Abstract
Heterotrimeric G-proteins couple metabotropic receptors to downstream effectors. In retinal ON bipolar cells, Go couples the metabotropic receptor mGluR6 to the TRPM1 channel and closes it in the dark, thus hyperpolarizing the cell. Light, via GTPase activating proteins, deactivates Go, opens TRPM1, and depolarizes the cell. Go comprises Gαo1, Gβ3 and Gγ13; all are necessary for efficient coupling. In addition, Gβ3 contributes to trafficking of certain cascade proteins and to maintaining the synaptic structure. The goal of this study was to determine the role of Gαo1 in maintaining the cascade and synaptic integrity. Using mice lacking Gαo1, we quantified the immunostaining of certain mGluR6-related components. Deleting Gαo1 greatly reduced staining for Gβ3, Gγ13, Gβ5, RGS11, RGS7, and R9AP. Gαo1’s deletion did not affect mGluR6, TRPM1, or PCP2. In addition, deleting Gαo1 reduced the number of rod bipolar dendrites that invaginate the rod terminal, similar to the effect seen in the absence of mGluR6, Gβ3, or the matrix-associated proteins, pikachurin, dystroglycan, and dystrophin, which are localized presynaptically to the rod bipolar cell. We therefore tested mice lacking mGluR6, Gαo1, and Gβ3 for expression of the matrix-associated proteins. In all three genotypes, staining intensity for these proteins was lower than in wild type, suggesting a retrograde trans-synaptic effect. We propose that the mGluR6 macromolecular complex is connected to the presynaptic rod terminal via a protein chain that includes the matrix-associated proteins. When a component of the macromolecular chain is missing, the chain may fall apart and loosen the dendritic tip adherence within the invagination.
Keywords: G-protein, Mouse, G-proteins, dystrophin, β-dystroglycan, pikachurin
Introduction
The key function of heterotrimeric G-proteins (comprising Gα and Gβγ subunits) is to couple metabotropic receptors to their downstream effectors. These downstream effectors (enzymes or channels) modulate processes as diverse as growth and differentiation to physiological signal processing. In the retina, an important G-protein-mediated signaling cascade occurs in a class of second order neurons, the ON bipolar cells, where it is essential for night vision and for detecting light increments in daylight vision. This cascade inverts the photoreceptor’s hyperpolarizing signal to a depolarizing response (Nawy and Jahr, 1990; Shiells and Falk, 1990). In the dark, glutamate released from photoreceptors binds the metabotropic glutamate receptor mGluR6, this activates a G-protein cascade that closes the non-selective cation channel TRPM1 and thereby hyperpolarizes ON bipolar cells (Koike et al., 2010; Masu et al., 1995; Morgans et al., 2010). Light hyperpolarizes the photoreceptors, reducing glutamate release, opening the TRPM1 channels, and thus depolarizing ON bipolar cells.
ON bipolar cells express Gαo, Gβ3 and Gγ13 (Huang et al., 2003; Ritchey et al., 2010; Vardi et al., 1993). While the three subunits in the heterotrimeric G-protein are thought to form a single functional unit that couples the receptor to an effector, it is becoming increasingly clear that each subunit also has a different function (Dhingra et al., 2012; Ramakrishnan et al., 2015). In ON bipolar cells, while deleting Gαo (Gαo1 and Gαo2) eliminates the light ON response (Dhingra et al., 2002; Dhingra et al., 2000; Okawa et al., 2010), deleting Gβ3 greatly reduces it but does not eliminate it, and deleting Gγ13 has a much milder effect (Dhingra et al., 2012; Ramakrishnan et al., 2015). In addition, deleting Gβ3 mislocalizes many of the cascade components and compromises the synapse while deleting Gγ13 has a milder effect on the expression and/or localization of these components and no effect on the synapse. The effect of deleting Gαo1, the predominant splice variant in ON bipolar cells, on the localization of mGluR6 cascade components has not yet been determined. In order to better understand how the G-protein subunits contribute to the maintenance of synaptic structure, we first tested the effect of deleting Gαo1 on the expression of mGluR6-related cascade components within the ON bipolar cells. We found that the most vulnerable cascade components are those that comprise the GTPase activating protein (GAP) complex. We also found that lack of Gαo1 compromises the synaptic structure. Testing the effects of deleting mGluR6, Gαo1 or Gβ3 on the expression of matrix-associated proteins in the rod to rod bipolar synapse, we found that their absence causes a retrograde trans-synaptic deficit in these proteins, and this likely leads to the compromised synaptic structure.
Methods
Ethical Approval
Procedures involving animals were performed in accordance with the guidelines of the National Institute of Health and the protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The total number of animals used for this study was 29 wild type (WT), 22 Gnao1 (gene encoding Gαo1) knockout (KO), 6 Grm6 (gene encoding mGluR6)-KO, and 6 Gnb3 (gene encoding Gβ3)-KO. For most experiments, mice were 4–7 weeks old, but we also used one 11 week old mouse for electron microscopy; both male and female mice were used. Mice were deeply anaesthetized with an intraperitoneal injection of ketamine and xylazine (100 and 10 μg/gm bodyweight). Eyes were enucleated and incised just below the lens.
Immunostaining of retinal cryosections
Eyeballs were fixed in 4% paraformaldehyde for 10 or 60 minutes (for Gβ3 staining), rinsed in phosphate buffer, soaked overnight at 4°C in 0.1M phosphate buffer containing 30% sucrose for cryoprotection and embedded in a mixture of two parts 20% sucrose in phosphate buffer and one part optimal cutting temperature (OCT) compound (Tissue Tek, Electron Microscopy Sciences, Hatfield, PA, USA). Radial sections (15–18 μm) were cut on a cryostat (Leica Biosystems, Buffalo Grove, IL, USA) at −20°C, and sections were collected on a superfrost plus slide (Fisher scientific, Pittsburgh, PA, USA). Sections were permeabilized and blocked with 10% normal goat serum, 5% sucrose and 0.5% Triton X-100 in phosphate buffer for 1 hour at 20°C. Sections were then incubated in primary antibodies (diluted in blocking solution) at 4°C overnight or 3 days for mGluR6. Retinas were washed 3 times in 0.1M phosphate buffer containing 5% sucrose at room temperature. Sections were then incubated in secondary antibodies at 20°C for 3 hours, washed, mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and cover slipped. For double labeling, sections were simultaneously incubated in both primary antibodies (raised in different hosts) followed by both secondary antibodies.
Imaging and quantification
Sections were photographed with a confocal laser scanning microscope (Olympus Fluoview 1000, Center Valley, PA, USA) under a 60x oil-immersion objective (NA=1.42). Immunostaining intensities were compared between littermate (or age-matched) WT and KO retinas that had been processed in parallel and imaged under the same settings (a KO and its littermate WT are referred to as a ‘set’). For images with single staining, regions of interest (ROI) were drawn around the different layers (outer plexiform, OPL; inner nuclear, INL; inner plexiform, IPL) and the background-subtracted average intensity per pixel for each ROI was measured using Volocity software (Improvision, PerkinElmer, Waltham, MA). For Gβ3 staining in cones, we drew ROIs around the outer segments of individual cones and determined the background-subtracted average intensity per pixel. For images with double stainings (ribbon and a matrix-associated protein), a user-defined MATLAB script was used to quantify the average intensity per pixel per punctum as described previously (Tummala et al., 2014). Briefly, the program identifies ribbons and isolates puncta in a single focal plane using a user-specified threshold for intensity. It then fits a 2-D Gaussian to each isolated punctum and determines the size (full width at half maximum, FWHM) as the number of pixels with intensities above the half maximum intensity of the punctum and the intensity of each punctum as the average intensity per pixel of these pixels. The average intensity of staining or punctum size for each retina is the average of values from at least three confocal images. For each metric, the values obtained for KOs were normalized with the average values obtained from all the WT mice.
