Abstract
Background
IL-6, which is reported to be elevated in association with mastocytosis, asthma and urticaria, is used in conjunction with stem cell factor (SCF) to generate human MCs (HuMCs) from progenitor (CD34+) cells. Despite these associations, the effects on, and mechanisms by which prolonged exposure to IL-6 alters HuMC number and function are not well understood.
Objectives
To study the effect of IL-6 on HuMC function, the mechanisms by which IL-6 exerts its effects, and the relationship of these findings to mastocytosis.
Methods
HuMCs were cultured in SCF with or without IL-6. The responses to FcεRI aggregation, and the expression of proteases and receptors including the soluble IL-6 receptor (sIL-6R) were then quantitated. Epigenetic changes in SOCS3 were determined using methylation specific PCR. Serum samples from healthy controls and patients with mastocytosis were assayed for IL-6, tryptase, and sIL-6R.
Results
IL-6 enhanced MC proliferation, maturation, and reactivity following FcεRI aggregation. IL-6 reduced expression of SOCS3, which correlated with methylation of the SOCS3 promoter, and increased expression and activation of STAT3. IL-6 also suppressed constitutive production of sIL-6R and serum levels of sIL-6R were similarly reduced in patients with mastocytosis.
Conclusion
IL-6 increases mast cell proliferation and formation of a more reactive phenotype enabled by suppressing proteolytic cleavage of sIL-6R from IL-6R and down regulation of the SOCS3 auto-inhibitory pathway. We suggest IL-6 blockade might ameliorate MC related symptoms and pathology in MC-related diseases associated with elevated IL-6 including mastocytosis.
Keywords: mast cells, signal transducer and activator of transcription 3, suppressor of cytokine signaling 3, stem cell factor, Interleukin 6, mastocytosis
Introduction
The pleiotropic cytokine, IL-6 is produced by T-cells, macrophages, and other cells in response to infection and acute inflammation and has been associated with the pathogenesis of several human mast cell (HuMC) related diseases.1, 2 These include the clinical observations that IL-6 levels relate to the severity of disease in systemic mastocytosis3, 4 acute5 and chronic urticaria,6 and asthma.7 In vitro, IL-6 promotes HuMC maturation,8 adhesion to extracellular matrix,9 chemokinesis,10 and survival, the latter through IgE dependent production of mast cell derived IL-6.11 IL-6 is also routinely used to supplement stem cell factor (SCF) to generate HuMCs from cord or peripheral blood progenitor (CD34+) cells.8, 12, 13 The consequences of long term exposure to IL-6 on HuMC function and the mechanisms by which IL-6 alters mast cell behavior have not been investigated.
The receptor for IL-6, IL-6R (IL-6Rα or CD126) is largely restricted to hematopoietic cells (reviewed by Mihara et al.2) while its signaling co-receptor, gp130 (CD130) is ubiquitously distributed. Even among the few types of cells that express IL-6R, gp130 is present in great excess.14 Cells that express gp130 but not IL-6R can also respond to IL-6 through its binding to the soluble form of IL-6R (sIL-6R) generated by alternative splicing or proteolytic cleavage of the membrane form.2, 15 The IL-6/sIL-6R complex then interacts with spare gp130 on the cell surface, referred to as trans-signaling in contrast to classic signaling initiated via IL-6R and gp130 at the cell surface. The formation of a dimeric IL-6/IL-6R/gp130 complex16 is followed by mutual transactivation of gp130 and Janus kinase 1 (JAK-1),18, 17 activation of the Ras/Raf/MEK/ERK pathway and the phosphorylation and dimerization of signal transducer and activator of transcription 3 (STAT3) which then induces transcription of genes including SOCS3 (SOCS3) which in turn modulates activation of these pathways. 19
We have investigated the effect of constant IL-6 exposure in vitro and in vivo and, as reported here, such exposure promotes development of not only a more mature but also a more reactive HuMC phenotype with significantly enhanced FcεRI-mediated signaling, degranulation, and cytokine production. The prolonged effects of IL-6 on HuMC function occurred in association with loss of SOCS3 auto-inhibition of the IL-6/JAK/STAT pathway and suppression of sIL-6R production. In vivo, IL-6 levels in mastocytosis correlated with serum tryptase and inversely correlated with serum sIL-6R. These data support the concept that lowering IL-6 levels in diseases such as mastocytosis might have a beneficial therapeutic effect.
METHODS
For detailed methods, including mice used, experimental protocols and procedures, clinical protocols, and statistical analysis, see the Methods section in this article’s Online Repository www.jacionline.org.
