Abstract
Persistent activation of NF-κB is a prerequisite for development of adult T cell leukemia-lymphoma (ATL) caused by human T cell leukemia virus type 1 (HTLV-1). HTLV-1 genome encodes a viral transforming protein named Tax, which constitutively activates the canonical IκB kinases (IKK), the central regulator of NF-κB signaling. However, the role of the non-canonical IκB kinases, TBK1 and IKKε, in the pathogenesis of HTLV-1-associated leukemia has not been evaluated. We here show that TBK1/IKKε are crucial pro-survival molecules by maintaining persistent activity of Stat3. Consistent with this finding, silencing Stat3 by the specific shRNA or by the chemical inhibitor ruxolitinib results in drastic impediment of leukemia cell growth. We further find that in HTLV-1-transformed T cells expressing Tax, TBK1 co-localizes with the canonical IκB kinases and Tax in the lipid raft microdomains. The wild type Tax, but not the Tax mutant defective in activating the canonical IKK, promotes the lipid raft translocation of TBK1. This phenomenon correlates with Tax activation of both NF-κB and Stat3. Tax does not interact directly with TBK1/IKKε, and it rather engages a molecular crosstalk between the canonical IKKs and TBK1/IKKε. Our data, therefore, demonstrate a key role of TBK1/IKKε in the survival and proliferation of HTLV-1-transformed T cells and implicate a potential therapy targeting TBK1/IKKε and Stat3 in controlling HTLV-1-mediated oncogenesis.
Keywords: TBK1/IKKε, IKK, NF-κB, Stat3, HTLV-1 Tax, T lymphocyte transformation
1. Introduction
Adult T cell leukemia and lymphoma (ATL) is an aggressive form of T lymphocyte malignancy, which is caused by chronic infection with human T cell leukemia virus type 1 (HTLV-1) (1,2). The molecular mechanism of HTLV-1-mediated T cell transformation remains elusive. Extensive studies show that the viral genome of HTLV-1 encodes two oncogenes, tax and hbz, and that the oncogenic cooperation of these two viral genes is proposed to play a key role in T cell transformation (3,4). Of note, Tax functions as a multifunctional viral protein that regulates viral and cellular gene expressions in both direct and indirect manners. Tax is the viral transactivator that promotes 5’-long terminal repeat (5’-LTR) of HTLV-1-directed expression of the structural proteins (Gag-Pol and Env) and regulatory proteins (Rex, p30 and Tax itself) (5,6). Tax also induces the transcription of the antisense gene hbz through the viral 3’-LTR (5). Hence, Tax is essential for mediating active viral replication in host cells. In addition, Tax is shown to act as a master regulator of host cell signaling including NF-κB and PI3 kinase/Akt pathways (7). Notably, constitutive activation of NF-κB signaling by Tax is a prerequisite of HTLV-1-mediated transformation of T cells. Tax stimulates the canonical IκB kinase complex (IKK) by interacting with IKKγ, the regulatory subunit of IKK, resulting in persistent activation of NF-κB (8,9). Furthermore, the molecular clone of HTLV-1 with the Tax mutant defective in activating NF-κB is unable to immortalize human primary T cells (10). This phenomenon further supports a key role of NF-κB signaling in HTLV-1-mediated oncogenesis. We recently demonstrate that Tax induces macroautophagy through lipid raft recruitment and activation of the canonical IκB kinases and the autophagy molecule Beclin1 to promote T cell survival and proliferation (11,12).
The canonical IKK complex constitutes two highly homologous catalytic subunits, IKKα and IKKβ, and one regulatory subunit, IKKγ (13). This kinase complex is the central regulator in NF-κB signaling. Two homologous non-canonical IκB kinases, TBK1 and IKKε, share 27% amino acid sequence homology with IKKα/β, but are not the components of the canonical IKK complex (14,15). Emerging evidence shows that IKKε is a defined cellular oncoprotein in breast cancer (16). Both TBK1 and IKKε are constitutively expressed in human T cells (17). Since TBK1/IKKε are implicated in functioning as the activators of NF-κB at certain biological conditions, we intend to determine if there is a role for TBK1/IKKε in activating NF-κB in the context of HTLV-1-mediated oncogenesis. In the present study, we demonstrate that TBK1/IKKε are crucial for maintaining constitutive activity of Stat3, and that Tax engages a molecular crosstalk between the canonical IKK complex and TBK1/IKKε. Our data, therefore, shed new insights into the oncogenic mechanism of HTLV-1-mediated cellular transformation and provide a rational for designing molecular therapeutics targeting TBK1/IKKε and Stat3 in treating HTLV-1-associated leukemia.
2. Materials and methods
2.1. Cell Lines, antibodies and reagents
FC36 cell line (kindly provided by Drs. Fiorenza Cocchi, Anthony DeVico, Alfredo Garzino-Demo, Suresh Arya, Robert Gallo, and Paolo Lusso) and MT-2 cell line (kindly provided by Dr. Douglas Richman) were obtained from the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (18,19). SLB-1, PTX4-1 and HEK293 cell lines as well as Tax antibody were described previously (11). PBMC cells were from healthy blood donors. Antibodies reacting to caveolin1, STAT3, p-ERK1, IKKα, IKKβ, IKKγ, GST, LAT, GFP and IKKε were purchased from Santa Cruz Biotechnology (CA, USA). Antibodies reacting to TBK1, p-STAT3, cleaved Caspase 3, 9, PARP, Mcl-1, BcL-xL, Bcl-2 and Bax were obtained from Cell Signaling Technology (Danvers, MA). Anti-ERK1 was from Transduction Lab. β-actin and Flag antibodies as well as protease and phosphatase inhibitor cocktails were obtained from Sigma (St. Louis, MO, USA).
2.2. Plasmids, DNA transfection and lentivirus transduction
The shRNAs specific for IKKε, STAT3 and TBK1 were purchased from GE Healthcare (Buckinghamshire, UK). The expression plasmids for Tax, M22 and M47, IKKβKA were described previously (11). The methods for lentivirus production, purification and transduction were reported previously (11).
2.3. Cell viability and apoptosis assays
Cells transduced with the lentiviruses expressing specific shRNAs were analyzed with the trypan blue exclusion assay to determine cell viability (11). Apoptotic cells were examined with Annexin V/7-AAD staining using the AnnexinV-APC/7-AAD Apoptosis Detection kit (Biolegend, USA). Flow cytometry analysis was performed on a FACS flow cytometer (Becton-Dickinson). Data were analyzed by Flowjo software.
