compared with corresponding vehicle-treated control cultures.
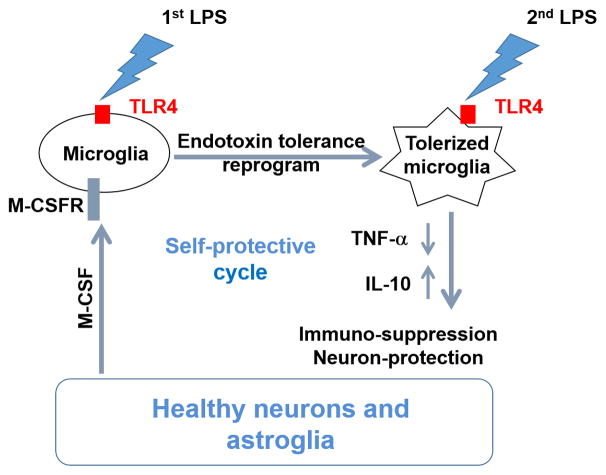
Figure 7. Neurons and astroglia govern microglial ET through M-CSFR-mediated ERK1/2 activation to prevent inflammation-induced neuronal damages.
Under endotoxin challenge, resting microglia could transform into tolerant phenotype where they produce less pro-inflammatory factors such as TNF-α when encountered a new LPS stimulation. Different from peripheral macrophages, microglia form endotoxin tolerance via a non-cell-autonomous mechanism. Microglia lose their endotoxin tolerance capacity in the absence of neurons and astroglia. The tolerant microglia occur at early stage of neuroinflammation; however they become non-tolerant at later stage of neuroinflammation when neurons and astroglia are injured. M-CSF secreted by neurons and astroglia acts on M-CSFR on microglia to modulate microglia endotoxin tolerance through downstream intracellular ERK1/2 signals.
4. Discussion
In this study, we identified a distinct and essential role of non-immune brain cells (neurons and astroglia) in microglial ET development. In the absence of neurons and astroglia, microglia-enriched cultures failed to form ET. M-CSF secreted by neurons and astroglia acted on microglial M-CSFR (CSF1R) to activate ERK1/2 to program microglial ET. During the neuroinflammatory process, the transition of microglia from tolerance to non-tolerance to repeated LPS exposure is strongly associated with decreased viability of neurons and astroglia. To the best of our knowledge, our study provides the first evidence that non-immune cells, neurons and astroglia, are able to influence the capacity of microglial ET. Together, this study revealed a novel regulatory interaction among neurons, astroglia, and microglia in the development of microglial ET.
ET formation in the brain and cultured brain slices or microglial cells has been reported by a few recent studies (Ajmone-Cat et al., 2013; Ajmone-Cat et al., 2003; Beurel and Jope, 2010; Schaafsma et al., 2015). However, the molecular mechanism underlying microglial ET has not been fully investigated. Dr. Eggen and his colleagues have recently reported for the first time that a single intraperitoneal or intracerebroventricular LPS injection leads to a reduced pro-inflammatory response to a subsequent LPS stimulation in mouse brain. Further mechanistic studies indicated that stable and long lasting epigenetical alterations in microglia modify their endotoxin sensitivity and tolerance to a subsequent endotoxin challenge (Schaafsma et al., 2015). In organotypic hippocampal slice cultures, LPS re-stimulation after a recovery time of 7 days from the previous LPS stimulation(s) promotes an anti-inflammatory response and microglial polarization toward M2-like phenotype but suppresses inflammatory response (Ajmone-Cat et al., 2013). Thus, in vivo (Schaafsma et al., 2015), ex vivo (Ajmone-Cat et al., 2013), and in vitro (Fig. 1) findings consistently show microglial ET formation in the presence of neurons and astroglia.