Antibodies
The following antibodies and concentrations were used: rabbit anti-Gγ13 (1:500, a gift from R. Margolskee); rabbit anti-Gβ3 (1:300, HPA005645; Sigma); guinea pig anti-mGluR6 (1:100, Neuromics Edina, MN Oregon); mouse anti-Gαo (1:100, MAB 3073; Millipore); rabbit anti-TRPM1 (1:100, a gift from T. Furukawa, Osaka Bioscience Institute, Osaka, Japan) (Koike et al., 2010; Kondo et al., 2011); rabbit anti-Gβ5 (1:500) and rabbit anti-RGS11 (1:1000, gifts from T. Wensel, Baylor College of Medicine, Houston, TX); rabbit anti-RGS7 (1:200, gift from K. Martemyanov, Scripps Research Institute, Jupiter, FL); rabbit anti-Ret-PCP2 (1:1,000, gift of Dr. Rod Feddersen; see Xu et al. 2008); rabbit anti-pikachurin (1:2,000, Wako 011-22631, Richmond,VA); mouse anti-dystroglycan (1:100, Abcam, ab49515, Cambridge, MA); mouse anti-dystrophin (1:100, Millipore MAB 1694, Billerica, MA); rabbit anti-RGS9 anchoring protein (R9AP) (1:100–500, a gift from V. Arshavsky, Duke University, Durham, NC); mouse anti-kinesin [1:100–200, Covance (currently Bio-legends)]; rabbit anti-ribeye (1:500, a gift from T. Sudhof, Stanford Universty, CA); sheep anti-TRPM1 (for Western blotting,1:1,000, Gift of Dr. Kirill Martemayanov, The Scripps Research Institute, Jupiter, Florida); and mouse anti-beta-actin (1:3,000, Clone AC-74 Sigma, St. Louis, MO).
Electron microscopy
Eyes were fixed in phosphate buffer (0.1 M, pH 7.4) containing 2% paraformaldehyde and 2% glutaraldehyde for 6–12 hours. Eyes were then rinsed in phosphate buffer, and small pieces of retina were taken (with the optic disk at one end). Tissues were incubated in 2% osmium tetroxide in 0.1 M phosphate buffer for 2 hours, rinsed with phosphate buffer, dehydrated in series of ethanol rinses and incubated sequentially with Embed 812:propylene oxide mixtures (1:1 and 2:1) and then in pure Embed 812 (Electron Microscopy Sciences, Hatfield, PA) overnight. Tissues were then embedded in fresh Embed 812 mixture at 60°C for 48 hours. Semi-thin sections (1 μm) were stained with toluidine blue. Ultrathin sections (90 nm) were counterstained with uranyl acetate and lead citrate, and were examined with a JEM 1011 transmission electron microscope (10K magnification). Images were captured by ORIUS 835.10W CCD camera (Gatan, Pleasanton, CA) and analyzed using ImageJ. For analysis, we quantified the number of rod terminal profiles at which we see a ribbon, a horizontal cell process, or a bipolar dendrite. Thus, we first numbered all the identifiable profiles of rod terminals in an image, and then determined the presence or absence of the relevant structures in each profile. While it is possible to analyze the structure in reference to ribbons or dyads, a study on Grm6-KO that compared results obtained from reconstruction to those obtained from the two different methods of sampling showed that when sampling, referencing the data to number of profiles rather than number of ribbons produces more comparable results (Tsukamoto and Omi, 2014).
Western Blotting
Retinas from Gnao1−/− and WT littermate mice were detached and frozen in liquid nitrogen. Tissue was homogenized using a Polytron homogenizer (Kinematica, Lucerne, Switzerland) in a lysis buffer containing 5 mM Tris-HCl, pH 7.5, 320 mM sucrose, 2 mM EDTA, 2.5 mM β-mercaptoethanol, and protease inhibitor mixture (P 8340, Sigma, St. Louis, MO). The homogenate was centrifuged for 10 min at 6000 × g. Proteins from the supernatant were assayed using BSA protein reagent (Bio-Rad, Hercules, CA). The proteins were run on 4–15% SDS-PAGE gels and transferred to a nitrocellulose membrane using a wet transfer apparatus (Bio-Rad, Hercules, CA). After a quick rinse in PBS, the blots were incubated sequentially in the following: Odyssey blocking buffer diluted with PBS (1:1, blocking buffer) at room temperature for 1 h; primary antibody diluted in blocking buffer containing 0.1% Tween 20 at 4°C overnight; washes in PBS+0.1% Tween 20 (PBST); IRDye-conjugated secondary antibodies (from LI-COR Biosciences, Lincoln, NE, 1:15,000 dilution in blocking buffer containing 0.1% Tween 20) at room temperature for 1 h; washes in PBST; and a final rinse in PBS. The blots were then scanned using the Odyssey Infrared Imaging system (LI-COR Biosciences) as per the manufacturer’s instructions. To control for equal loading, antibody against β-actin (as a loading control protein) was also included along with test antibody. The two antibodies used together were chosen such that they differed in the host species to enable their discrimination by different secondary antibodies. The band intensities were quantified using Odyssey software, adjusted for background and normalized against β-actin levels. Student’s t-test was used to compare a quantity in WT and null KOs.
Results
Deleting Gαo1 reduces Gβ3 and Gγ13 expression in ON bipolar cell dendritic tips
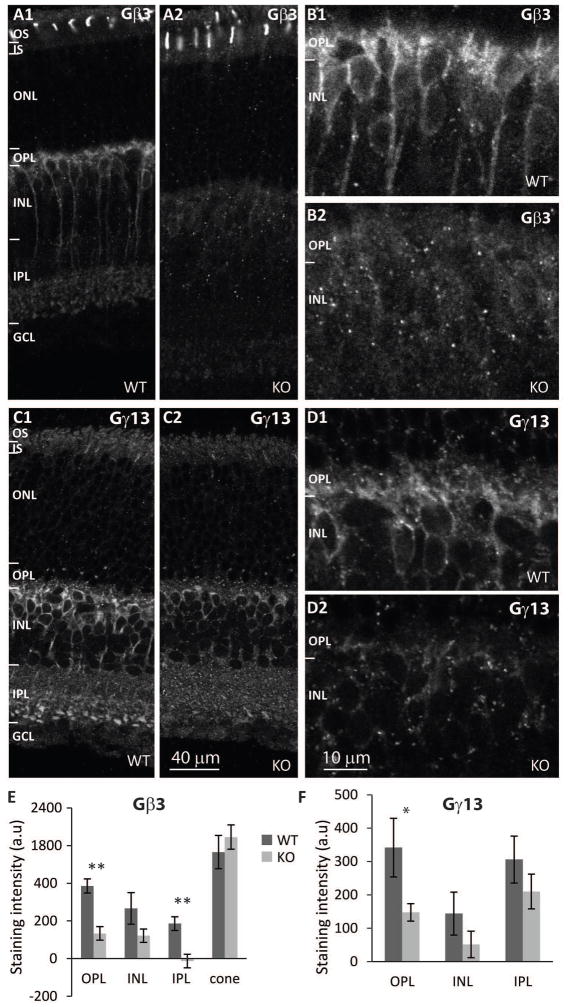
Expression of G-protein subunits in a heterotrimeric complex often depends on each other. We have previously shown that in ON bipolar cells, deleting Gβ3 dramatically reduces Gαo and Gγ13 (Dhingra et al., 2012). Surprisingly, deleting Gγ13 greatly reduces Gβ3, but hardly affects the expression of Gαo (Ramakrishnan et al., 2015). To test whether these relationships are reciprocal, we tested how the deletion of Gαo1, the primary splice variant in ON bipolar cells, affects its partners. Immunostaining of WT and Gnao1-KO retinas (4 sets) for Gβ3 was dramatically reduced in all compartments of ON bipolar cells lacking Gαo1 (Fig. 1A–B). In the OPL, the staining was reduced to about 35% of WT and the difference was highly significant (p=0.003) (Fig. 1E). Significant reduction was also seen in the IPL (p=0.008). In contrast, staining for Gβ3 in cones was unaffected (Fig. 1A, E). Staining for Gγ13 (6 WT and 7 KO mice) was also reduced substantially in all layers; in the OPL staining was reduced to 43% of WT (p=0.044; Fig. 1C, D, F). Thus, Gαo1 is required for normal expression of its partners.