2.4. Western blot, immunoprecipitation and GST pulldown assay
Cells were collected and lysed in RIPA buffer containing protease and phosphatase inhibitor mixtures at 4°C for 30 min. Equal amounts of cellular proteins were analyzed by SDS-PAGE, followed by immunoblot. Anti-β-actin blot was used for the protein loading control. The immunoprecipitation and GST pulldown assays were reported previously (11)
2.5. Electrophoretic Mobility Gel Shift Assay (EMSA)
Nuclear extracts were prepared from various cell lines using NE-PER nuclear and cytoplasmic extraction reagents (Pierce). The oligonucleotide was 5’-end-labeled with biotin (Integrated DNA Technologies) and annealed to its complementary strand. The binding activities were examined by EMSA using a light shift chemiluminescent EMSA kit (Pierce) following the protocol reported previously (22). The oligonucleotide probes are for STAT3 (5’-GATCCTTCTGGGAATTCCTAGATC-3’), NF-κB (5’-GATCCGGCAGGGGAATCTCCCTCTC-3’) and AP-1 (5’-CGCTTGATGACTCAG-CCGGAA-3’).
2.6. Lipid raft fractionation assay
Cells in 10 cm dishes with 95% confluence were lysed in 1.2 ml of extraction buffer (20 mM Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100 plus protease inhibitor mixture). Lysates were sheared by 50 passages through a 22-gauge needle and mixed with the OptiPrep density gradient medium (Iodixanol solution, final concentration, 40% vol/vol; AXIS-SHIELD PoC AS, Oslo, Norway), and placed at the bottom of a 12-ml tube. By overlaying 4 ml of 30% and 4 ml of 5% of OptiPrep medium, a discontinuous OptiPrep gradient was formed. Ultracentrifugation was performed at 28500 rpm for 4 hours at 4 °C in SW41Ti rotor. 1 ml of each fraction from top to bottom was collected and the protein samples in each fraction were subjected to immunoblot analysis.
3. Results
3.1. TBK1 is required for survival and proliferation of HTLV-1-transformed T cells
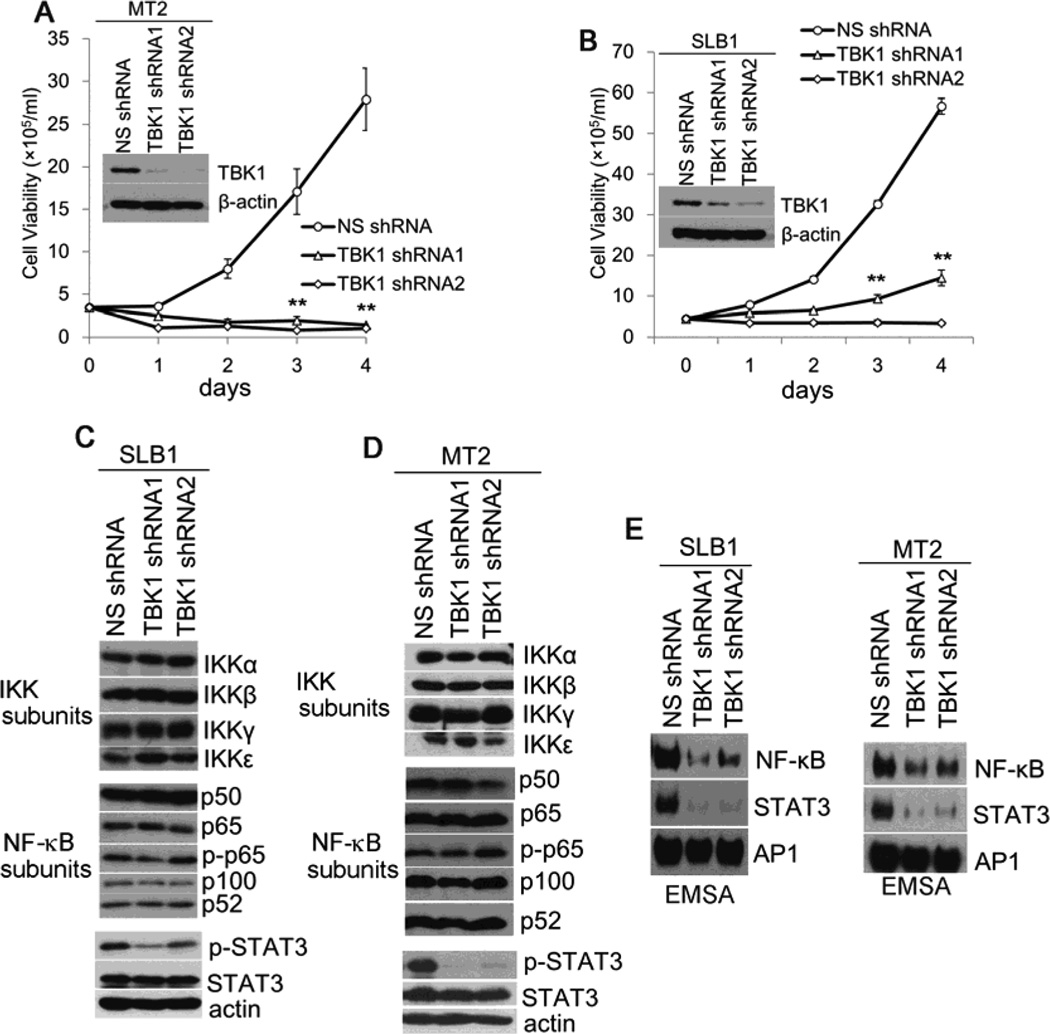
To define the role of TBK1 in HTLV-1-associated oncogenesis, we first determined if TBK1 plays a role in promoting survival and proliferation of HTLV-1-transformed T cells. We utilized lentiviral delivery of shRNAs specific to TBK1 and found that silencing TBK1 drastically affected viability of two HTLV-1-transformed T cell lines, MT-2 and SLB-1 (Fig. 1A and 1B). Since constitutive activity of NF-κB is crucial for survival of HTLV-1-transformed T cells, we next determined if depletion of TBK1 would lead to altered expression of NF-κB signaling molecules. As shown in Fig. 1C and 1D, the protein levels of the subunits of the canonical IKK complex including IKKα, IKKβ and IKKγ, together with the NF-κB subunits including p65, p50, p100 and p52, were not altered in TBK1-depleted MT-2 and SLB-1 cells. However, the depletion of TBK1 resulted in reduced levels of phosphorylated Stat3 in these virally transformed T cells (Fig. 1C and 1D). The activity of STAT3 was significantly impaired while the NF-κB activity was also reduced at certain degrees in the TBK1-depleted T cells (Fig. 1E). In contrast, the activity of AP-1 was not altered regardless of the levels of TBK1 in both MT-2 and SLB-1 cells (Fig. 1E). Together, these data provided a newly identified evidence for a crucial role of TBK1 in mediating persistent activation of Stat3 and NF-κB.