In the present study, we found that non-immune brain cells, neurons and astroglia, are essential for brain microglia to form ET (Fig. 1). This is different from ET development in peripheral monocytes/macrophages, where other cell types are not required. It is generally believed that microglia arise from mesodermal (myeloid) tissues during embryonic and fetal development (Kreutzberg, 1996). Microglia and peripheral macrophages are derived from the same primitive myeloid progenitors and perform similar function upon activation (e.g. phagocytosis, proliferation, and inflammatory/cytotoxic secretion). However, microglia and peripheral macrophages are quite different in turnover rate, antigen presentation capacity, and spatial/temporal regulation (Gao and Hong, 2008; Kreutzberg, 1996). We found isolated peripheral macrophages formed tolerance when exposed to repeat LPS challenges (Fig. 1), which is consistent with reported studies (Kraatz et al., 1998; Zingarelli et al., 2008); however, enriched microglia failed to form ET (Fig. 1). The surrounding microenvironment may also contribute to the functional disparity of macrophages and microglia (Gehrmann et al., 1995). Tight interactions among neurons, microglia, and astroglia not only guarantee the effective network of energy metabolism and signal transmission, but also maintain the status of immune homeostasis in the brain. MCS-F secretion by peripheral macrophages/monocytes (Rambaldi et al., 1987) but not by brain microglia (Fig. 2) may partially explain the observed difference in ET formation in cultured macrophages and microglia.
The distinct non-cell autonomous regulation of microglial ET found in our study (Fig. 1) seems to disagree with the autonomous regulation of microglial ET reported by three previous studies (Ajmone-Cat et al., 2003; Beurel and Jope, 2010; Schaafsma et al., 2015). Difference in LPS strain/contamination, culture methods, repeated treatment schemes (dose, duration, interval and recovery in fresh medium), sample collection times, and other unrecognized reasons may contribute to the observed discrepancies in ET formation in microglia-enriched cultures. Microglia respond differently to the same stimulus if other stimuli precede, co-exist with or follow it. This prepares microglia for an enhanced or decreased response to a second challenge. Whether possible difference in contamination of nucleic acids (common contaminants of phenol-extracted LPS and known activators of innate immune cells including microglia) in different LPS in the four studies may differently affect microglial endotoxin sensitivity and ET formation is unknown. Interestingly, LPS-preconditioning of neonatal or adult mouse microglial cultures results in reduced responsiveness to a subsequent LPS stimulation (Schaafsma et al., 2015). M-CSF-containing L929 fibroblast-conditioned medium (33% in the mixed glial medium) or conditioned medium collected from the mixed glial cultures (50% in microglial medium) was used to stimulate microglia proliferation during culturing neonatal microglia or treating microglia, respectively (Schaafsma et al., 2015). We found that microglia-enriched cultures, when incubated with NGCM or M-CSF protein, developed ET upon repeated LPS treatments (Fig. 2b, 2d). Thus, we may speculate that the seemingly autonomous regulation of microglial ET (Schaafsma et al., 2015) may have involvement of astroglia-derived soluble factors (e.g. M-CSF).
Accumulating evidence has demonstrated that both direct cell-cell contact and indirect volume transmission through soluble factors play important roles in regulation of neuroinflammation and progressive neuronal loss (Block and Hong, 2005; Fuxe et al., 2015; Gundersen et al., 2015; Jiang et al., 2015). In studying mechanisms of non-cell autonomics regulation of microglial ET, we found that pre-incubation of microglia-enriched cultures with NGCM partly restored the ET of microglia. The release of M-CSF from cultured neurons/astroglia but not microglia, the induction of microglial ET by M-CSF recombinant protein, and the attenuation in microglial ET by neutralization of M-CSF in NGCM together indicate an important role of M-CSF in modulating microglial ET (Fig. 2d–f). However, incomplete blockage of microglial ET (Fig. 2f) by complete neutralization of M-CSF in the NGCM (Fig. 1d), along with incomplete microglial ET in the presence of NGCM (Fig. 2b), suggests involvement of direct cell-cell contact or other soluble factor(s) in microglial ET formation. The finding that physical separation of microglia from neurons and astroglia did not abolish microglial ET development (Fig. 2a) indicates direct cell-cell contact is not absolutely required for microglial ET. But, direct cell-cell contact between microglia and neurons/astroglia might also contribute to microglial ET regulation. Future studies on effects of gene ablation of neuron-glia adhesion molecules such as CD200R, CD172a and CD45 on microglial ET will help to address this question. Several common inflammatory factors detected in our neuron-glia culture systems, including TNF-α, IL-1β, PGE2, and NADPH oxidase-derived free radicals, did not alter microglial ET development (Supplemental Fig. 1). By contrast, these inflammatory factors have been reported to participate in the development of ET in peripheral macrophages (Medvedev et al., 2000). The disparity in ET regulation in macrophages and microglia not only supports a distinct non-cell autonomics mechanism of microglial ET but also indicates a unique role of M-CSF in microglial ET.