RESULTS
Exposure to IL-6 enhances HuMC proliferation and maturation
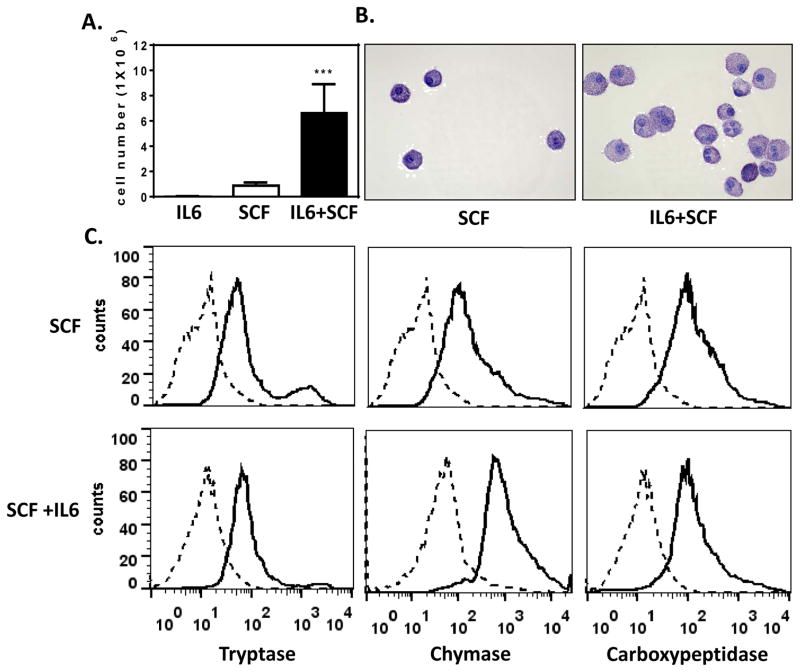
HuMCs proliferated to a significantly greater extent (P<0.001) when grown in the presence of IL-6 and SCF than cells grown in SCF alone (Fig 1, A). However, SCF itself was obligatory as cells failed to proliferate when cultured in IL-6 alone (Fig 1, A). This observation is similar to previous reports of the effect in IL-6 on cord blood derived mast cells20, 21 but differ from a single report that IL-6 decreases the growth of human mast cells from cord blood where the varied results were attributed to differing culture conditions.8 Once cells had reached their most mature state at 6 weeks, those exposed to IL-6 exhibited greater cell size and granularity (Fig 1, B). Examination by flow cytometry of the major MC-specific granule proteases namely, tryptase, chymase, and carboxypeptidase22, 23 indicated that all three were expressed regardless of growth conditions although the chymase content was substantially increased in IL-6 conditioned cells (Fig 1, C). These data are thus consistent with and extend previous reports that IL-6 increases the number13, 21, 20, 24 and maturity8 of human mast cells in culture
FIG 1. IL-6 enhances proliferation, alters cell morphology, and increases chymase content in HuMCs.
HuMCs were cultured for 7 weeks in SCF, with or without IL6, or IL6 alone (100 ng/ml of each). (A) Mean cell count +/− SEM (cultures from five donors, *** P<0.001). (B) Cytocentrifuged preparations of cells stained with toluidine blue. (C) Tryptase, chymase and carboxypeptidase content by flow cytometry. Representative results from one of three donors are shown in B and C.
HuMCs derived from cord blood CD34+ cells express the SCF receptor (KIT, CD117), FcεRI25, gp1308 and IL-6R at the cell surface. Examination of HuMCs derived from CD34+ cells from peripheral blood samples by flow cytometry indicated that IL-6 did not alter the surface expression of KIT, the FcεRI α-subunit, gp130, and IL-6R (Fig E1, A). Western blots also indicated similar expression of IL-6R as well as gp130 whether cultures were grown in the presence or absence of IL-6 (Fig E 1, B). Therefore, HuMCs grown under either condition express similar levels of the necessary receptors for responses to SCF, antigen/IgE, and IL-6.
Culture in IL-6 leads to more robust responses to FCεRI ligation
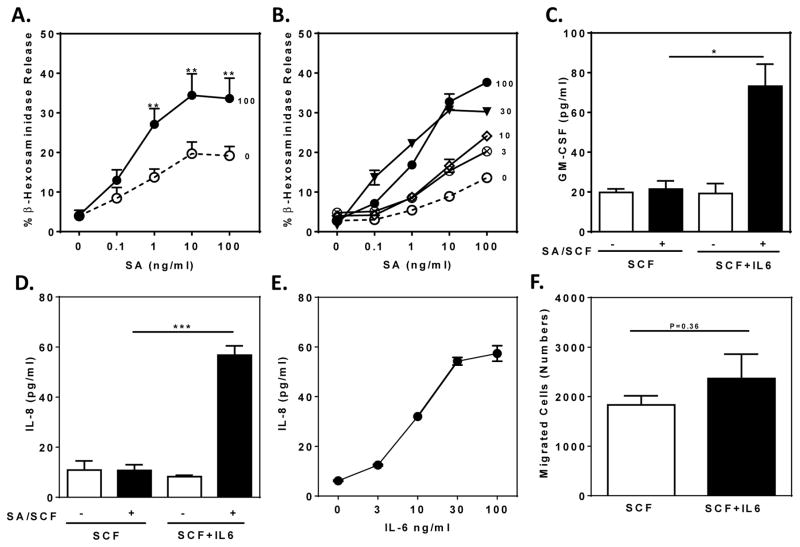
Stimulation of biotinylated IgE-sensitized HuMCs with graded concentrations of SA revealed significant enhancement of degranulation in response to concentrations of SA greater than 0.1 ng/ml in SCF/IL-6 cultured HuMCs, with a maximal response approximately twice that of cells cultured in SCF alone (Fig 2, A; P<0.01). The effect of IL-6 was concentration dependent with significant enhancement with as little as 3 ng/ml and maximal enhancement at 30 ng/ml IL-6 (Fig 2, B). As described in more detail later, the onset of IL-6 action was time dependent with significant increases in degranulation by 12 hours (data not shown).25 Conditioning of HuMCs with IL-6 also augmented production of the cytokines GMCSF and IL-8 (Fig 2, C and D) with optimal effects at 30 ng/ml IL-6 (Fig 2, E). In this experiment, cytokine production was induced by co-stimulation with SA and SCF as these stimulants individually elicit limited cytokine production.26 SCF-induced chemotaxis of HuMCs27 was not significantly affected by IL-6 (Fig 2, F).