Fig. 1.
TBK1 is required for survival and proliferation of HTLV-1-transformed T cells. (A) and (B) The viability of the MT2 and SLB1 cells transduced with non-specific (NS)- or TBK1-specific shRNAs. The protein levels of TBK1 were examined with anti-TBK1 immunoblot. (C) and (D) The protein levels of the IκB kinase subunits, various NF-κB subunits and the phosphorylated STAT3 (Tyr705) were examined with immunoblot in the SLB-1 and MT-2 cells transduced with NS- or TBK1-specific shRNAs. Total protein lysates were analyzed with antibodies specific for IKKα, IKKβ and IKKγ, IKKε, p65, p50, p-p65, p100 and p52 as well as the phosphorylated STAT3 (Tyr705) and total STAT3. β-actin was used as protein loading control. (E) The activities of STAT3 and NF-κB in TBK1-depleted MT-2 and SLB-1 cells were examined with EMSA. ** : p < 0.01 as determined by 2-tail student t-test.
3.2. Silencing IKKε leads to an impaired activity of STAT3 in HTLV-1-transformed T cells
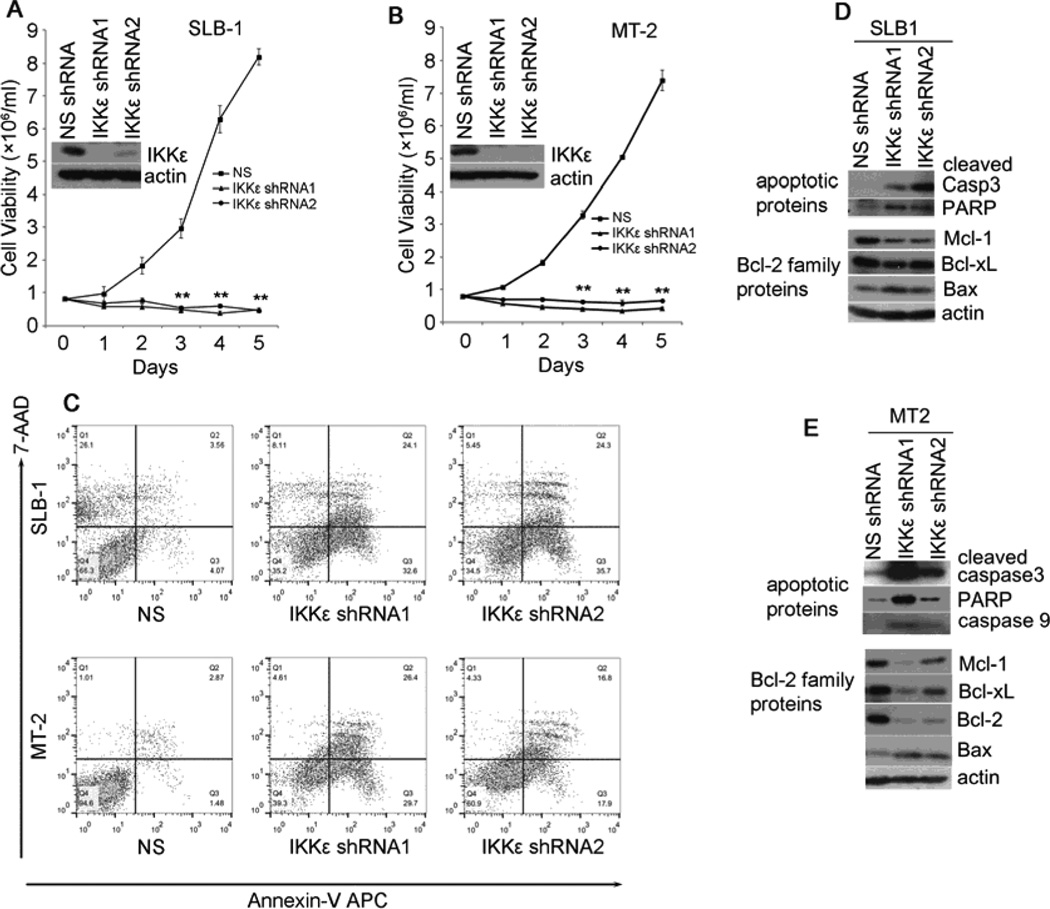
IKKε is highly homologous to TBK1. Both kinases are known to play important roles in interferon alpha signaling and to activate NF-κB in certain biological conditions. To evaluate if IKKε plays a role in NF-κB activation in addition to TBK1 or the canonical IκB kinases, we expressed IKKε-specific shRNAs in both SLB-1 and MT-2 cells. We found that similar to TBK1-depleted cells, silencing IKKε caused drastic reduction of the viability of HTLV-1-transformed T cells (Fig. 2A and 2B). Increased annexin-V-staining positive cells were also observed following IKKε silencing (Fig. 2C), and the depletion of IKKε led to increased levels of the cleaved caspase 3 and PARP (Fig. 2D and 2E). These results suggested that the removal of IKKε caused apoptotic death of HTLV-1-transformed T cells, and that IKKε played an essential role in facilitating survival of HTLV-1 transformed T cells. We further analyzed the expression levels of Bcl-2 family proteins and found that the pro-survival Mcl-1 was reduced while Bax, a pro-apoptotic Bcl-2 protein, was increased upon IKKε silencing (Fig. 2D and 2E). These results validated a pro-survival role of IKKε in HTLV-1-transformed T cells.
Fig. 2.
