Abstract
A phospho-null Ala substitution at protein kinase C (PKC)-targeted cardiac troponin I (cTnI) S43/45 reduces myocyte and cardiac contractile function. The goal of the current study was to test whether cTnIS43/45N is an alternative, functionally conservative substitution in cardiac myocytes. Partial and more extensive endogenous cTnI replacement was similar at 2 and 4 days after gene transfer, respectively, for epitope-tagged cTnI and cTnIS43/45N. This replacement did not significantly change thin filament stoichiometry. In functional studies, there were no significant changes in the amplitude and/or rates of contractile shortening and re-lengthening after this partial (2 days) and extensive (4 days) replacement with cTnIS43/45N. The cTnIS43/45N substitution also was not associated with adaptive changes in the myocyte Ca2+ transient or in phosphorylation of the protein kinase A and C-targeted cTnIS23/24 site. These results provide evidence that cTnIS43/45N is a functionally conservative substitution, and may be appropriate for use as a phospho-null in rodent models designed for studies on PKC modulation of cardiac performance.
Keywords: Troponin, Myofilament, Phosphorylation, Heart
INTRODUCTION
Protein kinase C (PKC) targets 5 residues in 3 clusters which are S23/24, S43/45 and T144 within cardiac troponin I (cTnI) [1,2,3]. Phosphorylation of cTnIS23/24 is established to accelerate relaxation by increasing the cardiac troponin C (cTnC) Ca2+ off rate [4–6]. Biophysical and biochemical studies indicate the phosphorylation of cTnIS43/45 and T144 independently modulate myofilament function [4,7–9], although their respective modulatory mechanism(s) are not well understood. Insight into their role in myofilament modulation is desirable given that elevated PKC and cTnI Ser43/45 and T144 phosphorylation are associated with human and animal models of heart failure [10–15].
To gain insight into the role played by the cTnIS43/45 site in modulating contractile function, investigators have used phospho-mimetic D and E or phospho-null A substitutions at S43/45. Myofilament extraction and replacement with cTnIS43/45 D or E indicate this cluster reduces Ca2+ sensitivity and maximum myofilament tension and sliding speed [9,16,17]. In a transgenic mouse model, complete replacement of endogenous cTnI with cTnIAllP, which contains phospho-mimetic D substitutions at all 3 PKC clusters also reduced myofilament Ca2+ sensitivity and maximum actomyosin ATPase activity [18]. This change in myofilament activation might be predicted to reduce peak pressure and accelerate relaxation in myocardium, and yet only a modest slowing of in vivo contractile pressure was observed in this mouse. Cellular studies have provided a partial bridge between the in vitro and in vivo work by showing that cTnIS43/45D expression initially impairs the amplitude and rates of shortening and relaxation [19]. Interestingly, the reduced shortening and re-lengthening rates returned toward control values when there was more extensive replacement with cTnIS43/45D, which could be attributed to the onset of a dynamic adaptive response within the myofilament [19].
Phospho-null substitutions at the same site are often necessary to verify the impact of phospho-mimetic substitutions, although this approach has led to disagreement about the role played by S43/45 in cTnI. In myofilament studies, a significant component of the PKC-targeted response was attributed to cTnIS43/45 [2]. Complete replacement with phospho-null cTnIAllAla at the same 3 sites as in the cTnIAllP mouse also resulted in a divergent phenotype than the one predicted from the work with phospho-mimetic substitutions. Specifically, myocytes from cTnIAllAla mice indicated that PKC activation at S43/45 phosphorylation accelerates pressure development and slows relaxation [20,21]. However, a hypertrophic phenotype developed in this mouse, and the remodeling process could contribute to this functional response. In another mouse model, partial replacement of endogenous cTnI with cTnIS43/45A had no significant impact on in vivo function [22,23], although myofilaments could theoretically still be functionally responsive to phosphorylation at the S43/45 site. Recent work in myocytes also demonstrated that extensive replacement with cTnIS43/45A reduces contractile function, and while not noted by previous investigators, these changes are clearly evident in the earlier studies utilizing cTnIS43/45A constructs [2,20,21]. Taken together, the functional phenotype emerging from studies performed using both phospho-mimetic and phospho-null cTnIS43/45A indicate that further work is needed to better understand this cluster under basal conditions and in response to physiological/pathophysiological states associated with elevated PKC activation, such as heart failure.
The previous work indicates a different phospho-null substitution is needed which should have little or no influence on contractile function. Towards this goal, the current study tests whether substitution of cTnIS43/45 with a polar N residue may act as a functionally conservative substitution at this PKC-targeted site in cTnI. These studies are anticipated to lead to more extensive work utilizing this substitution as a phospho-null to gain insight into the role played by S43/45 in response to PKC and provide the rationale for using this substitution in rodent models.