Fig. 1. Deletion of Gαo1 greatly reduces expression of Gβ3 and Gγ13 in ON bipolar cells.
A. Immunostaining for Gβ3 in wild type (A1) and Gnao1-KO (A2) retinas shows that localization is unaffected in cones and is greatly reduced in ON bipolar cells. B. Higher magnification of ON bipolar cells in WT (B1) and KO (B2). C and D. Lower and higher magnification images showing staining for Gγ13 in WT (C1, D1) and Gnao1-KO retinas (D1, D2). E, F. Quantification of staining intensity (average intensity per pixel) in OPL, INL, IPL, and in cones (for Gβ3). * indicates p value<0.05, and ** indicates p<0.01. For all figures: OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; WT wild type mice, KO, Gnao1 knockout.
Deleting Gαo1 does not affect the expression of mGluR6 and TRPM1
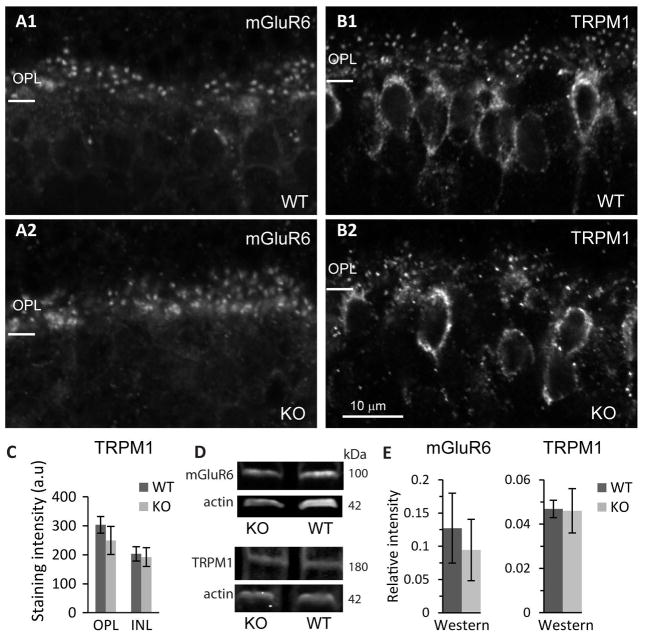
Next we tested the effect of Gαo1 deletion on the localization of the receptor mGluR6 and the channel TRPM1. For mGluR6, in both WT and KO (6 sets), we observed the characteristic punctate staining where each dot represents 2 rod bipolar dendritic tips. However, staining was variable between animals, and the puncta in some KOs appeared less organized (Fig. 2A). For TRPM1 (5 WT and 6 KO), staining was observed both in the dendritic tips in a punctate pattern and in the ON bipolar cells somas. Here too, staining in KOs was variable and appeared less organized than in WT (Fig. 2B). Quantitatively, the average intensity in the OPL of KO was lower than in WT, but the variability was higher and the difference did not reach statistical significance (Fig. 2C). Because mGluR6 and TRPM1 are restricted to ON bipolar cells, we could also assess expression by Western blotting; this showed that these two cascade components were expressed at similar amounts in WT and KO retinas (4 sets of WT and KO for mGluR6, p=0.6; 3 sets for TRPM1, p=0.7; Fig. 2D, E and Supplementary Fig. 1A, B).
Fig. 2. Localization and expression of mGluR6 and TRPM1 may be only mildly affected.
A. Immunostaining for mGluR6 in WT and Gnao1-KO as indicated. B. Immunostaining for TRPM1 in WT and KO as indicated. C. Average staining for TRPM1 in OPL and INL was not significantly different between WT and KO. D. Western blots for mGluR6 and actin (top) and TRPM1 and actin (bottom) for WT and KO as indicated. E. Quantification of Western blots for mGluR6 and TRPM1. Total band intensity for mGluR6 (or TRPM1) was divided by that for actin in each sample (three replicates per sample, sample was taken from 1–2 retinas from each mouse) and then averaged across samples to yield relative intensity (n=4 mice for mGluR6 and n=3 mice for TRPM1).
Deleting Gαo1 dramatically reduces expression of the GTPase activating proteins, but not other Gαo1 modulators
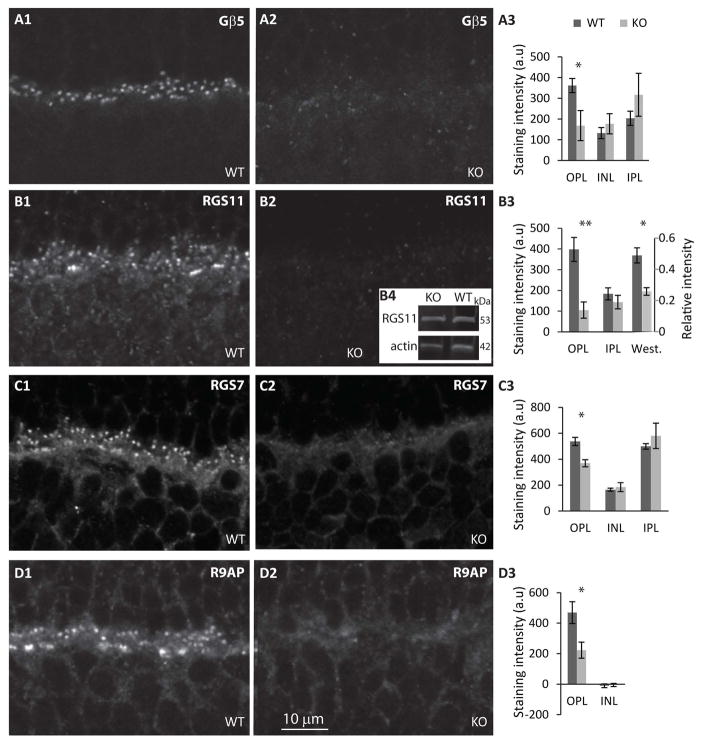
In Gnb3- and in Gng13 (gene encoding Gγ13)-KO mice, the most affected cascade components are those of the GTPase activating protein (GAP) complex, which are necessary for producing the rising phase of the light response (Cao et al., 2012; Dhingra et al., 2004; Orlandi et al., 2012; Shim et al., 2012; Zhang et al., 2010). To determine if the absence of Gαo1 results in a similar deficiency, we immunostained retinas for Gβ5, RGS11, RGS7, and R9AP. Indeed, for all these components the staining in the OPL was drastically reduced (Fig. 3). For Gβ5, staining intensity in KO OPL was 47% of that in WT (p=0.043; 5 WT and 6 KO); for RGS11, it was 26% (p=0.002; 5 WT and 6 KO); for RGS7 it was 69% (p=0.022; 4 sets); and for R9AP it was 47% (p=0.021; 5 WT and 6 KO). To test whether the reduced localization is due to reduced expression level in ON bipolar cells, we used Western blotting to determine total expression of RGS11, the only protein out of these 4 that, in retina, is restricted to ON bipolar cells. We found that RGS11 in KO is significantly reduced to 54% of WT (p=0.013) (Fig. 3B3, B4 and supplementary Fig. 1C). Reduced expression in OPL suggests that Gαo1 is needed for correct localization at the tips and reduced total expression suggests that Gαo1 is also needed to stabilize the protein.