Silencing IKKε leads to apoptosis of HTLV-1-transformed T cells. (A) and (B) The viability of the MT2 and SLB1 cells transduced with NS- or IKKε-specific shRNAs. (C) The apoptosis of the MT2 and SLB1 cells transduced with NS- or IKKε-specific shRNAs was examined using AnnexinV-APC/7-AAD Apoptosis Detection kit. The cleaved caspase 3, 9 and PARP, the pro-survival proteins (Mcl-1, Bcl-2, Bcl-Xl) and pro-apoptotic protein Bax were examined by immunoblot using relevant antibodies in the SLB1 (D) and MT2 (E) cells that are transduced with NS- or IKKε-specific shRNAs. ** : p < 0.01 as determined by 2-tail student t-test.
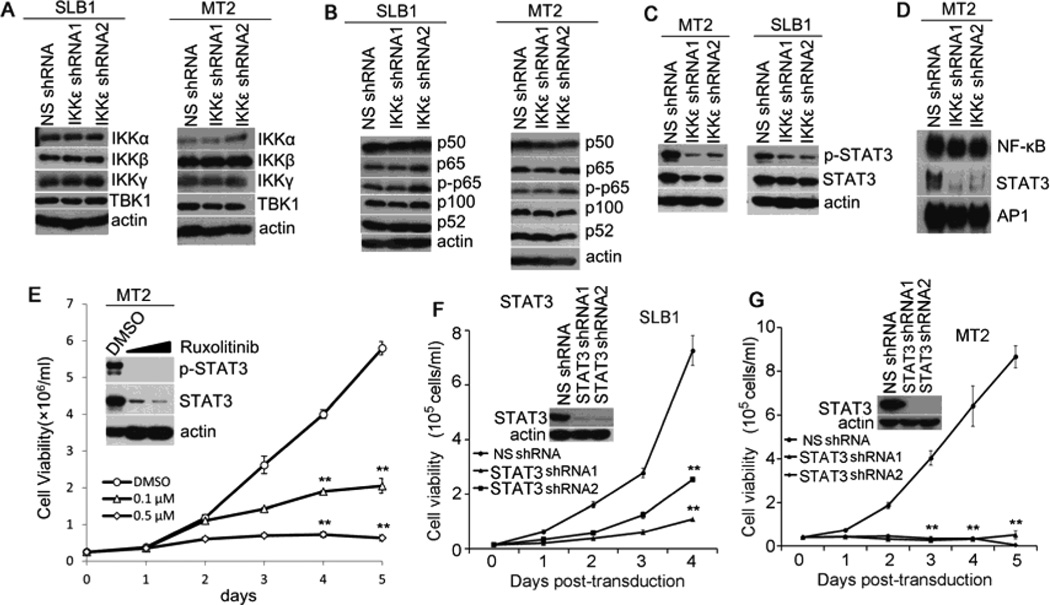
We next examined a possible pro-survival mechanism of IKKε by analyzing the protein levels of NF-κB signaling molecules. As shown in Fig. 3A, the protein levels of TBK1, along with the canonical IκB kinas including IKKα, IKKβ and IKKγ, were not altered in the IKKε-depleted cells as compared to the NS shRNA-transduced SLB-1 and MT-2 cells. Similarly, various NF-κB subunits, including p65/RelA, p50, p100 and p52, and the phosphorylation status of p65 were not changed by IKKε depletion (Fig. 3B). However, we found that the phosphorylation of Stat3 and the DNA binding activity of the nuclear translocated Stat3 were significantly impaired in the IKKε-depleted T cells (Fig. 3C and 3D). Unexpectedly, the NF-κB activity was not affected by knocking down IKKε (Fig. 3D). To determine if Stat3 plays a role in supporting survival of HTLV-1-transformed T cells, we first applied the chemical inhibitor, ruxolitinib. We found that treatment of MT-2 cells with ruxolitinib caused reduction of the STAT3 protein levels and consequently, decreased phospho-STAT3 levels (Fig. 3E). Correlating with impaired activity of Stat3 following ruxolitinib treatment, the viability of MT-2 cells was diminished (Fig. 3E). Next, we found that silencing STAT3 with the specific shRNAs indeed caused drastic reduction of the viability of SLB-1 and MT-2 cells (Fig. 3F and 3G). From these findings, we concluded that IKKε supported T cell survival by maintaining the persistent activity of STAT3 in HTLV-1-transformed T cells.
Fig. 3.
Silencing IKKε leads to an impaired activity of STAT3 in HTLV-1-transformed T cells. (A) Total protein lysates from the MT-2 and SLB-1 cells transduced with NS- or IKKε-specific shRNAs were analyzed with anti-IKKα, anti-IKKβ, anti-IKKγ and anti-TBK1. (B) The protein levels of NF-κB subunits, including p65/RelA, p50, p100 and p52, and the phosphorylation status of p65 were examined in the MT2 and SLB1 cells transduced with NS- or IKKε-specific shRNAs. (C) The phosphorylation status of STAT3 in IKKε-depleted SLB-1 and MT-2 cells. (D) The activities of Stat3 and NF-κB in MT-2 cells were detected by EMSA following IKKε depletion. (E) MT-2 cells were treated with DMSO or with ruxolitinib at does of 0.1 µM and 0.5 µM for 5 days. The levels of phosphorylated Stat3 and total STAT3 were examined using relevant antibodies. The viability of these cells was examined by Trypan blue exclusion assay. (F) and (G) The viability of the MT-2 and SLB-1 cells transduced with NSor STAT3-specific shRNAs. ** : p < 0.01 as determined by 2-tail student t-test.