FIG 2. IL-6 enhances HuMC degranulation and cytokine production.
(A and B) SA-induced release of β-hexosaminidase from HuMCs cultured in 100 ng/ml SCF and the indicated concentrations of IL-6 (A, n=7; B. n=1). (C–E) GMCSF and IL-8 production by HuMCs cultured in SCF (100 ng/ml) with or without IL-6 (100 ng/ml or as indicated) in response to SA and SCF (10 ng/ml each) (C, n=2; D, n=3; E, n=1). (F) Chemotactic response to 10 ng/ml SCF (n=3). Values are mean ± SEM (A, C, D, and F) or of replicate determinations of one HuMC donor (B and E). *P<0.05, **P<0.01, ***P<0.001.
Production of sIL-6R by HuMCs is inhibited by IL-6
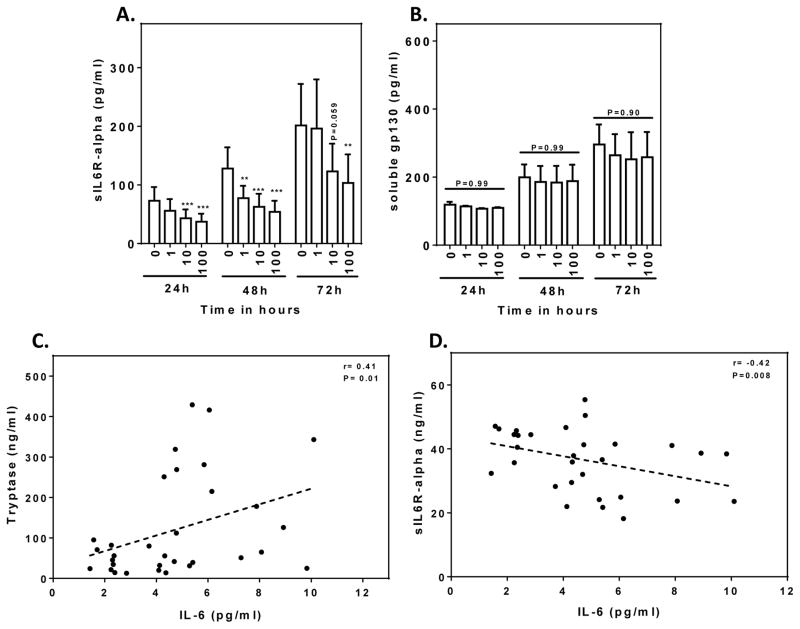
We next investigated the production of sIL-6R and sgp130 by HuMCs to determine whether the effects of IL-6 were due to classical or trans-signaling and the potential impact in inflammatory disease.28 HuMCs produced sIL-6R and sgp130 spontaneously in approximately equimolar concentrations (Fig 3, A and B). The notable finding was that production of sIL-6R (Fig 3, A), but not of sgp130 (Fig 3, B), was significantly suppressed by IL-6 in a concentration-dependent manner including marked suppression at a concentration of 100 ng/ml IL-6 that is normally used for HuMC culture. This action of IL-6 would minimize trans-signaling due to a decrease in formation of IL-6/sIL6R complexes. Production of sIL-6R was also inhibited by IL-6 in the human LAD2 mast cell line with an IC50 of ~1 ng/ml. (Fig E2, A). GI254023X and GW280264, inhibitors of ADAM10 and ADAM17 the metalloproteases responsible for cleavage of IL-6R to form sIL-6R (see Scheller et al.28), suppressed production of sIL-6R by LAD2 cells, consistent with the conclusion that sIL-6R resulted largely from proteolytic cleavage (Fig E2, B).29, 30
FIG 3. Effect of IL-6 on release of sIL-6R and sgp130 from cultured HuMCs and in patients with mastocytosis.
(A and B) Release of sIL-6R (n=3) and sgp130 (n=4) from SCF-conditioned HuMCs after transfer to medium containing SCF and IL-6; concentrations and times of exposure to IL-6 are indicated. Positive and negative correlations between serum IL-6 and MC tryptase (C) or sIL-6R and IL-6 (D) in patients with mastocytosis. Mean values ± SEM and significant differences are shown: *P<0.05, **P<0.01, ***P<0.001.
Evidence was obtained that IL-6 may similarly suppress sIL-6R production in vivo from studies of patients with systemic mastocytosis where serum levels of IL-6 correlate with serum tryptase3 levels used as a measure of mast cell burden.31 Extending this study, we found that while there was a positive correlation between serum IL-6 and tryptase levels as we and other have reported3 (Fig 3, C), there was a negative correlation between serum sIL-6R and IL-6 levels (Fig 3, D). No significant correlation was observed between serum sgp130 levels and serum tryptase or, as in MC cultures, with IL-6 levels (data not shown). This data supports the possibility that IL-6 levels in vivo may also suppress the production of sIL-6R and thus limit trans-signaling in vivo.