Fig. 3. Localization of GAP proteins in dendritic tips of ON bipolar cells is greatly reduced.
A1, 2. Immunostaining for Gβ5 in WT (A1) and in Gnao1-KO (A2) shows greatly reduced staining in KO. A3. Quantification of staining intensities for Gβ5 in different retinal layers; staining in IPL is mainly in amacrine cells. B. Immunostaining for RGS11 in WT (B1) and KO (B2). B4. An example of Western blots for RGS11 and actin.B3. Quantification of immunostaining (left axis) and of Western blots (right axis). C, D. Immunostaining and quantification for RGS7 (C) and R9AP (D) organized as in A and as indicated.
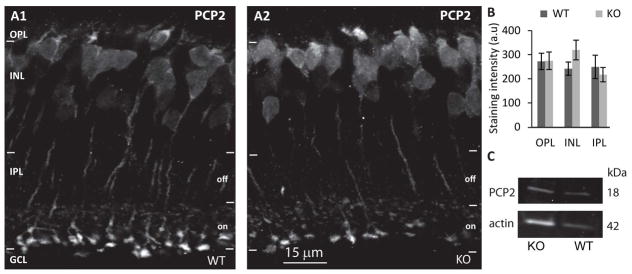
We also tested the expression of PCP2, a known interactor of Gαo that in the retina is expressed only in ON bipolar cells and serves to accelerate the light response (Xu et al., 2008). Expression and localization of this protein was only mildly changed with a small but not significant increase in INL (30%; p=0.167; 5 WT and 6 KO mice) (Fig. 4A, B). Expression of PCP2 as measured by Western blotting (Fig. 4C), showed about 31% increase in KO (4 sets) but measurements were highly variable, perhaps due to the small size of the protein (not shown). Thus, while the localization and perhaps expression level of the G-protein subunit partners of Gαo1 and its GAPs are affected by Gαo1’s expression, some cascade components are unaffected.
Fig. 4. PCP2 is not affected by deleting Gαo1.
A. Immunostaining for PCP2 in WT (A1) and Gnao1-KO (A2); staining is present in all compartments of the ON bipolar cell. B. Quantification of staining intensity in the different layers. C. Western blots for PCP2 in WT and KO.
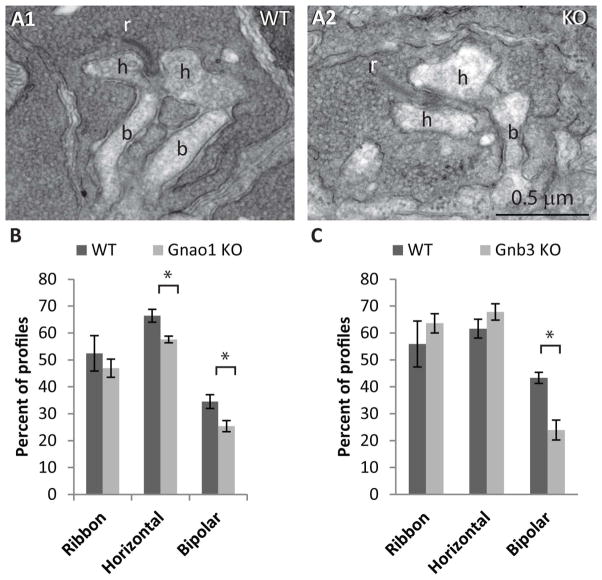
In Gnao1-KO, the ultrastructure of the synapse between rods and rod bipolar dendrites is slightly altered
Rods contact rod bipolar cells in a synaptic structure called tetrad. In this synaptic complex, the rod terminal contains a synaptic ribbon, on which the synaptic vesicles are tethered. The ribbon is presynaptic to four postsynaptic processes that invaginate the rod terminal: two lateral processes that belong to horizontal cells, and two central dendrites that belong to bipolar cells. We have previously qualitatively examined the synapse between the rod terminal and ON bipolar dendrites in Gnao-KO and found them to be similar, but because the evaluation was not quantitative, in this study, we re-examined the ultrastructure in Gnao1-KO. We analyzed 1370 profiles of rod terminal from 4 WT retinas and 1656 profiles from 4 littermates KO retinas of 4–7 week old mice and one 11 week old KO in a total retinal length of 1.5 mm for WT and 2.2 mm for KO. For all profiles, we counted the number that contained: a synaptic ribbon, at least one horizontal cell process, and at least one bipolar dendrite. We found that in KO, both horizontal cell processes and bipolar dendrites were represented in a lower fraction of profiles compared to WT (Fig. 5A, B). Horizontal cells in KO were reduced to 88% of WT (58% in KO vs 66% in WT, p= 0.010) and bipolar dendrites were reduced to 71% of WT (25% in KO vs 34% in WT; p=0.026). The percentage of profiles in which ribbons were observed was also smaller in KO (47% vs 52%), but this difference was not significant (p=0.450). In comparison, analyzing the effect of deleting Gβ3 on the ultrastructure showed no effect on percent of ribbons or horizontal cells and a greater effect on bipolar cells whose percentage was reduced to 55% of WT (3 sets of retinas from littermate mice, p=0.01; Fig. 5C).
Fig. 5. The structure of the rod terminal is slightly compromised.
A. Electron micrographs of rod terminal profiles in WT and Gnao1-KO. r- ribbon, h- horizontal cell process found lateral to the ribbon and b- bipolar dendrite. B. A bar graph showing the percentage of profiles in which we identified a ribbon, at least one horizontal cell process, or at least one bipolar dendrite in Gnao1 KO and in littermate WT. C. Similar to B, but evaluation was made from Gnb3-KO and littermate WT.