3.3. TBK1 is recruited to the lipid raft microdomains in Tax-expressing T cells
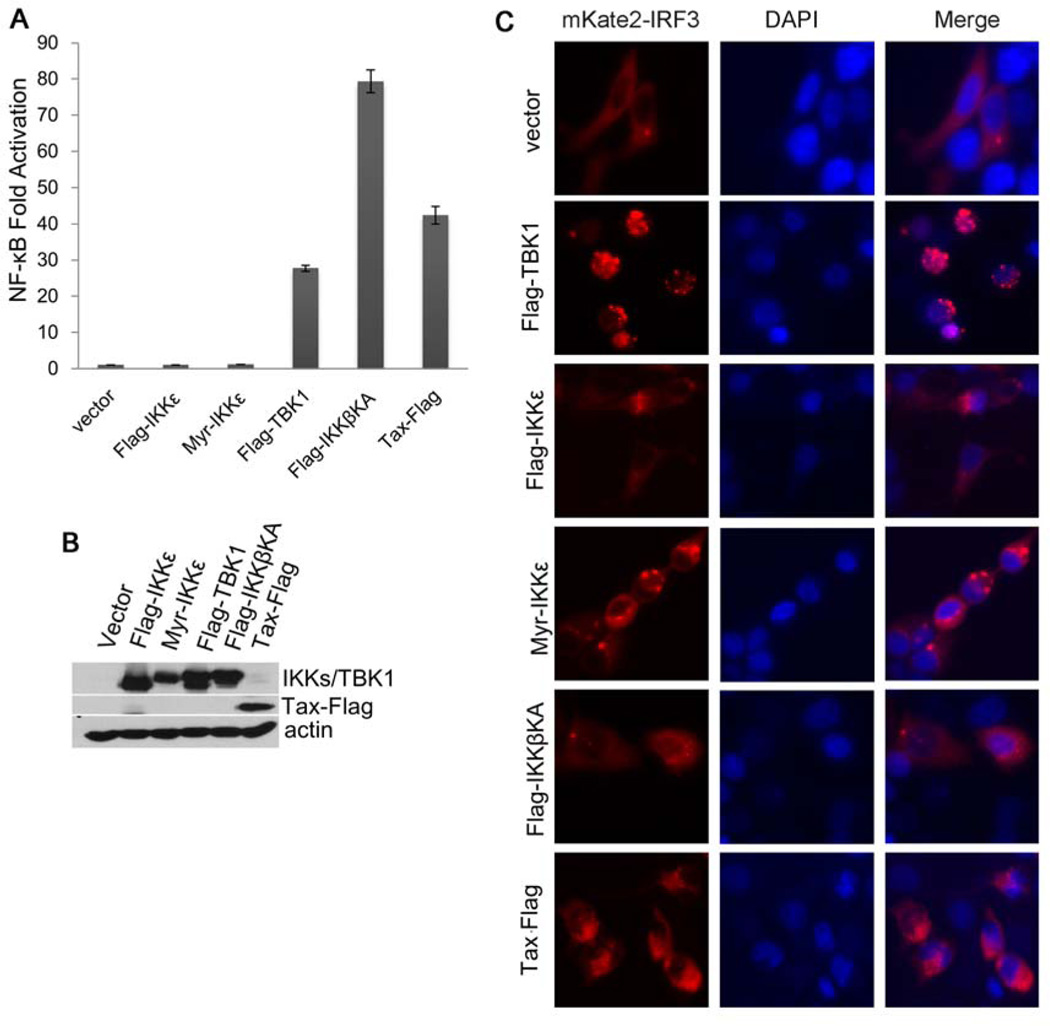
To verify the role of TBK1 and IKKε in the regulation of NF-κB and STAT3, we first performed NF-κB reporter assay. TBK1, but neither the wild type IKKε nor the myristoylated IKKε (Myr-IKKε), induced activation of NF-κB in transiently transfected HEK293 cells (Fig. 4A and 4B). Expectedly, the constitutively active form of IKKβ (IKKβKA) and Tax promoted robust NF-κB activation (Fig. 4A and 4B). Further, the expression of TBK1 induced nuclear translocation of IRF3 whereas IKKε, IKKβKA and Tax failed to do so (Fig. 4C). Since it was previously shown that IKKε has a role in activating IRF3 in interferon signaling, the failure to detect the IRF3-inducing activity by IKKε in this setting indicated that the intrinsic kinase activity of IKKε requires extracellular stimulation. Taken together, these results are consistent with the finding that TBK1, in addition to the canonical IκB kinases, plays a crucial role in NF-κB activation in the context of HTLV-1 transformation of T lymphocytes.
Fig. 4.
TBK1 induces activation of NF-κB and nuclear translocation of IRF3. (A) NF-κB reporter assay in HEK293 cells transfected with vector, Flag-IKKε, Myr-IKKε, Flag-TBK1, Flag-IKKβKA and Tax-Flag, together with pNF-κB-luciferase reporter plasmid. (B) Expression levels of various proteins in transfected HEK293 cells seen in (A). (C) Fluorescence imaging analysis of the subcellular localization of IRF3 in HEK293 cells co-transfected with mKate2-IRF3 together with vector, Flag-IKKε, Myr-IKKε, Flag-TBK1, Flag-IKKβKA or Tax-Flag.
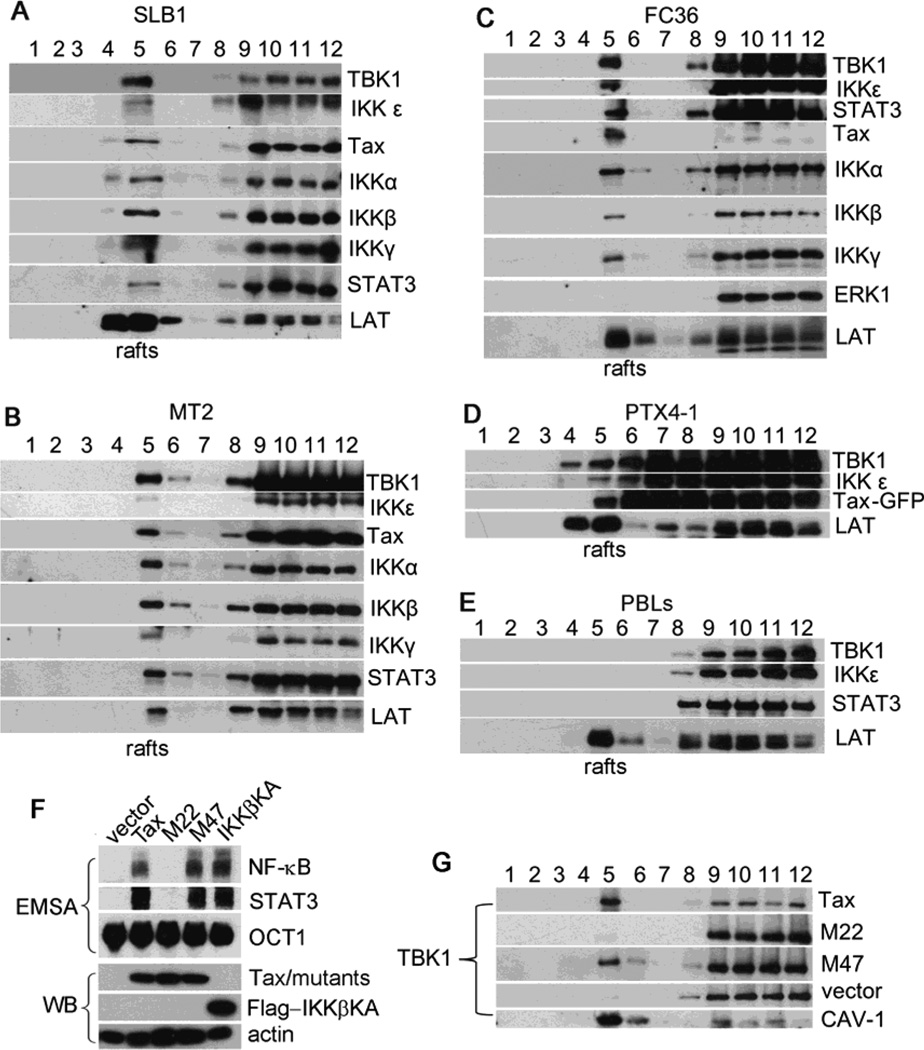
The viral Tax protein and the canonical IκB kinases are persistently resided in the lipid raft microdomains where these kinases are activated in promoting constitutive activation of NF-κB in HTLV-1-transformed T cells (11). To investigate the activation mode of TBK1/IKKε, we performed density gradient ultracentrifugation to determine if these non-canonical IKKs were present in the rafts. We found that significant portions of TBK1, along with the canonical IKKs and Tax, were