Increased FcεRI, KIT, and IL-6R mediated signaling in IL-6 conditioned HuMCs
To determine whether the enhanced responses in the presence of IL-6 could be attributed to stronger signaling through relevant pathways, we initially investigated the effects on calcium signaling which is essential for FcεRI-mediated degranulation and is augmented on co-stimulation with SCF.32, 33 A substantial strengthening of the calcium signal was apparent in IL-6-conditioned cells whether cells were stimulated with SA or the combination of SA and SCF (Fig E3, A). IL-6 conditioning had minimal, if any, effect on the calcium signal induced by SCF alone (Fig E3, B) or that generated by the calcium mobilizing agent thapsigargin (Fig E3, C) indicating that that MCs had not become intrinsically more responsive to all stimulants.
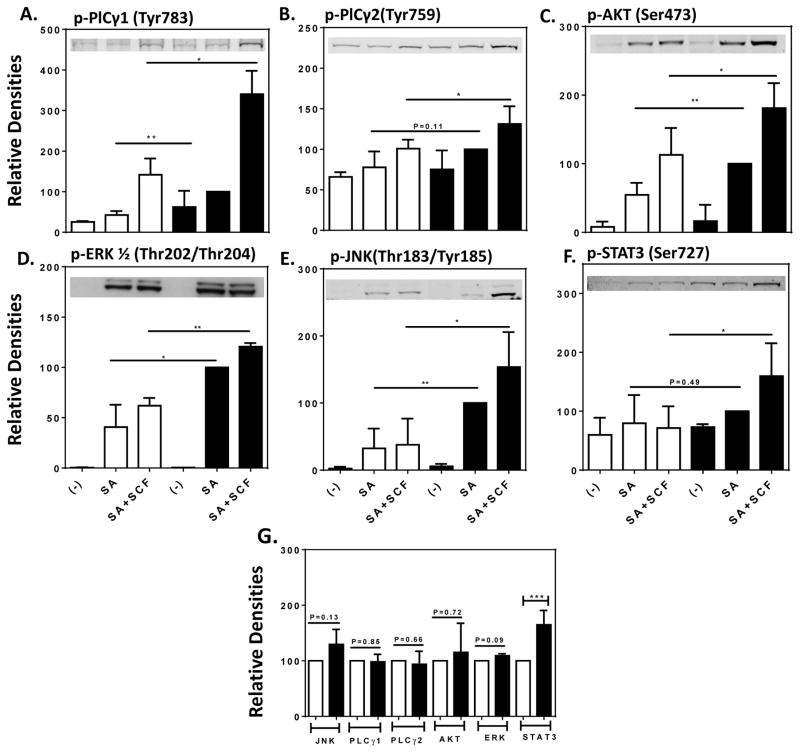
We next examined FcεRI-mediated phosphorylation of signaling molecules critical for HuMC activation. Activating phosphorylations of phospholipase Cγ (PLCγ)1 and 2, Akt a downstream target of phosphoinositide 3-kinase (PI3K), and the MAP kinases ERK and JNK were all enhanced in IL-6/SCF conditioned cells as compared to SCF conditioned cells after stimulation with SA or SA plus SCF (Fig 4, A–E and Fig E4)) even though the levels of these proteins remained the same in both sets of cells (Fig 4, G and Fig E4).
FIG 4. Increased FcεRI-mediated phosphorylation of signaling proteins in IL-6 conditioned HuMCs.
HuMCs grown in SCF in the absence (open columns) or presence (solid columns) of IL-6 were stimulated or not with SA or SA with SCF (10 ng/ml of each) for 2 minutes. Representative immunoblots as well as the mean ± SEM values of densitometric data for the indicated proteins and phosphorylated proteins for HuMCs from three donors are shown. Significant differences, * P<0.05, ** P<0.01, *** P<0.001.
In addition, SCF and IL-6 activate the JAK/STAT pathways which may complement the signals generated via FcεRI for cytokine production.32 When compared to non-conditioned cells, the extent of phosphorylation of STAT3 in response to stimulation with SA and SCF was substantially enhanced in IL-6 conditioned cells (Fig 4, F). In contrast to the other signaling molecules examined, the levels of STAT3 protein were significantly higher in IL-6 conditioned cells (Fig 4, G and Fig E4). Therefore, the exposure to IL-6 resulted not only in stronger signaling through the FcεRI and KIT canonical pathways but also in increased levels of STAT3 and phosphorylated STAT3.