Absence of mGluR6, Gαo1 or Gβ3 affects expression of presynaptic proteins
The finding that the synaptic structure is compromised in several mice lines in which the ON bipolar cascade components are deleted or mutated (such as mGluR6, Gβ3, RGS7+RGS11) (Cao et al., 2009; Dhingra et al., 2012; Ishii et al., 2009; Shim et al., 2012; Tsukamoto and Omi, 2014) suggests that the deletion of these cascade components affects not only proteins directly involved in the cascade, but also proteins that are responsible for maintaining a normal synaptic structure. Three extracellular matrix-associated proteins are known to localize at the rod-to-rod bipolar synapse. These are pikachurin, dystroglycan, and dystrophin (reviewed by Constantin, 2014; Pillers, 1999 and). Deleting either pikachurin or dystroglycan impedes complete invagination of the rod bipolar dendrites, similar to that seen after deleting the mGluR6 cascade components (Omori et al., 2012; Sato et al., 2008). This raises the possibility that the observed deficiency in synaptic structure after deleting a cascade component is secondary to its effect on the expression or localization of any of these matrix-associated proteins.
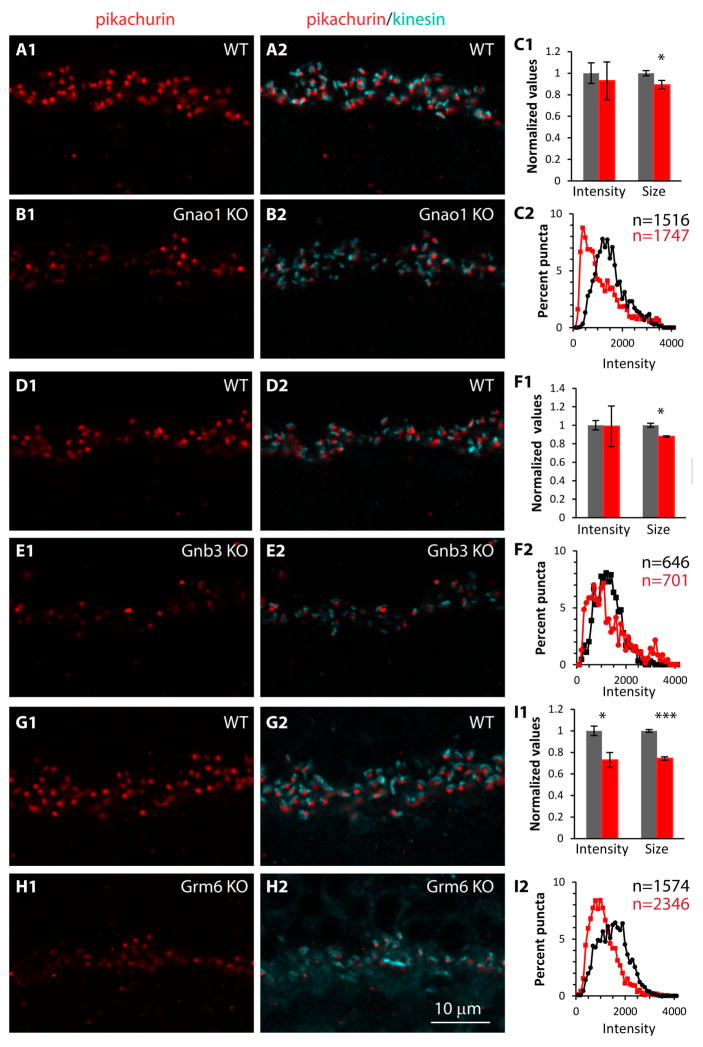
To determine the effect of deleting a cascade component on the localization of the matrix-associated proteins, we double stained retinas of WT and three different KO genotypes (Gnao1-KO, Gnb3-KO, and Grm6-KO) for each of these proteins and also for a ribbon marker (ribeye or kinesin). First we stained for pikachurin; this protein is similar to agrin and perlecan with three laminin G domains (Sato et al., 2008). It is produced by photoreceptors where it is cleaved and released into the extracellular space between the photoreceptor and the ON bipolar dendrite (Han and Townes-Anderson, 2012). In wild type mice, immunostaining for pikachurin showed the expected punctate appearance in the outer plexiform layer (Fig. 6A). In Gnao1-KO, staining appeared reduced, but the variability in punctate staining was higher than in WT (Fig. 6B). Using the staining for a presynaptic ribbon to localize the expected location of its postsynaptic bipolar dendritic tip, for each punctum, we quantified the average staining intensity per pixel. This method is more sensitive than that in which we quantified the average intensity per pixel in the OPL because when considering only the puncta, the average intensity is not diluted by empty spaces in the OPL. We also estimated the size of each punctum (see methods). Note however that a change in punctum size does not necessarily mean smaller apposition between the rod membrane and the dendritic tip because the computed size depends on the staining intensity of the punctum. Using these metrics we found that the average intensity for pikachurin in Gnao1-KO was slightly lower than in WT (Fig. 6C1), but the difference was not statistically significant (p=0.73; 7 sets). The average punctum size, however, was slightly smaller in KO, and the difference was significant (p = 0.047), probably because of the lower variability in this metric. Given the large variability in the staining intensities of the puncta, we plotted the distribution of the intensities. We binned intensity values from all the 7 retinas into 41 bins and plotted the proportion of puncta in each bin (the intensity values ranged from 0-4095; each bin included 100 values). We found that in WT, the puncta intensities distributed normally and peaked at 1200 units, but in KO most puncta had low intensities, and the distribution peaked at 400 units (Fig. 6C2). The distribution in KO also showed that the proportion of puncta with high intensity was slightly higher than in WT, thus explaining why the average intensity was similar, despite appearing lower in the KO.
Fig. 6. In mutants missing mGluR6-related cascade elements, pikachurin in the synaptic cleft between photoreceptor terminals and ON bipolar cells is greatly reduced.
Comparison of staining in WT and littermate Gnao1-KO (A–C), in WT and littermate Gnb3-KO (D–F), and in WT and aged-matched Grm6-KO (G–I). On the left (X1) are images of pikachurin staining (red) in WT and KO as indicated; in the middle (X2), same images merged with staining for kinesin to show synaptic ribbons; and on the right (C, F, I) are the quantitative data for each genotype. C1, F1, I1. Bar graphs shows average normalized values for intensity per pixel in a punctum and punctum size (values were normalized to average WT; gray bars are WT and red bars are KOs). C2, F2, I2. Curves show the intensity distribution of all quantified puncta for WT (black) and KOs (red); the values on the y-axis are % of puncta for each intensity bin, where the total puncta are 100%; the actual numbers of puncta analyzed (n) for each curve are color code next to each graph.
Evaluating pikachurin expression in Gnb3-null retinas, we found similar results: the average staining intensity was similar in WT and KO (3 mice each) with higher variability in KO (Fig. 6D–F), the puncta size in KO was slightly (88%) but significantly (p=0.020) lower than in WT, and the puncta distribution in Gnb3-KO was shifted to the left, similar to that in Gnao1-KO (Fig. 6F). Finally, in Grm6-KO (Fig. 6G–I), the average intensity was significantly lower than in WT (74% of WT; p=0.01;4 WT and 6 KO mice), and the average size was highly significantly lower (73%; p<0.0001). The KO distribution was greatly shifted to the left of WT, and very few puncta showed high intensity.