METHODS
Site-directed Mutagenesis and Recombinant Virus Construction
Site-directed mutagenesis QuikChange, Agilent Tech, Inc., Santa Clara, CA) of wildtype rat cTnI cDNA in pGEM3Z was utilized to replace Ser43/45 (S43/45) with Asn (N) residues ([24–26]. The mutagenesis primers, 5′gccaagaaaaagtctaagatcaacgccaacagaaagcttcagttg-3′ (sense) and 5′caactgaagctttctgttggcgttgatcttagactttttcttggc-3′ (anti-sense) were used to produce cTnIS43/45N and cTnIS43/45NFLAG substitution mutants (underline = nucleotide changes), and then individually sub-cloned into a pDC315 shuttle vector. Recombinant adenovirus was produced by homologous recombination of each shuttle vector with pBHGLoxΔE1,3Cre (Microbix) in HEK293 cells [25,27]. In addition to cTnIS43/45N with and without FLAG, previously prepared recombinant adenoviruses for cTnI, cTnIFLAG and cTnIS43/45D also were utilized during gene transfer experiments [19].
Cardiac Myocyte Isolation, Gene Transfer and Culture
The isolation of Ca2+-tolerant adult rat myocytes from adult Sprague-Dawley rats was performed as described previously [19]. Protocols and procedures for myocyte isolation were approved by the University Committee for the Use and Care of Animals (UCUCA) at the University of Michigan. After isolation, myocytes were re-suspended in Dulbecco’s Modified Eagle (DMEM) media containing 5% fetal bovine serum (FBS), penicillin (50 U/ml) and streptomycin (P/S; 50 μg/ml), and attached to laminin-coated coverslips for 2 hrs in a 37°C incubator. Media was gently replaced with recombinant adenovirus re-suspended in M199 +P/S at 37°C to achieve efficient gene transfer [25], and 1 hr later each an additional 2 ml aliquot of M199 + P/S media was added to each coverslip. Media was changed the next day and every other day after gene transfer.
Myofilament Protein Expression and Sarcomere Incorporation
Western blot analysis was used to determine the replacement of endogenous cTnI with FLAG and non-FLAG-tagged cTnI and cTnIS43/45N 2 and 4 days after gene transfer [26]. For these studies, proteins were separated on a 12% or 4–20% SDS-PAGE gels, and then electrophoretically transferred to PVDF membranes. Membranes were then probed using the primary and secondary antibody pairs described below and detected using enhanced chemilumenescence (ECL). Expression on these Western blots and the silver (Ag)-stained gel or Sypro-stained blot were quantified using Quantity One® software [26]. Thin filament stoichiometry also was analyzed by Western blot [19,26].
Total cTnI expression and replacement of endogenous protein was evaluated 2 and 4 days post-gene transfer using a monoclonal troponin I (TnI) primary antibody (Ab; MAB1691; 1:4000; Millipore; Billerica, CA), horseradish-peroxidase (HRP)-conjugated goat anti-mouse (GAM) secondary Ab. The percent replacement of endogenous cTnI with cTnIFLAG or cTnIS43/45NFLAG was determined from the ratio of FLAG-tag/Total cTnI. Total cTnI and Tm expression in each lane are compared to non-treated myocytes (e.g. control), which was set to 1.0 and then normalized for the protein loading detected using Ag-stained gels or Sypro stained blots [19,26]. Membranes also were probed for tropomyosin (Tm) and normalized to the same Ag or Sypro stains (Tm311; 1:10,000; Sigma-Aldrich; St. Louis, MO). Immunoblot detection of total cTnI and Tm were used to assess myofilament protein stoichiometry. In addition, adaptive changes in phosphorylation of cTnIS23/24 was evaluated using pS23/24 Ab (1:1000; Cell Signaling Technology; Boston, MA) and HRP-conjugated goat-anti-rabbit (GAR) secondary Ab and ECL detection.
Sarcomere incorporation was evaluated by immunohistochemical staining of myocytes 4 days after gene transfer of cTnIS43/45NFLAG, as described previously [28]. Briefly, detergent-permeabilized myocytes were fixed in 3% paraformaldehyde, blocked with 20% normal goat serum (NGS; Sigma) in phosphate buffered saline containing 0.5% triton-X100 (PBS + TX-100), and then labeled with primary TnI-specific monoclonal (MAB1691) and anti-FLAG polyclonal (1:1000, Cell Signaling) antibodies (Abs). Myocytes were then washed in PBS + TX-100, blocked in 20% NGS and then immunolabeled with GAM secondary Ab conjugated to Texas Red (TR, 1:500, Thermo) and GAR Ab conjugated to fluorescein isothiocyanate (FITC, 1:500, Thermo). After rinsing in PBS, coverslips were mounted on slides with ProLong Gold antifade reagent. Projection images were obtained with a Fluoview 500 laser scanning confocal microscope (Olympus; Center Valley, PA) and de-convoluted using AutoQuant X software (Media Cybernetics; Rockville, MD).