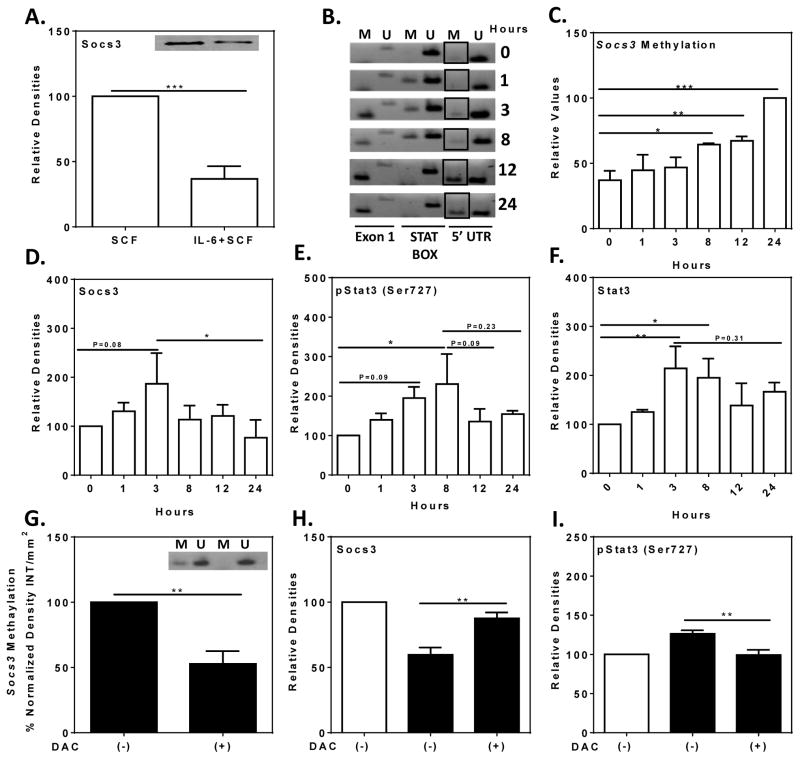
Sustained IL-6 exposure reduces SOCS3 expression and increases methylation of the SOCS3 promoter
IL-6 induces production of STAT334 and phosphorylated STAT3 can in turn induce expression of SOCS3 which acts as an auto-inhibitory regulator of the IL-6/STAT3 pathway (reviewed by Babon et al .35). Unexpectedly, we observed that SOCS3 levels were substantially reduced in HuMC cultures grown in SCF and IL-6 as compared to their counterparts grown in SCF alone (Fig 5, A). The methylation status of the SOCS3 promoter was then examined as hypermethylation of the promoter is associated with epigenetic silencing of SOCS3 in tumor cells.36–45 Methylation of the 5′UTR region of the SOCS3 promoter increased progressively over 24 hours after addition of IL-6 to HuMCs grown in SCF alone (Fig 5, B and C). SOCS3 levels initially increased and then declined substantially after 3 hours (Fig 5, D). The levels of phosphorylated STAT3 and STAT3 increased over the course of 3 to 8 hours and declined thereafter but not to a statistically significant extent (Fig 5, E and F). These events were blocked by 5-aza-2′-deoxycytidine, an inhibitor of DNA methyltransferases (DNMT) and SOCS3 promoter methylation.36 This compound suppressed methylation of the SOCS3 promoter, restored SOCS3 expression, and suppressed phosphorylation of STAT3 to levels observed in cultures without IL-6 (Fig 5, G–I).
FIG 5. Effects of IL-6 on SOCS3, methylation of SOCS3 promoter, STAT3, and phosphorylated STAT3.
SCF-conditioned HuMCs were exposed to IL-6 (100 ng/ml) for the hours indicated or maintained in SCF and IL-6 continuously. (A) Representative immunoblot and densitometric data for levels of SOCS3 under both conditions (n=3). (B) Representative gel image obtained for methylation of the SOCS3 promoter of the 5′UTR domain (M and U indicate methylated and unmethylated domain specific primer sets) and (C) densitometric data from two donors. (D–F) Relative levels of SOCS3, STAT3, and phosphorylated STAT3 as determined by immunoblotting and densitometry (n=5). (G–I) Effect of 5-aza-2′-deoxycytidine (+/−DAC) pretreatment after addition of IL-6 (solid columns) or not (open columns). (G) A representative gel image (inset) and relative densitometric values (n=3) for SOCS3 promoter methylation (G) and relative densitometric values of immunoblots for SOCS3 (H, n=4) and phosphorylated STAT3 (I. n=5). Values are mean ± SEM of values. Significant differences: * P<0.05, ** P<0.01, *** P<0.001.
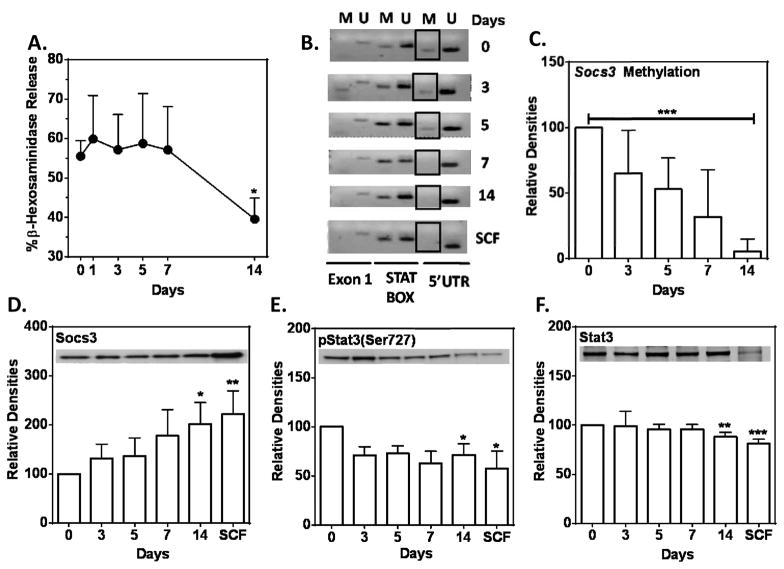
Withdrawal of IL-6 from SCF/IL-6 conditioned HuMC cultures had the reverse effect. Degranulation in response to SA declined between 7 to 14 days after withdrawal of IL-6 (Fig 6, A). Methylation of the 5′UTR region of the SOCS3 promoter (Day 0 in Fig 6, B) progressively diminished over the 14 day period after IL-6 withdrawal (Days 3–14 in Fig 6, B and C) with an accompanying increase in SOCS3 and a decrease in STAT3 and phosphorylated STAT3 levels (Fig 6, D–F).