M-CSF and its receptor M-CSFR play critical roles in microglial differentiation and proliferation during the embryonic period (Otero et al., 2009; Sasaki et al., 2000; Smith et al., 2013). Elegant studies have revealed fewer brain microglia in M-CSF-deficient mice than wildtype mice and no brain microglia in M-CSFR-deficient mice (Ginhoux et al., 2010). Mutations in gene encoding M-CSFR cause hereditary diffuse leukoencephalopathy with spheroids (Rademakers et al., 2012). Inhibition of M-CSFR activity blocks glioma progression by altering macrophage/microglia polarization (Pyonteck et al., 2013) and slows neurodegeneration and disease progression by suppressing microglial proliferation in models of Creutzfeldt-Jakob disease and Alzheimer’s disease (Gomez-Nicola et al., 2013). M-CSF improves cellular growth, survival, and phagocytic activity of microglia under physiopathological conditions (Boissonneault et al., 2009; Elmore et al., 2014; Liu et al., 1994; Mitrasinovic et al., 2001; Mitrasinovic et al., 2003). M-CSF suppresses microglial response to LPS (Lodge and Sriram, 1996), augments β-amyloid-induced IL-1, IL-6, and NO production by microglia (Murphy et al., 1998), and regulates programmed death of cultured rat microglia (Gehrmann, 1995). In the present study, we elucidated a novel function of M-CSF in modulating microglial ET formation beyond their known roles in early development. Our results also indicated that modulation of microglial ET by M-CSF is M-CSFR dependent (Fig. 3). We found exogenous IL-34, another ligand for M-CSFR, was also able to induce ET in microglia-enriched cultures (Fig. 3d). Recent studies have shown that IL-34 is primarily produced by neurons, specifically directs the differentiation of myeloid cells in the developing CNS and determines the phenotype discrepancy in M-CSF-deficient and M-CSFR-deficient mice described above (Mizuno et al., 2011; Nandi et al., 2012; Wang et al., 2012). IL-34 deficiency mainly affects development but not the ability of microglia to produce inflammatory cytokines (Greter et al., 2012; Nakamichi et al., 2013; Wang and Colonna, 2014). IL-34 was undetectable in our culture system (data not shown). Whether IL-34 participates in microglial ET regulation warrants further investigation. Binding of M-CSF to M-CSFR triggers activation of several signal pathways such as PI3K-Akt and ERK1/2 to turn on expression of genes that modulate multiple cellular behaviors (Imai and Kohsaka, 2002). Increased expression of M-CSFR (CSF1R) in microglia and neurons in patients and/or mouse models of ischemic or traumatic brain injury implies the clinical relevance of this receptor in CNS diseases (Du Yan et al., 1997; Mitrasinovic et al., 2005). More interestingly, microglia with increased expression of M-CSFR are neuroprotective against NMDA-induced neurotoxicity in a microglial-hippocampal organotypic co-culture system (Mitrasinovic et al., 2005).
Tolerant microglia appeared M2-like phenotype and protected neurons against the LPS-induced neuronal damage (Fig. 4). Those results, combined with a necessary role of neurons/astroglia in microglial ET formation (Fig. 1), imply that a positive self-protective cycle exists between healthy neurons and M2-like tolerant microglia in the early stage of neuroinflammatory process (Figure 7). Conversely, tolerant microglia lost their tolerance to the subsequent LPS stimulation when surrounding neurons and astroglia are injured (Fig. 6), thereby losing protection against excessive inflammation. Loss of ET may be an important pathogenetic factor of inflammation-associated neuronal damages. Our studies also describe a potential relationship among initial microglial activation, later microglial ET development, and possible prevention of excessive damages to the CNS in case of a recurrent immune challenge.
The present study revealed that astroglia by releasing M-CSF and other possible factors participated in the regulation of microglial ET (Fig. 1g and Fig. 2c). Data collected from experiments involving neuron-glia cultures or NGCM (Fig. 1b, c; Fig. 2b, e, f; and Fig. 4b–d) also support involvement of astroglia in microglial ET regulation and neuroinflammatory process. Interestingly, a recent study has reported inflammatory tolerance in astroglia (Beurel and Jope, 2010). Further studies on molecular mechanisms of astroglial ET regulation will advance our understating of brain immune tolerance and its function in prevention of excessive neuronal damages.