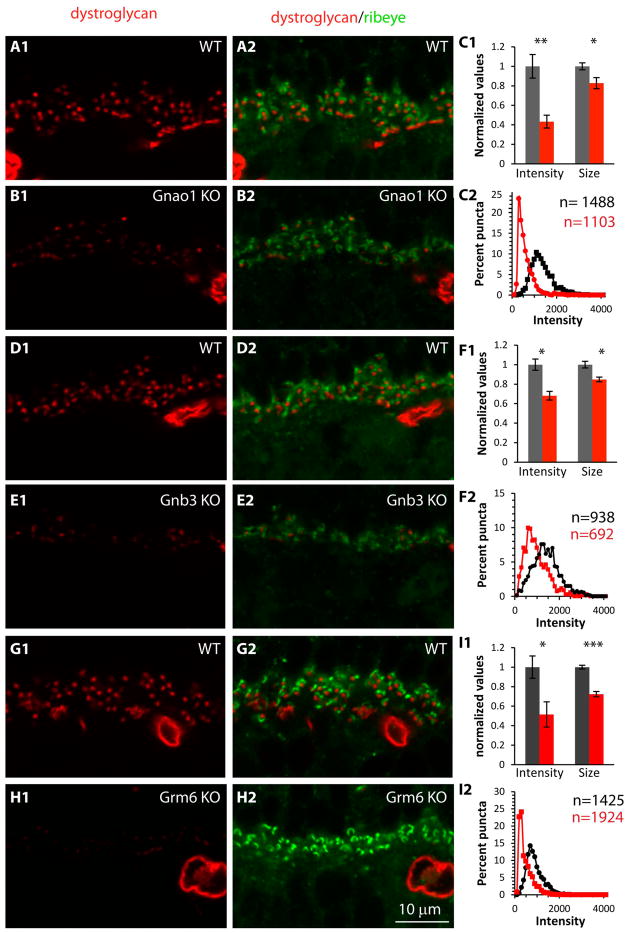
Next we tested localization of β-dystroglycan, a glycoprotein with one transmembrane domain that is cleaved into α and β dystroglycan, and localizes with pikachurin at the electron-dense region of the photoreceptor membrane that apposes the ON bipolar dendrite (Blank et al., 1999; Drenckhahn et al., 1996; Hu et al., 2011; Jastrow et al., 2006; Kanagawa et al., 2010; Montanaro et al., 1995; Omori et al., 2012). In this case, we used Ctbp2 (Ribeye) as the pre-synaptic ribbon marker. The staining pattern for β-dystroglycan was also punctate, and the intensity and the punctum average size were significantly reduced in all three genotypes. In Gnao1-KO, the intensity decreased to 43% of WT (p=0.003; 6 mice each) and the size decreased to 83% of WT (p=0.033) (Fig. 7A–C). In Gnb3-KO, these ratios were 68% for intensity (p=0.013; 3 sets) and 85% for size (p=0.025) (Fig. 7D–F). In Grm6-KO, the intensity in KO was reduced to 51% of WT (p=0.025; 4 WT and 5 KO mice) and the size was reduced to 72% (p<0.001) (Fig. 7G–I). The distribution curves in all three KO genotypes were shifted to the left.
Fig. 7. In mutants missing mGluR6-related cascade elements, β-dystroglycan associated with the photoreceptor terminals to ON bipolar dendrite synapse is greatly reduced.
The organization of this figure is as for Fig. 6, but showing staining for dystroglycan. Here, ribeye (green) was used to localize ribbons. Gray bars and black curves are for WT, red bars and red curves for KOs.
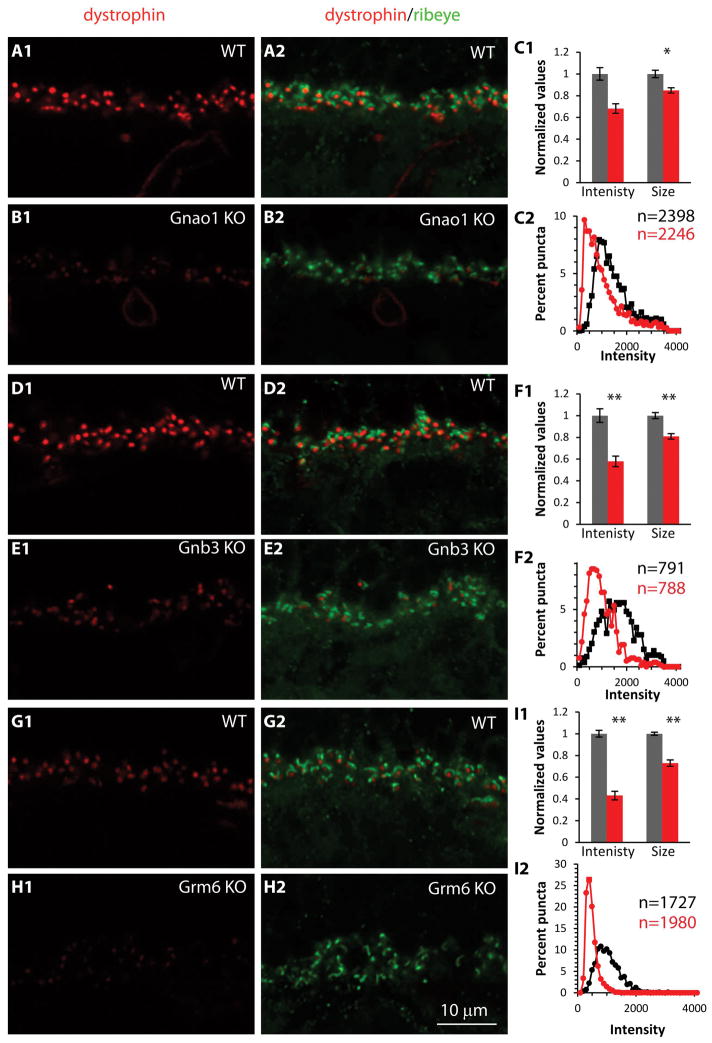
Finally we tested immunoreactivity for dystrophin, a dystroglycan ligand that is expressed in the photoreceptor terminals and whose deficiency causes a slow and reduced ERG b-wave in both humans and mice (Blank et al., 1999; Dalloz et al., 2001; Drenckhahn et al., 1996; Green et al., 2004; Haenggi and Fritschy, 2006; Kameya et al., 1997; Lenk et al., 1996; Omori et al., 2012; Pillers et al., 1995; Satz et al., 2009; Wersinger et al., 2011). Similar to pikachurin and dystroglycan, immunostaining for dystrophin was punctate. In Gnao1-KO, staining intensity per punctum was reduced to 67% of wild type (p= 0.085; 5 WT and 6 KO mice) and the size was reduced to 86% (p=0.022) (Fig. 8A–C). In Gnb3-KO these ratios were 58% (p=0.007; 3 sets) and 81% (p=0.007) correspondingly (Fig. 8D–F) and in Grm6-KO, these were 43% (p<0.001; 4 WT and 6 KO mice) and 73% (p<0.001) (Fig. 8G–I). Thus all the three matrix-associated proteins were reduced by removing mGluR6-related cascade components.
Fig. 8. In mutants missing mGluR6-related cascade elements, dystrophin present at the photoreceptor terminals in apposition to ON bipolar dendrites is greatly reduced.
The organization of this figure is as for Fig. 7 with staining for dystrophin. Gray bars and black curves are for WT, red bars and curves for KOs.