localized in the lipid raft fractions in Tax-expressing HTLV-1-transformed T cell lines including SLB-1, MT-2 and FC36 as well as in Tax-immortalized T cell line PTX4-1 (Fig. 5A–5D). TBK1 was detected exclusively in soluble fractions in normal peripheral blood lymphocytes (PBLs) (Fig. 5E). A small fraction of IKKε and STAT3 were also detected in the rafts as compared to the levels of these proteins expressed in normal PBLs (Fig. 5A–5E), indicating that the translocation of IKKε and STAT3 to lipid rafts occurred in a rapid turnover rate.
Fig. 5.
TBK1 is recruited to the lipid raft microdomains in Tax-expressing T cells. Lipid raft fractionation analysis of TBK1, IKKε, Tax, IKKα, IKKβ, IKKγ and STAT3 in SLB1 cells (A), MT-2 cells (B), FC36 cells (HTLV-1 infected CD8+ T cells) (C), PTX4-1 cells (Tax-immortalized T cells) (D), and PBLs (normal peripheral blood lymphocytes) (E). (F) Tax, M22, M47 and IKKβKA were transfected into HEK293 cells. The nuclear extracts of the transfected cells were examined for the activities of STAT3 and NF-κB using EMSA. (G) Tax, M22, M47 and the vector control were transfected into HEK293 cells. Lipid raft fractionation analysis of the transfected cells was analyzed for the lipid raft translocation of TBK1 with immunoblot. LAT and Caveolin 1 (CAV-1) are the lipid raft marker proteins.
To determine if the activity of TBK1 correlates with the canonical IKK activation, we applied the wild type Tax and its mutants, M22 and M47. M22 retains the ability to activate CREB but is defective in inducing NF-κB activity (11). In contrast, M47 retains the capacity to activate the IKK complex but is deficient in promoting CREB activation (11). We found that the wild type Tax, M47 and IKKβKA, but not M22 nor the vector control, were able to activate both NF-κB and Stat3 (Fig. 5F). Correlating with these activities, Tax and M47 promoted lipid raft translocation of TBK1 (Fig. 5G). Unlike TBK1, IKKε was only constitutively expressed in certain tissues and was not detected in HEK293 cells. This suggested that the activity of TBK1 was executed independently of IKKε.
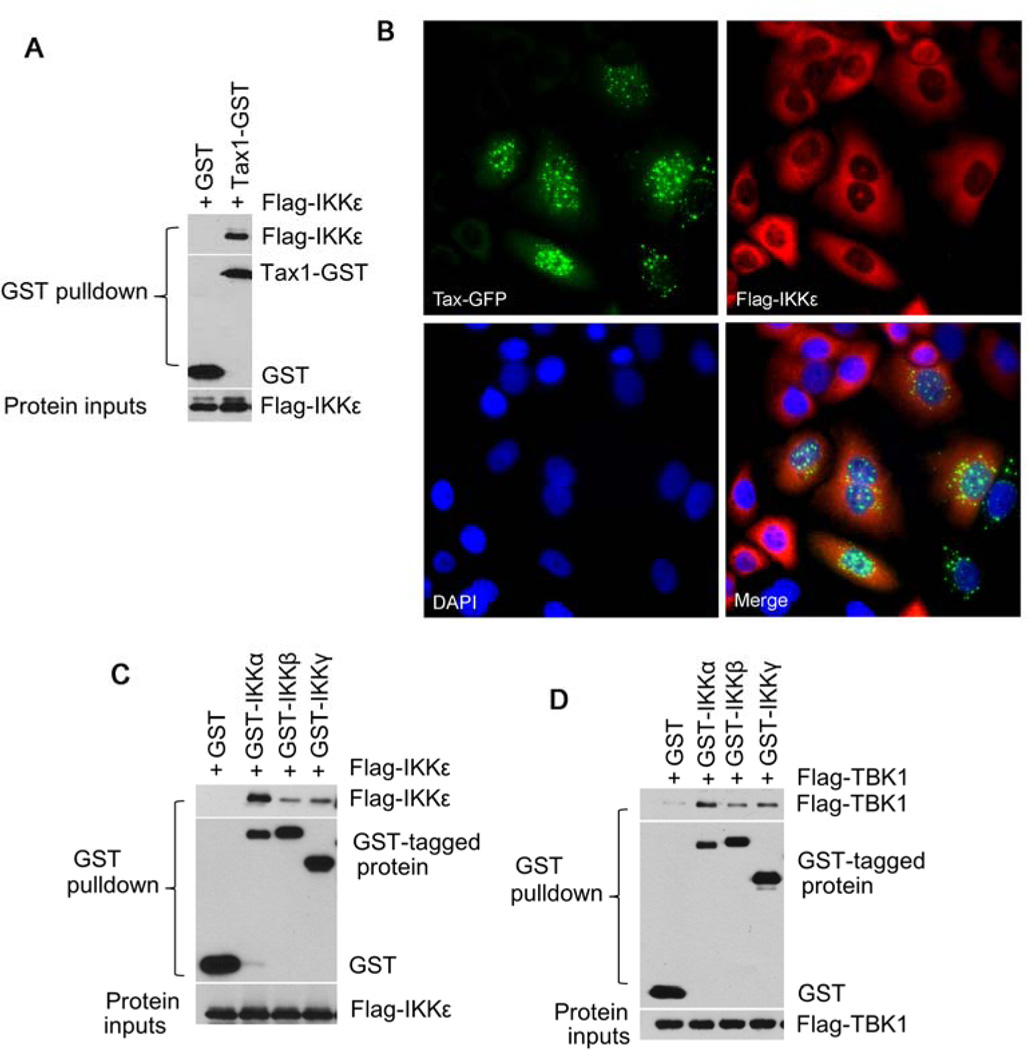