Contractile Function and Ca2+ Transient Measurement
Myocytes were transferred to stimulation chambers and paced at 0.2 Hz starting 1 day after plating, and media was changed every 12 hrs for these myocytes [26]. Myocyte contractile function was analyzed 2 and 4 days after gene transfer using signal-averaged traces collected with a video-based CCD camera system (Ionoptix; Beverly, MA). Resting sarcomere length, peak shortening amplitude, shortening rate, re-lengthening rate, time to 50% re-lengthening (TTR50%) were determined from each signal-averaged trace [26]. The same platform was used to measure Ca2+ transients in myocytes loaded with Fura-2AM [26]. The basal and peak Ca2+ ratios, rates of Ca2+ rise and decay, and the time to 50% decay (TTD50%) were calculated from these signal-averaged traces 4 days after gene transfer [26].
Data analysis
Results are presented as mean ± SEM. Quantitative data was analyzed by a one-way analysis of variance (ANOVA) with statistical significance set to p<0.05 (*), unless otherwise noted.
RESULTS
Expression and myofilament incorporation of cTnIS43/45N
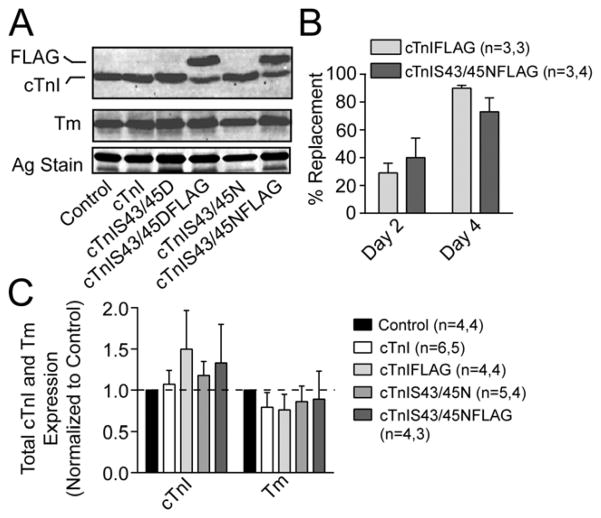
Experiments with cTnIFLAG and cTnIS43/45NFLAG showed there was partial replacement of endogenous cTnI by 2 days (<40%) and more extensively replacement by 4 days (>75%) after gene transfer (Fig. 1A,B). This replacement is comparable with the expression achieved with phospho-mimetic cTnIS43/45DFLAG and the phospho-null cTnIS43/45AFLAG (Fig. 1A, [19, 26]). Non-tagged versions of these earlier constructs are presumably similar, and in earlier work, the expression of non-tagged constructs caused alterations in myofilament function [19, 26]. Quantitative analysis of Western blots also indicates there are no significant changes in total cTnI and Tm expression in myocytes expressing tagged (FLAG) or non-tagged cTnI and cTnIS43/45N compared to non-treated controls (Fig. 1C). The maintenance of cTnI and Tm expression after gene transfer of cTnI and cTnIS43/45N shows that thin filament stoichiometry is preserved in these myocytes. In agreement with earlier work, gene transfer of cTnIS43/45NFLAG also produced a striated distribution pattern when myocytes were immunostained to label TnI (Fig. 2A), and the FLAG tagged expression (Fig. 2B). Most importantly, the immunolabeling patterns for TnI and FLAG overlapped with each other (Fig. 2C), which is consistence with sarcomere incorporation of the exogenous cTnIS43/45N within myocytes.
FIGURE 1. Expression of cTnIS43/45N replaces endogenous cTnI without changing thin filament protein stoichiometry.