Discussion
We present here four key findings. (1) The absence of Gαo1, the predominant subunit of Go, greatly reduces the staining of its partners (Gβ3 and Gγ13) throughout the cell and the staining of its GAPs in rod bipolar dendritic tips. (2) The absence of Gαo1 leads to slight reduction and disorganization of stainings for mGluR6 and TRPM1. (3) In the absence of Gαo1, fewer rod bipolar dendrites invaginate the rod terminal. (4) In retinas lacking Gαo1, Gβ3, or mGluR6, genotypes with a phenotype of reduced invaginating rod bipolar dendrites, stainings for the presynaptic extracellular matrix-associated proteins are reduced.
Differential function of Go’s subunits
The primary function of the heterotrimeric G-protein is to couple the receptor to its effector; however, our previous work showed that the heterotrimer is not obligatory. Thus, while deleting Gαo eliminates the light ON response (Dhingra et al., 2002; Dhingra et al., 2000; Okawa et al., 2010), deleting Gβ3 greatly reduces it but does not eliminate it (Dhingra et al., 2012). Furthermore, deleting Gγ13 has a much smaller effect (Ramakrishnan et al., 2015). The ability to generate a light response in the absence of Gβ3 suggests that Gαo can cycle between its inactive GDP-bound form to its active GTP-bound form without a Gβγ dimer. This interpretation is also supported by the finding that in cones lacking Gβ3, response sensitivity is reduced, but the maximal light response is as in WT (Nikonov et al., 2013). Similar results were also obtained in rods lacking Gγ1 (Kolesnikov et al., 2011; Lobanova et al., 2008). These data suggest that the function of Gβ and Gγ is not necessarily to permit coupling, but to increase its efficiency. An increase in efficiency may be achieved by locally facilitating conformational changes in the macromolecular complex comprising the receptor, the G-protein, and the effector. But an increase in efficiency can also be achieved by ensuring that the molecules comprising the macromolecule are trafficked to the same intracellular locus and maintained there. We previously postulated that Gβ3 is crucial for either trafficking or maintaining the integrity of the mGluR6 macromolecular complex because in its absence, both mGluR6 and TRPM1 do not concentrate in the dendritic tips as they do in WT. Here we surmise that this trafficking function may not be shared by Gαo since the absence of Gαo1 only slightly affects the localization of the receptor and channel, and this could happen indirectly by the reduction of Gβ3. Further support for Gβ’s role in trafficking is provided by the observation that surface expression of Kir2.4 and Kir3.2 increases after exogenously expressing Gβγ (Rubinstein et al., 2009; Sulaiman et al., 2013). Additionally, in the absence of Gβ3, Gαt2, which typically concentrates in the cone outer segments, is localized diffusely throughout the cone compartments, suggesting that its trafficking to the outer segment also requires a Gβ (Nikonov et al., 2013). As for Gγ13, while the absence of Gαo1 greatly reduces expression of Gβ3 and Gγ13, and the absence of Gβ3 reduces expression of Gαo and Gγ13, the absence of Gγ13 reduces Gβ3, but not Gαo1. This suggests a minor function for Gγ13 in stabilizing the heterotrimer.
Susceptibility of the GAP proteins in ON bipolar cells
We showed here that deleting Gαo1 dramatically reduces the GAP proteins (RGS11, RGS7, Gβ5, and R9AP) in the dendritic tips of ON bipolar cells; similar results were obtained when Gβ3 or mGluR6 were deleted. This is different from cone photoreceptors, in which the absence of Gβ3 does not affect the expression of R9AP (Nikonov et al., 2013). These observations raise the question as to why the localization of the GAPs in the dendritic tips of ON bipolar cells is so susceptible to the absence of the receptor or the key coupling subunits. We speculate that in ON bipolar cells, active Gαo recruits the GAPs to the dendritic tips. Thus, in the absence of Gαo1, which occurs both when Gnao1 or Gnb3 are deleted, there is no active subunit and GAPs are diffused throughout the cell. In case of Grm6-KO, although Gαo is still expressed (Xu et al., 2012), the absence of mGluR6 renders Gαo inactive at all times, once again preventing the GAPs from being recruited to the tips. It is currently unknown whether the GAPs are also affected in the absence of TRPM1; however, our hypothesis predicts that their localization in the tips would not be affected.
Retrograde trans-synaptic communication from rod bipolar cells to rod photoreceptors
We showed that without Gαo1, the number of rod bipolar dendrites that invaginate the rod terminal is reduced. Qualitatively similar results were obtained when the following genes were mutated or deleted: Grm6, Gnb3, Gnb5 (gene encoding Gβ5), and RGS7+RGS11 (Rao et al., 2007; Cao et al., 2009; Ishii et al., 2009; Dhingra et al., 2012; Shim et al., 2012; Tsukamoto and Omi, 2014). These findings raise an important question: what is the mechanism that causes the deficit? In principle, the deficit can arise during development, in which case, one would propose that the postsynaptic cascade elements affect developmental processes. Or, it is possible that development proceeds normally, but the structure cannot be maintained. In one of these mutants, Gnb5-KO, the loss of invaginating bipolar dendrites was attributed to deficits in the developmental process (Rao et al., 2007); however because Gβ5 is also expressed in photoreceptors, the deficits may not be due to alterations in the mGluR6 cascade. In the Gnb3-KO, the loss of invaginating bipolar dendrites was more severe in the adult than in early development, indicating that the structural deficit is due to the failure of synaptic maintenance (Dhingra et al., 2012).
To start understanding why the synaptic structure is affected by deleting postsynaptic cascade components, we stained for three matrix-associated proteins known to play a role in maintaining the structure of the rod to rod bipolar synapse. Our findings that these proteins are greatly reduced in three genotypes that show deficits in invaginating rod bipolar dendrites suggest that the lack of proper expression of these proteins causes withdrawal of the invaginating dendrites. Thus, we envision that certain proteins of the mGluR6-related cascade interact directly and others interact indirectly with the matrix-associated proteins. Together, they form a protein chain that connects the postsynaptic rod bipolar membrane and the cascade components to the presynaptic rod membrane (Fig. 9). When a protein in the chain is missing, the chain breaks apart, leading to reduced adherence between the rod and the rod bipolar cell, and then to retraction of the dendrite from the invagination. Thus, although the rod bipolar dendrites can develop normally by a genetic program and invaginate the rod terminal using for example the interaction between the cell adhesion protein ELFN1 and mGluR6 (Cao et al., 2015), a stable adhesion requires the full complement of pre- and postsynaptic complexes that interact with the matrix proteins. A large number of extracellular matrix proteins have been localized to the rod synapse (reviewed by Mercer and Thoreson, 2011); these likely form several microdomains (such as the apposition between rod and horizontal cells or apposition between rod and bipolar cells). The identity of the protein on the rod bipolar that directly interacts with the extracellular matrix-associated proteins is unknown, but is likely one of the proteins that contain an extracellular domain. Nyctalopin could be a candidate, but staining for dystrophin in a mouse lacking nyctalopin appears normal (Ball et al., 2003). Other candidates are GPR179, LRIT3, mGluR6, TRPM1, and Cacna1S (Fig. 9). Although the function of such a chain appears to be primarily to create a stable morphological connection between the pre and the post synaptic cells, reminiscent of what is seen in the neuromuscular junction (Nishimune et al., 2004), it inherently contributes to the size and proper timing of the rod bipolar response to light as seen when pikachurin, dystroglycan, or dystrophin are deleted or mutated (Pillers et al., 1999; Sato et al., 2008; Omori et al., 2012).