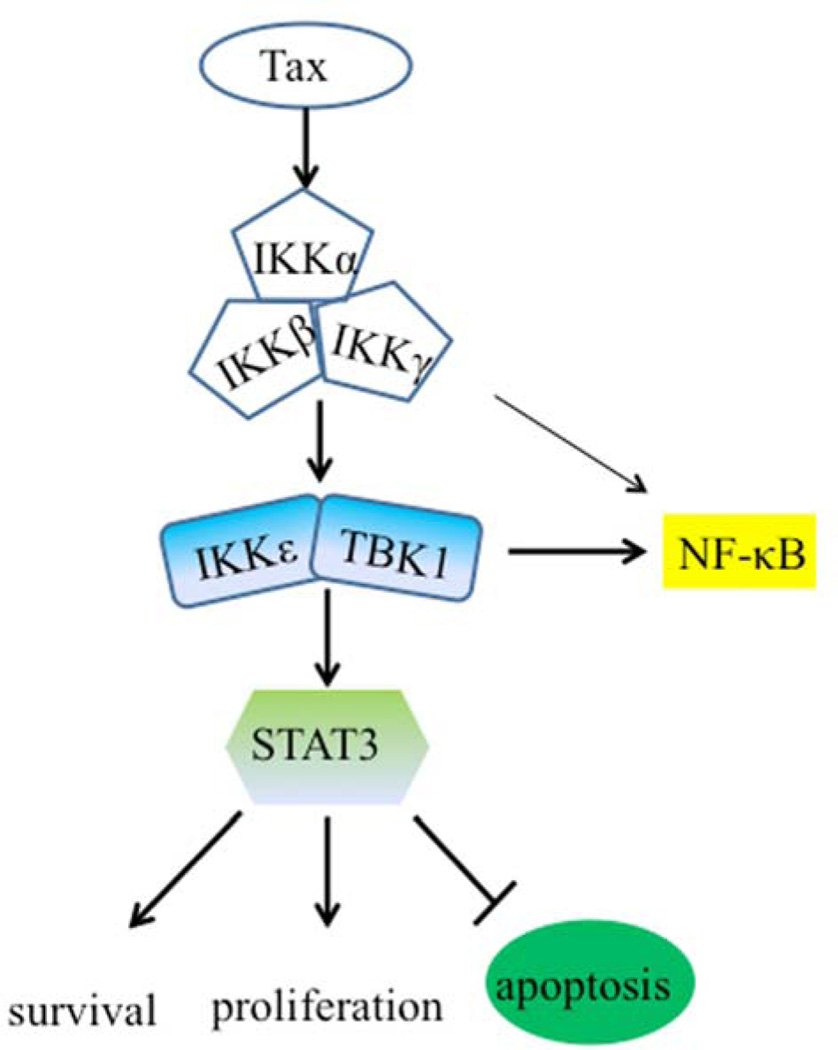
Tax is known to interact with the IKK complex through its direct binding to IKKγ. A recent report shows that Tax interacts with TBK1/IKKε in transfected HEK293 cells (20). Consistent with this finding, we found that Tax was co-precipitated with IKKε (Fig. 6A), but the interaction of Tax with TBK1 was not detected. We also failed to detect the interaction of the endogenously expressed Tax and TBK1/IKKε in HTLV-1-transformed T cells using co-immunoprecipitation assay. We next evaluated potential co-localization of Tax and IKKε in transfected HeLa cells. Tax was distributed in both the cytoplasm and the nucleus with speckled patterns, while IKKε was evenly distributed in the cytoplasm, and two fluorescence signals did not overlap (Fig. 6B). This physical evidence suggested that Tax was likely to affect the activity of TBK1/IKKε in an indirect manner. To test a possible interaction of TBK1/IKKε with the canonical IκB kinases, we co-transfected TBK1 or IKKε with the canonical IκB kinases. As shown in Fig. 6C and 6D, IKKε/TBK1 were found to interact with predominantly with IKKα subunit of the IKK complex. Taken all above results together, we proposed that TBK1/IKKε acted downstream of the canonical IκB kinases and that Tax engaged a molecular crosstalk between TBK1/IKKε and the canonical IκB kinase complex in mediating survival, anti-apoptosis and oncogenesis in the context of HTLV-1 transformation of T lymphocytes (Fig. 7).
Fig. 6.
TBK1/IKKε interact with the canonical IκB kinases. (A) Co-precipitation of IKKε with Tax in transfected HEK293 cells. The GST pulldown was analyzed with anti-FLAG antbody for detecting FLAG-tagged proteins and with anti-GST antibody for detecting GST-tagged proteins. (B) Fluorescence imaging of HeLa cells expressing Tax-GFP and Flag-IKK. (C) Interaction of IKKε with IKKα, IKKβ or IKKγ in co-transfected HEK293 cells was analyzed with GST pulldown assay. (D) Interaction of TBK1 with IKKα, IKKβ or IKKγ in co-transfected HEK293 cells was analyzed with GST pulldown assay.
Fig. 7.
A hypothetical model of Tax activation of TBK1/IKKε in the context of HTLV-1-transformed T lymphocytes.