In conclusion, our study identifies a novel non-cell autonomous regulatory mechanism of microglial ET by neurons and astroglia. Released M-CSF by these non-immune cells acted on M-CSFR and activated downstream ERK1/2 signals to regulate microglial ET. A better understanding of the heterogeneity of microglial activation including ET might provide a glia-based therapeutic strategy to prevent excessive damages to the CNS in case of a recurrent immune challenge.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NIH/NIEHS (ES090082) to J.-S. H, the National Natural Science Foundation of China (No. 31471006 and No. 21577004), the national high technology research and development program of China (863 program; No. 2014 AA021601), and the award to high-level innovative and entrepreneurial talents of Jiangsu Province of China to H.-M. G. We thank Anthony Lockhart for the assistance with animal colony management and maintenance of the timed pregnant mice.
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
References
- Ajmone-Cat MA, Mancini M, De Simone R, Cilli P, Minghetti L. Microglial polarization and plasticity: evidence from organotypic hippocampal slice cultures. Glia. 2013;61:1698–1711. doi: 10.1002/glia.22550. [DOI] [PubMed] [Google Scholar]
- Ajmone-Cat MA, Nicolini A, Minghetti L. Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. Journal of neurochemistry. 2003;87:1193–1203. doi: 10.1046/j.1471-4159.2003.02087.x. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Glycogen synthase kinase-3 regulates inflammatory tolerance in astrocytes. Neuroscience. 2010;169:1063–1070. doi: 10.1016/j.neuroscience.2010.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in immunology. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in neurobiology. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain: a journal of neurology. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, Hong JS. Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods in molecular biology. 2013;1041:231–240. doi: 10.1007/978-1-62703-520-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CH, Chen SH, Wang Q, Langenbach R, Li H, Zeldin D, Chen SL, Wang S, Gao H, Lu RB, Hong JS. PGE2 Inhibits IL-10 Production via EP2-Mediated beta-Arrestin Signaling in Neuroinflammatory Condition. Molecular neurobiology. 2015;52:587–600. doi: 10.1007/s12035-014-8889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, Schmidt AM. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmi H, Chaby R. Differential recovery of macrophages from endotoxin-tolerant states elicited by lipopolysaccharide and enzymatic treatments. Immunological investigations. 1994;23:243–258. doi: 10.3109/08820139409066821. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Marcoli M, Borroto-Escuela DO. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochemical research. 2015 doi: 10.1007/s11064-015-1574-5. [DOI] [PubMed] [Google Scholar]
- Gao H, Zhou H, Zhang F, Wilson B, Kam W, Hong J. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends in immunology. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989;9:183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J. Colony-stimulating factors regulate programmed cell death of rat microglia/brain macrophages in vitro. Journal of neuroimmunology. 1995;63:55–61. doi: 10.1016/0165-5728(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Gehrmann J. Microglia: a sensor to threats in the nervous system? Research in virology. 1996;147:79–88. doi: 10.1016/0923-2516(96)80220-2. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain research Brain research reviews. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert Gosse S, Bourgin C, Liu WQ, Garbay C, Mouchiroud G. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cellular signalling. 2005;17:1352–1362. doi: 10.1016/j.cellsig.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33:2481–2493. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial Transmission. Physiological reviews. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- Hoppstadter J, Kessler SM, Bruscoli S, Huwer H, Riccardi C, Kiemer AK. Glucocorticoid-Induced Leucine Zipper: A Critical Factor in Macrophage Endotoxin Tolerance. Journal of immunology. 2015;194:6057–6067. doi: 10.4049/jimmunol.1403207. [DOI] [PubMed] [Google Scholar]
- Huang F, Cao J, Liu Q, Zou Y, Li H, Yin T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1beta induced in human endometriosis stromal cells in vitro. International journal of clinical and experimental pathology. 2013;6:2129–2136. [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Jiang L, Chen SH, Chu CH, Wang SJ, Oyarzabal E, Wilson B, Sanders V, Xie K, Wang Q, Hong JS. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia. 2015;63:1057–1072. doi: 10.1002/glia.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraatz J, Clair L, Bellingham J, Wahlstrom K, Rodriguez JL, West MA. Lipopolysaccharide pretreatment produces macrophage endotoxin tolerance via a serum-independent pathway. The Journal of trauma. 1998;45:684–691. doi: 10.1097/00005373-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Liu W, Brosnan CF, Dickson DW, Lee SC. Macrophage colony-stimulating factor mediates astrocyte-induced microglial ramification in human fetal central nervous system culture. The American journal of pathology. 1994;145:48–53. [PMC free article] [PubMed] [Google Scholar]