FIG 6. Reversibility of IL-6 effects.
Data indicate days after withdrawal of IL-6 from SCF/IL-6-conditioned HuMC cultures. (A) Degranulation with SA, 10 ng/ml (n=2), (B) methylation of SOCS3 promoter (5′UTR domain, boxed region) in a representative culture or (C) densitometric data from all donors (n=3), and (D–F) expression of SOCS3 (n=5), STAT3 (n=6), and phosphorylated STAT3 (n=7) as indicated by representative immunoblots and densitometric values for all donors. Values are relative mean values ± SEM (day zero equals 100). * P<0.05, **P<0.01, ***P<0.001.
The above data are consistent with the conclusion that sustained exposure to IL-6 leads to hypermethylation of the SOCS3 promoter, reduced SOCS3 expression, and increased expression of STAT3 and phosphorylated STAT3. Consequently, suppression of SOCS3 expression allows optimal IL-6 mediated signaling throughout cell culture and thus alters HuMC phenotypic properties.
DISCUSSION
In this study, we report that IL-6 not only enhances the development of MCs from human peripheral blood CD34+ cells but also MC reactivity. The effect on maturation was apparent from increased granularity, cell size, and chymase content (Fig 1) while the enhanced reactivity was evident from increased FcεRI-mediated degranulation and cytokine production (Fig 2) as well as more robust signaling via calcium mobilization (Fig E3) and phosphorylation of PLCγ, Akt, ERK, JNK, and STAT3 (Fig 4). This hyper-reactive state was long lived and reverted back to a less active state over the course of 7 to 14 days after withdrawal of IL-6 (Fig 6). The ability of IL-6 to promote HuMC maturation and function over an extended period is associated with suppression of SOCS3 expression (Fig 5A) and the shedding of sIL-6R (Fig. 3A) thus favoring signaling through the classical pathway. Down regulation of SOCS3 coincided with methylation of the SOCS3 promoter region (Fig 5B) and was associated with increased levels of STAT3 protein and serine phosphorylated STAT3 (Fig 5 E, F). Our finding that these effects are prevented by the DNMT inhibitor, 5-aza-2′-deoxycytidine (Fig 5, G–I) and are reversed by withdrawal of IL-6 (Fig 6) imply that SOCS3 regulation is disrupted through methylation of the SOCS3 promoter to enable optimal phosphorylation and induction of STAT3 by IL-6. Aberrant sustained IL-6 signaling through STAT3 along with diminished SOCS3 expression has been observed in human tumor cells36, 37, 46 and attributed to increased expression of DNMT1 and, in turn, SOCS3 promoter methylation.36 The restoration of SOCS3 expression by 5-aza-2′-deoxycytidine (Fig 5, H) suggests that DNMT1 may play a similar role in IL-6 cultured HuMCs. Also the increased levels of STAT3 in IL-6-conditioned HuMCs might be a consequence of diminished SOCS3-mediated degradation of STAT3 as SOCS3 is reported to promote degradation of signaling proteins such as the JAKs and STATs.47, 48
SOCS3 autoregulation of the IL-6/JAK2/STAT3 pathway is dependent on SOCS3 binding to tyrosine phosphorylated gp130 and to the kinase inhibitory region of JAK (reviewed in 49). Without this negative regulation the inflammatory and pro-oncogenic effects of STAT3 and IL-6 such as cell proliferation and survival2 predominate as in the case of ulcerative colitis-related colorectal cancer 36 and other cancers.50 The disabling of the autoregulatory SOCS3 loop when HuMCs are cultured in IL-6 along with SCF may, therefore, have bearing on the pathogenesis of mastocytosis because of the constitutive activation of KIT51 and elevated serum IL-6 levels in mastocytosis.3
As both IL-6R and gp130 are expressed at the cell surface in MCs derived from human peripheral (Fig E1) and cord blood8 CD34+ cells it is likely that IL-6 acts directly via these receptors. We suspect this would be true for MCs from other sources because of their reactivity towards IL-6.9–11, 52 It is possible that IL-6 also acts via sIL-6R but this is less likely, at least in cell culture, because the concentrations of IL-6 required to transform HuMCs to a more reactive phenotype (3 to 100 ng/ml, Fig 2, B) also suppress production of sIL-6R (Fig 3, A and Fig E, 2A). This would favor formation of the neutralizing IL-6/sIL-6R/sgp130 complex rather than the IL-6/sIL-6R trans-activating complex. Shedding of the ectodomain through proteolytic cleavage by ADAM10 and ADAM 1729 is thought to account for most of the sIL-6R produced in human serum28 and this appears to be true for MCs (Fig E2, B). Both proteases are expressed at high levels in human lung MCs.53 A potential mechanism for the suppression of sIL-6R production is that IL-6R is protected from proteolytic cleavage when bound to IL-6 and held as a complex with gp130.