4. Discussion
The present study has identified a new role of TBK1/IKKε in functioning as pro-survival molecules in HTLV-1-transformed T cells. Both TBK1 and IKKε are crucial for maintaining the constitutive activity of STAT3 since silencing one of them significantly impairs the Stat3 activation. Consistently, silencing STAT3 leads to drastic impediment of the leukemia cell growth, thereby validating the pro-survival function of TBK1/IKKε in HTLV-1-transformed T cells. The STAT3 activity in ATL cells has not been well studied previously. We have reproducibly observed the STAT3 activity by EMSA and by the specific phosphorylation of STAT3 in Tax-expressing HTLV-1-transformed T cells (11,19). In fact, Tax alone is able to activate STAT3 in transfected HEK293 cells and in Tax-immortalized T cells (11, 19). Since TBK1/IKKε are involved in IFNα induction, one would reason that the activated TBK1/IKKε induce the STAT3 activity through soluble factors such as IFNα. This appears not to be the case in this setting, as we have found that soluble factors have no role in STAT3 activation in the HTLV-1-transformed T cells (21).
Although the precise mechanism of the TBK1/IKKε’s action in the maintenance of the persistent STAT3 activity remains to be further investigated, our data show that this action is likely mediated through the molecular crosstalk between the canonical IKK complex and TBK1/IKKε. This notion is supported with following observations. First, silencing IKKγ, the regulatory subunit of the canonical IKK complex, leads to the impaired activities of both NF-κB and STAT3 (19), implying that the activity of STAT3 correlates with the NF-κB activation in HTLV-1-transformed T cells. Second, using the wild type Tax and its mutants with employing the lipid raft fractionation assay and EMSA, we confirm that the activity of TBK1 is correlated with the activation of the canonical IκB kinases. Third, silencing IKKε only affects the Stat3 activity, suggesting that IKKε is a downstream molecule of the canonical IKK complex.
Here, we propose a hypothetical model of Tax-induced activation of TBK1/IKKε. Tax promotes the recruitment of the canonical IκB kinases along with TBK1/IKKε into the lipid raft microdomains where these kinases become persistently activated. The microdomains in T cells serve as signal transduction platform where the signaling molecules including the canonical IκB kinases are recruited to the plasma membrane-associated lipid rafts upon T cell receptor (TCR) engagement, in transmitting activation signals. Tax imitates the TCR activation mode by bypassing the upstream events of TCR, directly targeting the canonical IκB kinases to the microdomains for activation. Consequently, we find, for the first time, that TBK1 also migrates to the microdomains and that the lipid raft translocation of TBK1 is apparently dependent on the canonical IκB kinases. Given the facts about the roles of STAT3 and IKKε/TBK1 in tumor induction and progression, our study also demonstrates that these non-canonical IκB kinases play a key role in HTLV-1-transformation of human T lymphocytes in addition to their pro-survival functions.
Highlights.
TBK1/IKKε promote survival and proliferation of HTLV-1-transformed T lymphocytes
TBK1/IKKε maintain persistent activity of STAT3 in HTLV-1-transformed T cells
HTLV-1 Tax engages a molecular crosstalk between TBK1/IKKε and the IKK complex
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award number R01AI090113 to Hua Cheng. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
HZ designed, performed experiments, analyzed the data and wrote the manuscript. LC performed parts of experiment and SHC analyzed data. HC analyzed the data and modified the manuscript.
Reference
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24:5986–5995. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- 4.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Journo C, Douceron E, Mahieux R. HTLV gene regulation: because size matters, transcription is not enough. Future Microbiol. 2009 May;4(4):425–440. doi: 10.2217/fmb.09.13. [DOI] [PubMed] [Google Scholar]
- 6.Kashanchi F, Brady JN. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene. 2005 Sep 5;24(39):5938–5951. doi: 10.1038/sj.onc.1208973. [DOI] [PubMed] [Google Scholar]
- 7.Boxus M, Twizere JC, Legros S, Dewulf JF, Kettmann R, Willems L. The HTLV-1 Tax interactome. Retrovirology. 2008 Aug 14;5:76. doi: 10.1186/1742-4690-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun SC, Ballard DW. Persistent activation of NF-kappa B by the Tax transforming protein of HTLV-1: hijacking cellular I kappa B kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 9.Chu ZL, DiDonato JK, Hawiger J, Ballard DW. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates I kappa B kinases containing IKK alpha and IKK beta. Journal of Biological Chemistry. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 10.Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999 Jun;73(6):4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren T, Takahashi Y, Liu X, Loughran TP, Sun S-C, Wang H-G, Cheng H. HTLV-1 Tax deregulates autophagy by recruiting autophagic molecules into lipid raft microdomains. Oncogene. 2015 Jan 15;34(3):334–345. doi: 10.1038/onc.2013.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JN, Ren T, Guan H, Jiang YX, Cheng H. HTLV-1 Tax Is a Critical Lipid Raft Modulator That Hijacks I kappa B Kinases to the Microdomains for Persistent Activation of NF-kappa B. Journal of Biological Chemistry. 2009;284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 13.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005 Oct;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 14.Verhelst K, Verstrepen L, Carpentier I, Beyaert R. IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer. Biochem Pharmacol. 2013 Apr 1;85(7):873–880. doi: 10.1016/j.bcp.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal. 2011 Aug 9;4(187):e39. doi: 10.1126/scisignal.2002355. [DOI] [PubMed] [Google Scholar]
- 16.Agami R. All roads lead to IKKepsilon. Cell. 2007 Jun 15;129(6):1043–1045. doi: 10.1016/j.cell.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol Rev. 2012 Mar;246(1):327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 18.Cocchi F, Devico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 19.Haertle T, Carrera CJ, Wasson DB, Sowers LC, Richman DD, Carson DA. Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2', 3'-dideoxyadenosine derivatives. J Biol Chem. 1988;263:5870–5875. [PubMed] [Google Scholar]
- 20.Diani E, Avesani F, Bergamo E, Cremonese G, Bertazzoni U, Romanelli MG. HTLV-1 Tax protein recruitment into IKKε and TBK1 kinase complexes enhances IFN-I expression. Virology. 2015 Feb;476:92–99. doi: 10.1016/j.virol.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Liu D, Zhang Y, Zhang H, Cheng H. The autophagy molecule Beclin 1 maintains persistent activity of NF-κB and Stat3 in HTLV-1-transformed T lymphocytes. Biochemical and Biophysical Research Communications. 2015;465(4):739–745. doi: 10.1016/j.bbrc.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]