- Lodge PA, Sriram S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. Journal of leukocyte biology. 1996;60:502–508. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- Lopez-Collazo E, del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Critical care. 2013;17:242. doi: 10.1186/cc13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. Journal of immunology. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Mendez C, Kramer AA, Salhab KF, Valdes GA, Norman JG, Tracey KJ, Carey LC. Tolerance to shock: an exploration of mechanism. Annals of surgery. 1999;229:843–849. doi: 10.1097/00000658-199906000-00011. discussion 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Grattan A, Robinson CC, Lapustea NB, Poon C, Ryan H, Phong C, Murphy GM., Jr Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system. J Neurosci. 2005;25:4442–4451. doi: 10.1523/JNEUROSCI.0514-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Perez GV, Zhao F, Lee YL, Poon C, Murphy GM., Jr Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. The Journal of biological chemistry. 2001;276:30142–30149. doi: 10.1074/jbc.M104265200. [DOI] [PubMed] [Google Scholar]
- Mitrasinovic OM, Vincent VA, Simsek D, Murphy GM., Jr Macrophage colony stimulating factor promotes phagocytosis by murine microglia. Neuroscience letters. 2003;344:185–188. doi: 10.1016/s0304-3940(03)00474-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, Sonobe Y, Takeuchi H, Suzumura A. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-beta neurotoxicity. The American journal of pathology. 2011;179:2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GM, Jr, Yang L, Cordell B. Macrophage colony-stimulating factor augments beta-amyloid-induced interleukin-1, interleukin-6, and nitric oxide production by microglial cells. The Journal of biological chemistry. 1998;273:20967–20971. doi: 10.1074/jbc.273.33.20967. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. Journal of bone and mineral metabolism. 2013;31:486–495. doi: 10.1007/s00774-013-0476-3. [DOI] [PubMed] [Google Scholar]
- Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Developmental biology. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nature immunology. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, Adamson J, Kouri N, Sundal C, Shuster EA, Aasly J, MacKenzie J, Roeber S, Kretzschmar HA, Boeve BF, Knopman DS, Petersen RC, Cairns NJ, Ghetti B, Spina S, Garbern J, Tselis AC, Uitti R, Das P, Van Gerpen JA, Meschia JF, Levy S, Broderick DF, Graff-Radford N, Ross OA, Miller BB, Swerdlow RH, Dickson DW, Wszolek ZK. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nature genetics. 2012;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A, Young DC, Griffin JD. Expression of the M-CSF (CSF-1) gene by human monocytes. Blood. 1987;69:1409–1413. [PubMed] [Google Scholar]
- Sasaki A, Yokoo H, Naito M, Kaizu C, Shultz LD, Nakazato Y. Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor. Neuropathology: official journal of the Japanese Society of Neuropathology. 2000;20:134–142. doi: 10.1046/j.1440-1789.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma W, Zhang X, van Zomeren KC, Jacobs S, Georgieva PB, Wolf SA, Kettenmann H, Janova H, Saiepour N, Hanisch UK, Meerlo P, van den Elsen PJ, Brouwer N, Boddeke HW, Eggen BJ. Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain, behavior, and immunity. 2015;48:205–221. doi: 10.1016/j.bbi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, Sutcliffe JG, Carson MJ. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. Journal of neurochemistry. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, Curtis MA, Faull RL, Dragunow M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. Journal of neuroinflammation. 2013;10:85. doi: 10.1186/1742-2094-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. European journal of immunology. 2014;44:1575–1581. doi: 10.1002/eji.201344365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nature immunology. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka M, Montaner LJ, Karp CL. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. Journal of immunology. 2005;174:7398–7402. doi: 10.4049/jimmunol.174.11.7398. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Fedoroff S. Neuron-microglia interactions in vitro. Acta neuropathologica. 1996;91:385–395. doi: 10.1007/s004010050440. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liao J, Aloor J, Nie H, Wilson BC, Fessler MB, Gao HM, Hong JS. CD11b/CD18 (Mac-1) is a novel surface receptor for extracellular double-stranded RNA to mediate cellular inflammatory responses. Journal of immunology. 2013;190:115–125. doi: 10.4049/jimmunol.1202136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B, Fan H, Ashton S, Piraino G, Mangeshkar P, Cook JA. Peroxisome proliferator activated receptor gamma is not necessary for the development of LPS-induced tolerance in macrophages. Immunology. 2008;124:51–57. doi: 10.1111/j.1365-2567.2007.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.