A. Representative Western blot showing non-tagged and FLAG-tagged cardiac troponin I (cTnI and FLAG; upper panel) and Tm (middle panel) protein expression in myocytes 4 days after gene transfer of cTnI, cTnIFLAG, cTnIS43/45N and cTnIS43/45NFLAG compared to non-treated control myocytes. A portion of the silver (Ag)-stained gel also is included to indicate protein loading in each lane (lower panel). B. Quantitative analysis of cTnIFLAG and cTnIS43/45NFLAG replacement of endogenous cTnI 2 and 4 days post-gene transfer. Replacement was calculated from the FLAG-taggedtotal cTnI ratio. C. Quantitative analysis showing total TnI and tropomyosin expression 4 days after gene transfer. The cTnI and Tm expression ratios were normalized to the non-treated control value, which was set to 1.0. A one-way ANOVA analysis indicated neither cTnI or Tm expression were significantly different from non-treated control values in myocytes expressing cTnI, cTnIFLAG, cTnIS43/45N or cTnIS43/45NFLAG (p>0.050).
FIGURE 2. Striated pattern of cTnIS43/45NFLAG incorporation into sarcomeres of adult rat cardiac myocytes.
Dual immunohistochemical detection of TnI (A) and FLAG (B) plus the overlay (C) demonstrate sarcomere incorporation of cTnIS43/45NFLAG 4 days after gene transfer. Primary antibody binding of MAB1691 (TnI) and FLAG were detected with secondary antibodies conjugated to TR (left) and FITC (left), respectively (bar = 25 μm). Insets are included to further illustrate the striated pattern of incorporation.
Myocyte contractile function and Ca2+ transients
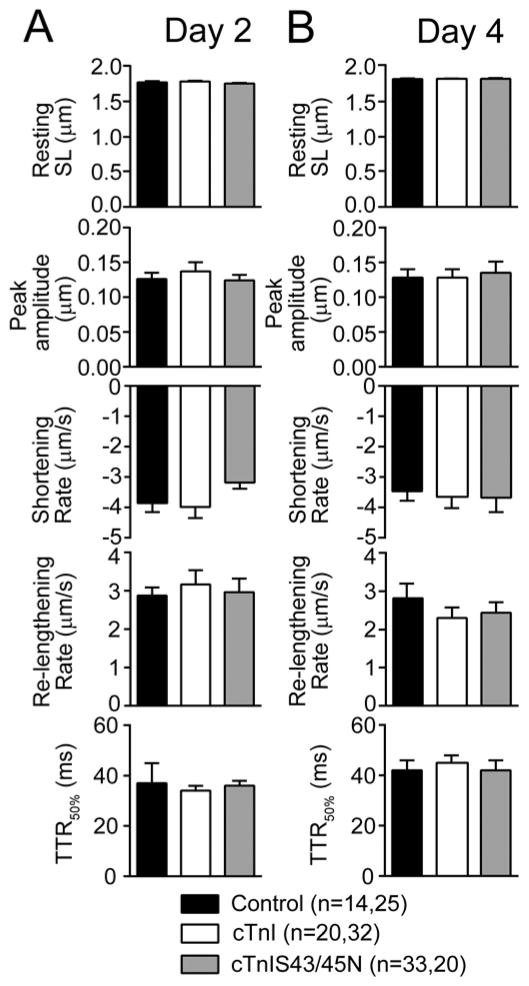
To determine whether expression of cTnIS43/45N produced functional alterations in myocytes, signal-averaged recordings of contractile function were analyzed at both 2 and 4 days post-gene transfer when there was partial (<50%) and more extensive replacement (>70%) with cTnIS43/45N in myocytes (Fig. 3A, B). The N substitution did not significantly alter resting or peak sarcomere length, the rates of shortening and re-lengthening nor the TTR50%. These observations are in contrast to previous studies in which reduced peak shortening and slowed shortening rates developed when <50% of endogenous cTnI was replaced by cTnIS43/45D [19]. Moreover, the significant reduction in shortening amplitude and accelerated TTR50% observed after extensive replacement with the phospho-null, cTnIS43/45Ala [26] also was absent in myocytes expressing cTnIS43/45N (Fig. 3B). Collectively, these findings suggest substitution with a polar N residue at cTnI Ser43/45 has little influence on basal myofilament function in intact cardiac myocytes.
FIGURE 3. Cardiac myocyte contractile function 2 and 4 days after gene transfer.
Quantitative analysis of myocyte contractile function measured 2 (A) and 4 (B) days after gene transfer. Contractile function was evaluated based on measurements of resting length, peak shortening amplitude, shortening and re-lengthening rates, and the TTR50%. There were no significant differences for myocytes expressing cTnIS43/45N compared to control and cTnI-expressing myocytes at either 2 or 4 days post-gene transfer (p>0.05 by 1-way ANOVA).