One possible consequence of reduction of sIL-6R shedding by IL-6 is that the inflammatory actions of IL-6 via IL-6/sIL-6 trans-signaling would be minimized, at least locally, during IL-6 induced maturation of MCs. IL-6/sIL-6R trans-signaling is thought to be primarily responsible for the inflammatory actions of IL-6.54 Thus, a polymorphism (Asp358Ala) within the proteolytic cleavage site of IL-6R results in enhanced shedding of sIL-6R,55, 56 elevated serum levels of sIL-6R, and a predisposition for persistent atopic dermatitis.57 Therefore, IL-6 may promote proliferation of more mature and antigen-reactive MCs but in doing so MCs become less able to propagate the inflammatory actions of IL-6 through suppression of sIL-6R.
In conclusion our studies highlight the long-term effects of IL-6 on HuMC, which are associated with expression of SOCS3 through methylation of SOCS3 promoter and suppression of the spontaneous production of soluble sIL-6R. Interestingly, the inverse relationship between serum levels of sIL-6R and IL-6 observed in mastocytosis (Fig 3, D) has been reported for colorectal cancer,58 neuroblastoma,59 and juvenile arthritis.60 Therefore, suppression of sIL-6 production by IL-6 may occur in other types of cells as well as MCs and may thus reduce formation of the trans-activating IL-6/sIL-6R pro-inflammatory complexes. These findings may have relevance in MC driven diseases associated with elevated levels of IL-6 including mastocytosis where treatment directed at lowering IL-6 levels could have a therapeutic benefit.
Supplementary Material
Key messages.
Stem cell factor dependent primary human mast cells increase in number and differentiate into a hyper-responsive phenotype upon co-culture in IL-6.
IL-6 suppresses spontaneous production of the soluble IL-6 receptor in vitro and in mastocytosis.
Sustained effect of IL-6 is attributable to disruption of feedback inhibition mediated by epigenetic silencing of the SOCS3 gene.
Acknowledgments
Supported by the Division of Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and the National Heart, Lung, and Blood Institute, National Institutes of Health.
Abbreviations
- HuMCs
CD34+-derived primary human mast cell cultures
- MC
mast cell
- PLCγ
phospholipase Cγ
- SA
streptavidin
- SCF
Stem Cell Factor
- SOCS3
Suppressor of Cytokine Signaling
- STAT3
Signal transducer and activator of transcription 3
- IL-6R
IL-6 Receptor
- sIL-6R
soluble IL-6R
Footnotes
Authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirano T. Interleukin 6 and its receptor: Ten years later. Intern Rev Immunol. 1998;16:249–84. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 2.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–59. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 3.Brockow K, Akin C, Huber M, Metcalfe DD. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–23. doi: 10.1016/j.clim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Mayado A, Teodosio C, Garcia-Montero AC, Matito A, Rodriguez-Caballero A, Morgado JM, et al. Increased IL6 plasma levels in indolent systemic mastocytosis patients are associated with high risk of disease progression. Leukemia. 2015:176. doi: 10.1038/leu.2015.176. [DOI] [PubMed] [Google Scholar]
- 5.Fujii K, Konishi K, Kanno Y, Ohgou N. Acute urticaria with elevated circulating interleukin-6 is resistant to anti-histamine treatment. J Dermatol. 2001;28:248–50. doi: 10.1111/j.1346-8138.2001.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 6.Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011;41:1386–91. doi: 10.1111/j.1365-2222.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 7.Morjaria JB, Babu KS, Vijayanand P, Chauhan AJ, Davies DE, Holgate ST. Sputum IL-6 concentrations in severe asthma and its relationship with FEV1. Thorax. 2011;66:537. doi: 10.1136/thx.2010.136523. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita T, Sawai N, Hidaka E, Yamashita T, Koike K. Interleukin-6 directly modulates stem cell factor-dependent development of human mast cells derived from CD34+ cord blood cells. Blood. 1999;94:496–508. [PubMed] [Google Scholar]
- 9.Schoeler D, Grutzkau A, Henz BM, Kuchler J, Kruger-Krasagakis S. Interleukin-6 enhances whereas tumor necrosis factor α and interferons inhibit integrin expression and adhesion of human mast cells to extracellular matrix proteins. J Invest Dermatol. 2003;120:795–801. doi: 10.1046/j.1523-1747.2003.12126.x. [DOI] [PubMed] [Google Scholar]
- 10.Misiak-Tloczek A, Brzezinska-Blaszczyk E. IL-6, but not IL-4, stimulates chemokinesis and TNF stimulates chemotaxis of tissue mast cells: involvement of both mitogen-activated protein kinases and phosphatidylinositol 3-kinase signalling pathways. APMIS. 2009;117:558–67. doi: 10.1111/j.1600-0463.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 11.Cruse G, Cockerill S, Bradding P. IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunol. 2008;9:2. doi: 10.1186/1471-2172-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–12. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 13.Lappalainen J, Lindstedt KA, Kovanen PT. A protocol for generating high numbers of mature and functional human mast cells from peripheral blood. Clin Exp Allergy. 2007;37:1404–14. doi: 10.1111/j.1365-2222.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25:241–53. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 15.Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, et al. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011;286:14804–11. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–9. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 18.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science. 2003;300:2101–4. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 19.Starr R, Hilton DJ. SOCS: suppressors of cytokine signalling. Int J Biochem Cell Biol. 1998;30:1081–5. doi: 10.1016/s1357-2725(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 20.Nakahata T, Tsuji K, Tanaka R, Muraoka K, Okumura N, Sawai N, et al. Synergy of stem cell factor and other cytokines in mast cell development. Biological and Molecular Aspects of Mast Cell and Basophil Differentiation and Function. 1995:13–24. [Google Scholar]
- 21.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–50. [PubMed] [Google Scholar]
- 22.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–34. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their á-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–67. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 24.Ochi H, Hirani WM, Yuan Q, Friend DS, Austin KF, Boyce JA. T Helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi T, Ishida S, Kinoshita T, Sakuma S, Sugawara N, Yamashita T, et al. IL-6 enhances IgE-dependent histamine release from human peripheral blood-derived cultured mast cells. Cytokine. 2002;20:200–9. doi: 10.1006/cyto.2002.2010. [DOI] [PubMed] [Google Scholar]
- 26.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–7. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 27.Smrz D, Bandara G, Beaven MA, Metcalfe DD, Gilfillan AM. Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells. Eur J Immunol. 2013;43:1873–82. doi: 10.1002/eji.201243214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, et al. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–71. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 30.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–95. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96:2702–10. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit.Rev. Immunol. 2009;29:155–86. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFκB. Genes Dev. 2007;21:1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babon JJ, Varghese LN, Nicola NA. Inhibition of IL-6 family cytokines by SOCS3. Semin Immunol. 2014;26:13–9. doi: 10.1016/j.smim.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis. 2012;33:1889–96. doi: 10.1093/carcin/bgs214. [DOI] [PubMed] [Google Scholar]
- 37.Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–96. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fourouclas N, Li J, Gilby DC, Campbell PJ, Beer PA, Boyd EM, et al. Methylation of the suppressor of cytokine signaling 3 gene (SOCS3) in myeloproliferative disorders. Haematologica. 2008;93:1635–44. doi: 10.3324/haematol.13043. [DOI] [PubMed] [Google Scholar]
- 39.He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, et al. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci U S A. 2003;100:14133–8. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011;122:241–51. doi: 10.1007/s00401-011-0832-0. [DOI] [PubMed] [Google Scholar]
- 41.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–17. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 42.Pierconti F, Martini M, Pinto F, Cenci T, Capodimonti S, Calarco A, et al. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate. 2011;71:318–25. doi: 10.1002/pros.21245. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, et al. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23:7726–33. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- 44.Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, et al. Methylation status of the SOCS3 gene in human malignant melanomas. Int J Oncol. 2007;30:689–94. [PubMed] [Google Scholar]
- 45.Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, et al. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24:6699–708. doi: 10.1038/sj.onc.1208818. [DOI] [PubMed] [Google Scholar]
- 46.Isomoto H. Epigenetic alterations in cholangiocarcinoma-sustained IL-6/STAT3 signaling in cholangio-carcinoma due to SOCS3 epigenetic silencing. Digestion. 2009;79(Suppl 1):2–8. doi: 10.1159/000167859. [DOI] [PubMed] [Google Scholar]
- 47.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–11. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 48.Morales JK, Falanga YT, Depcrynski A, Fernando J, Ryan JJ. Mast cell homeostasis and the JAK-STAT pathway. Genes Immun. 2010;11:599–608. doi: 10.1038/gene.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. SOCS, Inflammation, and Autoimmunity. Front Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–28. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 51.Brockow K, Metcalfe DD. Mastocytosis. Chem Immunol Allergy. 2010;95:110–24. doi: 10.1159/000315946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traum D, Timothee P, Silver J, Rose-John S, Ernst M, LaRosa DF. IL-10-induced gp130 expression in mouse mast cells permits IL-6 trans-signaling. J Leukoc Biol. 2012;91:427–35. doi: 10.1189/jlb.0411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, et al. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol. 2005;206:279–90. doi: 10.1002/path.1780. [DOI] [PubMed] [Google Scholar]
- 54.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 2004;5:513–6. doi: 10.1038/sj.gene.6364120. [DOI] [PubMed] [Google Scholar]
- 56.Garbers C, Monhasery N, Aparicio-Siegmund S, Lokau J, Baran P, Nowell MA, et al. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim Biophys Acta. 2014;1842:1485–94. doi: 10.1016/j.bbadis.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Esparza-Gordillo J, Schaarschmidt H, Liang L, Cookson W, Bauerfeind A, Lee-Kirsch MA, et al. A functional IL-6 receptor (IL6R) variant is a risk factor for persistent atopic dermatitis. J Allergy Clin Immunol. 2013;132:371–7. doi: 10.1016/j.jaci.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 58.Okugawa Y, Miki C, Toiyama Y, Yasuda H, Yokoe T, Saigusa S, et al. Loss of tumoral expression of soluble IL-6 receptor is associated with disease progression in colorectal cancer. Br J Cancer. 2010;103:787–95. doi: 10.1038/sj.bjc.6605827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res. 2008;14:7028–34. doi: 10.1158/1078-0432.CCR-07-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peake NJ, Khawaja K, Myers A, Nowell MA, Jones SA, Rowan AD, et al. Interleukin-6 signalling in juvenile idiopathic arthritis is limited by proteolytically cleaved soluble interleukin-6 receptor. Rheumatology (Oxford) 2006;45:1485–9. doi: 10.1093/rheumatology/kel154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.