Adaptive signaling in myocytes expressing cTnIS43/45N
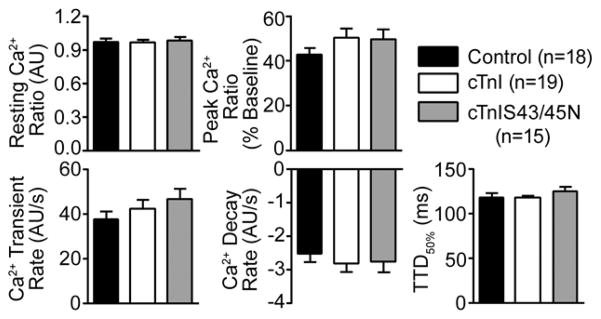
Our previous studies in myocytes expressing cTnIS43/45D and cTnIS43/45A indicated adaptations in signaling blunt the direct functional impact when substitutions are made at S43/45 [19]. Adaptative behavior also is reported in other cardiac myofilament substitution models [29, 30], and this adaptative behavior tends to return myofilament function toward the original set point or steady state. This adaptive behavior includes changes in the phosphorylation of other phosphorylation sites in cTnI and/or alterations in the cellular Ca2+ transient [19,26]. Thus, it remained a possibility that the absence of change in contractile function observed in myocytes expressing cTnIS43/45N also could be explained by similar adaptative responses. To test for this possibility, Ca2+ transients and phosphorylation at the cTnI S23/24 site were examined in myocytes expressing cTnIS43/45N because they appear to be early and consistent responders when there are changes made to cTnI within the myofilament. Thus, Ca2+ transients were analyzed in Fura-2AM-loaded cells 4 days after gene transfer of cTnIS43/45N. There were no significant differences in any of the transient measurements between myocytes expressing cTnIS43/45N compared to cTnI-expressing myocytes or non-treated controls, including baseline and peak Ca2+ transient ratios, nor the rate of Ca2+ rise and decay (Fig. 4).
FIGURE 4. Quantitative analysis of Ca2+ transients in myocytes 4 days after cTnIS43/45N gene transfer.
Basal and peak Ca2+ ratios (upper panel) as well as the Ca2+ release and decay rates, and the TTD50% (lower panel) were measured from signal-averaged Ca2+ transients in Fura-2AM-loaded control and cTnI- and cTnIS43/45N-expressing myocytes 4 days after gene transfer. These variables were not significantly different in myocytes expressing cTnIS43/45N compared to control or cTnI-expressing myocytes (p>0.05).
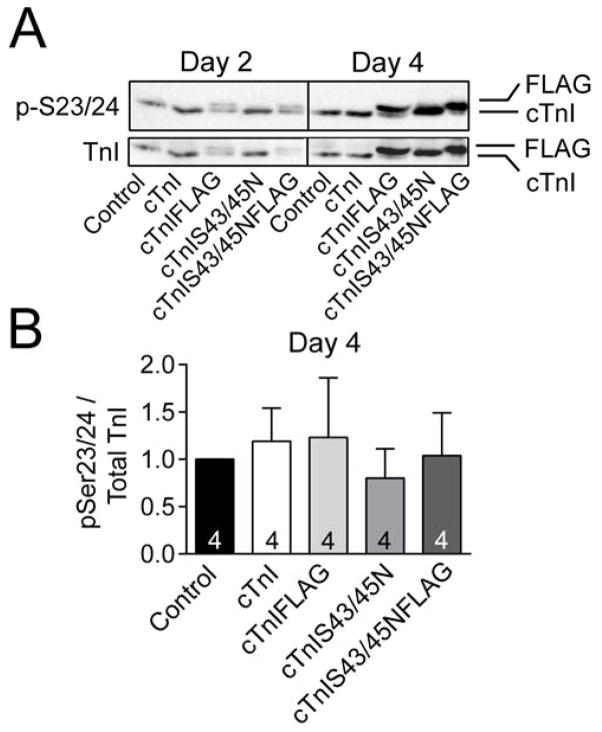
Earlier studies also demonstrated that cTnI phosphomimetic cTnIS43/45D expression led to increased phosphorylation of cTnISer23/24, and this response was closely associated in time with adaptive changes in contractile function [19]. However, cTnIS23/24 phosphorylation remained unchanged from non-treated control values in myocytes expressing cTnIS43/45N or cTnI in the present study (Fig. 5). Taken together, there appears to be an absence of change in known adaptive signaling targets when myocytes express cTnIS43/45N. These observations also are consistent with this substitution acting as a functionally conservative substitution in myocytes.
FIGURE 5. Phosphorylation of cTnI S23/24 in myocytes 4 days after gene transfer.
Representative detection (A) and quantitative analysis (B) of phosphorylated cTnI S23/24 relative to total cTnI expression. A. Representative immunoblot showing phosphorylated cTnI S23/24 (pS23/24; upper panel) relative to total cTnI expression (lower panel) detected by Western analysis. B. Quantitative analysis of pS23/24 relative to total cTnI expression in control, cTnI-, cTnIFLAG-, cTnIS43/45N- and cTnIS43/45NFLAG-expressing myocytes 4 days after gene transfer. The number of preparations analyzed is indicated within each column. Ratios in each group were normalized to the non-treated control ratio, which was set to 1.0 [26]. A 1-way ANOVA indicated there were no statistically significant differences in pS23/24 among myocytes from the different groups (p>0.05).
DISCUSSION
Previous studies on intact myocytes expressing phospho-mimetic cTnIS43/45D provided insight into the ability of this cluster to act as a modulatory brake on contractile function, and also illustrated that adaptive secondary changes contribute to a dynamic PKC response [19]. The reduced contractile function detected in intact myocytes temporally coincided with reductions Ca2+-sensitivity of isometric force in myocytes expressing either TnIS43/45D or cTnIS43/45A [19, 26]. The development of a more functionally conservative construct than cTnIS43/45A [26] became highly desirable to gain additional insight, and provided the rationale for the current studies using cTnIS43/45N. Expression of this construct met important initial criteria, as temporal sarcomere replacement with cTnIS43/45N is comparable to cTnI (Figs. 1,2), and to the cTnIS43/45D and cTnIS43/45A studied in earlier work [19, 26]. Most importantly, the short-term expression of cTnIS43/45N in myocytes did not change intact myocyte measures of shortening and re-lengthening (Fig. 3), and adaptive alterations in the Ca2+ transient and myofilament phosphorylation were absent at the same time point detected in myocytes expressing cTnIS43/45D (Figs. 4,5).
Modulation of contractile function by PKC-targeted cTnIS43/45
The specific in vivo role played by cTnIS43/45 phosphorylation continues to be difficult to define, even though PKC expression and activation, and phosphorylation at PKC-targeted cTnI residues are consistently linked to cardiac dysfunction and/or heart failure [10–15]. Genetic animal models have largely focused on the functional role played by PKC-targeted cTnIS43/45 using phospho-mimetic and phospho-null substitutions at Ser43/45 in combination with the other PKC-targeted residues, S23/24 and T144 [18,20,21,31]. The integrated role played by all these residues is important, especially given that cTnI S198 is now identified as an additional phosphorylation target for PKC [32]. However, studies combining substitutions in all 3 PKC-targeted clusters have led to controversies about the specific changes produced by S43/45 phosphorylation. This question is further complicated by disparities between the observed in vivo cardiac phenotypes and outcomes predicted from myofilament and/or biochemical analysis [2,9,33,34], and divergent cardiac phenotypes observed in mice expressing phospho-mimetic D, E and phospho-null A substitutions [18,20,21,31]. In addition, transgenic mice expressing ~50% cTnI as cTnIS43/45A also developed alterations in Ca2+ cycling [22,23], and it remains unclear whether this phenotype is due to the A substitution or reduced PKC-related phosphorylation of cTnIS43/45. The present results in adult myocytes expressing cTnIS43/45N are encouraging and provide a solid rationale for developing a mouse model expressing this construct. Future studies in a genetic cTnIS43/45N model are essential for determining whether in vivo cardiac function is maintained during longer-term expression and/or in response to load.
Insights into the cTnIS43/45 modulatory mechanism
The current results with cTnIS43/45N together with earlier work on cTnIS43/45D and cTnIS43/45A indicate the amino acid substitutions at this site can significantly impact the troponin switch mechanism. Substitution of S with N is not common but this substitution has been utilized in other proteins [35]. Multiple studies now indicate that the more typical phospho-null A substitution produces functional changes at S43/45 [9,21,36[, but is a functionally conservative substitution at S23/24 [4,20]. The future use of N or other residues at cTnI phosphorylation sites should be evaluated on a case-by-case basis.
Several mechanisms have been proposed to explain a transduction mechanism for cTnIS43/45 phosphorylation. Biophysical studies showed cTnIS43/45D destabilizes the C-lobe of cTnC [37], while cTnIS43/45D, and cTnIS45E alone and in combination with S43E and T144E reduce troponin Ca2+ affinity [33,34]. The impact on Ca2+ affinity led to predicted transduction via altered interactions with the N-lobe of cTnC [17,33]. A polar R group at S43/45 may contribute to interactions with the C- or N-lobe of cTnC, and the present results with cTnIS43/45N indicate weak interactions with a more polar R group, such as the hydroxyl in S or amide in N may be important for basal troponin switch function compared to A substitutions. The addition of negatively charged phosphate also should alter these interactions, but it remains unclear how the lack of a polar R group in cTnIS43/45A reduces troponin Ca2+ sensitivity and myofilament function [26,34].
Alternatively, the cTnI S43/45 cluster may play a critical role in defining the function of the I-T arm within troponin. The S43 residue is located at the N-cap and S45 at the n+2 position of the H1 alpha helix within cTnI. This H1 helix together with the H2 helix and C-terminal cTnT helices form the I-T arm (residues 202–271; [38]), and all 3 helices are highly conserved (>90–95%) in mammalian myocardium. Phosphorylation of N-cap and n+2 residues stabilize alpha helices [39], which could either modulate thin filament positioning and/or the downstream inhibitory peptide and switch domains (e.g. H3 helix) within cTnI. The use of D or A substitutions also should stabilize the helix [40] and would be predicted to produce the observed decrease in contractile function attributed to phosphorylation. In contrast, the N substitutions at these critical amino-terminal positions of the coil may maintain weak internal interactions without further stabilizing the coil. The present results showing that cTnIS43/45N does not alter contractile function support this potential modulatory mechanism. However, extensive biophysical, biochemical and in vivo work will be needed to validate and define the downstream impact of S43/45 on troponin switch and/or thin filament function in future studies.
Conclusions
The present group of experiments demonstrates contractile function is maintained without adaptive responses after short-term replacement of endogenous cTnI with cTnIS43/45N. Phosphorylation of the cTnIS43/45 cluster is predicted to act as a modulatory brake on contractile function, but also triggers compensatory signaling in the dynamic myofilament scaffold [19]. Further validation of cTnIS43/45N as a functionally conservative substitution under in vivo conditions could have therapeutic implications. For example, gene transfer of this construct and/or delivery of small molecules to prevent its phosphorylation may help to blunt contractile dysfunction during chronic PKC activation.
Highlights.
Cardiac troponin I (cTnI) S43/45N is expressed in the sarcomeres of myocytes
Thin filament stoichiometry is not changed by cTnIS43/45N expression in myocytes
Short-term cTnIS43/45N expression in myocytes does not change contractile function
Expression of cTnIS43/45N does not cause adaptive signaling changes in myocytes
Acknowledgments
The technical assistance of Gail Romanchuk, Briana Dumond, and Erin Keyes is gratefully acknowledged. This work was supported by National Institutes of Health Grant R01-HL-067254, and an American Heart Association pre-doctoral award 12PRE8830022 (SEL). This work utilized the Morphology and Image Analysis Core Facilities of the Michigan Diabetes Research and Training Center (supported by National Institutes of Health Grant P60-DK20572).
ABBREVIATIONS
- ANOVA
Analysis of variance
- Ab
antibody
- Abs
antibodies
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- DMEM
Dulbecco’s Modified Eagle Medium
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAM
goat anti-mouse
- GAR
goat anti-rabbit
- HRP
horseradish peroxidase
- NGS
normal goat serum
- P/S
penicillin/streptomycin
- PBS
phosphate buffered saline
- PKC
protein kinase C
- Ag stain
silver stain
- TR
Texas Red
- TTR50%
time to 50% re-lengthening
- TTD50%
time to 50% decay
- Tx-100
triton X-100
- Tm
tropomyosin
- TnI
troponin I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Noland TA, Jr, Raynor RL, Kuo JF. J Biol Chem. 1989;264:20778–20785. [PubMed] [Google Scholar]
- 2.Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, Kuo JF. J Biol Chem. 1995;270:25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 3.Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, Sievert G, Balke CW, Feinmark SJ, Solaro RJ, Steinberg SF. J Biol Chem. 2008;283:22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Circ Res. 2007;101:377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- 5.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- 6.Dong WJ, Jayasundar JJ, An J, Xing J, Cheung HC. Biochemistry. 2007;46:9752–9761. doi: 10.1021/bi700574n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Zhao J, Mandveno A, Potter JD. Circ Res. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 8.Noland TA, Jr, Kuo JF. J Mol Cell Cardiol. 1993;25:53–65. doi: 10.1006/jmcc.1993.1007. [DOI] [PubMed] [Google Scholar]
- 9.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. J Biol Chem. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Circulation. 2012;126:1828–1837. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 12.Walker LA, Walker JS, Ambler SK, Buttrick PM. J Mol Cell Cardiol. 2010;48:1180–1186. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong X, Sumandea CA, Chen YC, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. J Biol Chem. 2012;287:848–857. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christopher B, Pizarro GO, Nicholson B, Yuen S, Hoit BD, Ogut O. J Muscle Res Cell Motil. 2009;30:111–123. doi: 10.1007/s10974-009-9180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Circulation. 2004;110:982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 16.Lu QW, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. J Biol Chem. 2010;285:11810–11817. doi: 10.1074/jbc.M109.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur MC, Kobayashi T, Chalovich JM. Biophys J. 2008;94:542–549. doi: 10.1529/biophysj.107.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, VanBuren P, Martin LA, Robbins J. J Biol Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 19.Lang SE, Schwank J, Stevenson TK, Jensen MA, Westfall MV. J Mol Cell Cardiol. 2015;79:264–274. doi: 10.1016/j.yjmcc.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. J Physiol. 2003;552:845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Circ Res. 2002;90:649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 22.MacGowan GA, Du C, Cowan DB, Stamm C, McGowan FX, Solaro RJ, Koretsky AP, Del Nido PJ. Am J Physiol Heart Circ Physiol. 2001;280:H835–843. doi: 10.1152/ajpheart.2001.280.2.H835. [DOI] [PubMed] [Google Scholar]
- 23.MacGowan GA, Evans C, Hu TC, Debrah D, Mullet S, Chen HH, McTiernan CF, Stewart AF, Koretsky AP, Shroff SG. Cardiovasc Res. 2004;63:245–255. doi: 10.1016/j.cardiores.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AM, Jones L, 2nd, Sims HF, Strauss AW. Biochemistry. 1991;30:707–712. doi: 10.1021/bi00217a018. [DOI] [PubMed] [Google Scholar]
- 25.Westfall MV, Rust EM, Albayya F, Metzger JM. Methods Cell Biol. 1997;52:307–322. [PubMed] [Google Scholar]
- 26.Lang SE, Robinson DA, Wu HC, Herron TJ, Wahr PA, Westfall MV. Arch Biochem Biophys. 2013;535:49–55. doi: 10.1016/j.abb.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampert SE, Devaney E, Westfall MV. In: Handbook of Molecular and Cellular Methods in Biology and Medicine. Cseke AKLJ, Kaufman PB, Westfall MV, editors. CRC Press; Boca Raton, Florida: 2011. pp. 557–578. [Google Scholar]
- 28.Michele DE, Albayya FP, Metzger JM. J Cell Biol. 1999;145:1483–1495. doi: 10.1083/jcb.145.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves ML, Dias FA, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RT, 3rd, Sadayappan S, Robbins J, Wieczorek DF, Solaro RJ, Wolska BM. Circ Cardiovasc Genet. 2014;7:132–143. doi: 10.1161/CIRCGENETICS.113.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guinto PJ, Haim TE, Dowell-Martino CC, Sibinga N, Tardiff JC. Am J Physiol Heart Circ Physiol. 2009;297:H614–626. doi: 10.1152/ajpheart.01143.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirk JA, MacGowan GA, Evans C, Smith SH, Warren CM, Mamidi R, Chandra M, Stewart AF, Solaro RJ, Shroff SG. Circ Res. 2009;105:1232–1239. doi: 10.1161/CIRCRESAHA.109.205427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kooij V, Zhang P, Piersma SR, Sequeira V, Boontje NM, Wijnker PJ, Jimenez CR, Jaquet KE, dos Remedios C, Murphy AM, Van Eyk JE, van der Velden J, Stienen GJ. PLoS One. 2013;8:e74847. doi: 10.1371/journal.pone.0074847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, Dong WJ, Burkart EM, Cheung HC, Solaro RJ. Biochemistry. 2004;43:5996–6004. doi: 10.1021/bi036073n. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Lopez JJ, Biesiadecki BJ, Davis JP. PLoS One. 2014;9:e86279. doi: 10.1371/journal.pone.0086279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZY, Wang F, Sellers JR, Korn ED, Hammer JA., III Proc Natl Acad Sci. 1998;95:15200–15205. doi: 10.1073/pnas.95.26.15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijnker PJM, Sequeira V, Witjas-Paalberends ER, Foster DB, dos Remedios CG, Murphy AM, Stienen GJM. J van der Velden Arch Biochem Biophys. 2014;554:11–21. doi: 10.1016/j.abb.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finley NL, Rosevear PR. J Biol Chem. 2004;279:54833–54840. doi: 10.1074/jbc.M408304200. [DOI] [PubMed] [Google Scholar]
- 38.Takeda S, Yamashita A, Maeda K, Maeda Y. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 39.Andrew CD, Warwicker J, Jones GR, Doig AJ. Biochemistry. 2002;41:1897–1905. doi: 10.1021/bi0113216. [DOI] [PubMed] [Google Scholar]
- 40.Doig AJ. Biophys Chem. 2002;101–102:281–293. doi: 10.1016/s0301-4622(02)00170-9. [DOI] [PubMed] [Google Scholar]