Fig. 9. Schematic diagram illustrates hypothetical protein chain that holds presynaptic rod membrane close to postsynaptic rod bipolar dendrite.
Matrix-associated proteins on the rod side are known: dystrophin binds actin and β-dystroglycan, β-dystroglycan binds α-dystroglycan which in turn binds pikachurin. We hypothesize that these elements link to proteins that reside on the rod bipolar membrane. On the rod bipolar side, the possible players (those which contain an extracellular domain) are: LRIT3, GPR179, Nyx, mGluR6, Cacna1s, and TRPM1. It is currently unknown which is the connecting protein, but clearly all are part of a large macromolecular complex that contains also the heterotimeric G-protein Go (in red). β-DG, α-DG are beta and alpha dystroglycan respectively; Nyx, nyctalopin. Dashed lines show some possible connections.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EY11105 (Noga Vardi), R21 EY021308 (Noga Vardi and Robert G Smith), EY 013333 (Michael A Freed) and NEI P30 EY01583 (Vision Research Core of the University of Pennsylvania). We thank Drs. Takahisa Furukawa, Robert Margolskee, Theodore G. Wensel, Vadim Y Arshavsky, Rod Feddersen, Thomas Sudhof, and Kirill Martemyanov for donating the antibodies used in this study. We also thank, Robert Smith, Michael Freed, Mikhail Y. Lipin, and Sergei Nikonov for multiple discussions and help over the course of this study.
Abbreviations
- WT
wild type
- KO
knockout
- GAP
GTPase activating proteins
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- INL
inner nuclear layer
- IPL
inner plexiform layer
- ROI
region of interest
- FWHM
full width at half maximum
References Cited
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci. 2003;20:267–272. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Blank M, Koulen P, Blake DJ, Kroger S. Dystrophin and beta-dystroglycan in photoreceptor terminals from normal and mdx3Cv mouse retinae. Eur J Neurosci. 1999;11:2121–2133. doi: 10.1046/j.1460-9568.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc Natl Acad Sci U S A. 2012;109:7905–7910. doi: 10.1073/pnas.1202332109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sarria I, Fehlhaber KE, Kamasawa N, Orlandi C, James KN, Hazen JL, Gardner MR, Farzan M, Lee A, Baker S, Baldwin K, Sampath AP, Martemyanov KA. Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron. 2015;87:1248–1260. doi: 10.1016/j.neuron.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim Biophys Acta. 2014;1838:635–642. doi: 10.1016/j.bbamem.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Dalloz C, Claudepierre T, Rodius F, Mornet D, Sahel J, Rendon A. Differential distribution of the members of the dystrophin glycoprotein complex in mouse retina: effect of the mdx(3Cv) mutation. Mol Cell Neurosci. 2001;17:908–920. doi: 10.1006/mcne.2001.0978. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004;24:5684–5693. doi: 10.1523/JNEUROSCI.0492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A, Vardi N. Gbeta3 is required for normal light ON responses and synaptic maintenance. J Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D, Holbach M, Ness W, Schmitz F, Anderson LV. Dystrophin and the dystrophin-associated glycoprotein, beta-dystroglycan, co-localize in photoreceptor synaptic complexes of the human retina. Neuroscience. 1996;73:605–612. doi: 10.1016/0306-4522(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG, Guo H, Pillers DA. Normal photoresponses and altered b-wave responses to APB in the mdx(Cv3) mouse isolated retina ERG supports role for dystrophin in synaptic transmission. Vis Neurosci. 2004;21:739–747. doi: 10.1017/S0952523804215085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenggi T, Fritschy JM. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cell Mol Life Sci. 2006;63:1614–1631. doi: 10.1007/s00018-005-5461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Townes-Anderson E. Cell specific post-translational processing of pikachurin, a protein involved in retinal synaptogenesis. PLoS One. 2012;7:e50552. doi: 10.1371/journal.pone.0050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Li J, Zhang Z, Yu M. Pikachurin interaction with dystroglycan is diminished by defective O-mannosyl glycosylation in congenital muscular dystrophy models and rescued by LARGE overexpression. Neurosci Lett. 2011;489:10–15. doi: 10.1016/j.neulet.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Ishii M, Morigiwa K, Takao M, Nakanishi S, Fukuda Y, Mimura O, Tsukamoto Y. Ectopic synaptic ribbons in dendrites of mouse retinal ON- and OFF-bipolar cells. Cell Tissue Res. 2009;338:355–375. doi: 10.1007/s00441-009-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrow H, Koulen P, Altrock WD, Kroger S. Identification of a beta-dystroglycan immunoreactive subcompartment in photoreceptor terminals. Invest Ophthalmol Vis Sci. 2006;47:17–24. doi: 10.1167/iovs.05-0597. [DOI] [PubMed] [Google Scholar]
- Kameya S, Araki E, Katsuki M, Mizota A, Adachi E, Nakahara K, Nonaka I, Sakuragi S, Takeda S, Nabeshima Y. Dp260 disrupted mice revealed prolonged implicit time of the b-wave in ERG and loss of accumulation of beta-dystroglycan in the outer plexiform layer of the retina. Hum Mol Genet. 1997;6:2195–2203. doi: 10.1093/hmg/6.13.2195. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Omori Y, Sato S, Kobayashi K, Miyagoe-Suzuki Y, Takeda S, Endo T, Furukawa T, Toda T. Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization. J Biol Chem. 2010;285:31208–31216. doi: 10.1074/jbc.M110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Rikimaru L, Hennig AK, Lukasiewicz PD, Fliesler SJ, Govardovskii VI, Kefalov VJ, Kisselev OG. G-protein betagamma-complex is crucial for efficient signal amplification in vision. J Neurosci. 2011;31:8067–8077. doi: 10.1523/JNEUROSCI.0174-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Sanuki R, Ueno S, Nishizawa Y, Hashimoto N, Ohguro H, Yamamoto S, Machida S, Terasaki H, Adamus G, Furukawa T. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6:e19911. doi: 10.1371/journal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk U, Oexle K, Voit T, Ancker U, Hellner KA, Speer A, Hubner C. A cysteine 3340 substitution in the dystroglycan-binding domain of dystrophin associated with Duchenne muscular dystrophy, mental retardation and absence of the ERG b-wave. Hum Mol Genet. 1996;5:973–975. doi: 10.1093/hmg/5.7.973. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY. Transducin gamma-subunit sets expression levels of alpha- and beta-subunits and is crucial for rod viability. J Neurosci. 2008;28:3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Mercer AJ, Thoreson WB. The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci. 2011;28:453–471. doi: 10.1017/S0952523811000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro F, Carbonetto S, Campbell KP, Lindenbaum M. Dystroglycan expression in the wild type and mdx mouse neural retina: synaptic colocalization with dystrophin, dystrophin-related protein but not laminin. J Neurosci Res. 1995;42:528–538. doi: 10.1002/jnr.490420411. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brown RL, Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Lyubarsky A, Fina ME, Nikonova ES, Sengupta A, Chinniah C, Ding XQ, Smith RG, Pugh EN, Jr, Vardi N, Dhingra A. Cones respond to light in the absence of transducin beta subunit. J Neurosci. 2013;33:5182–5194. doi: 10.1523/JNEUROSCI.5204-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Okawa H, Pahlberg J, Rieke F, Birnbaumer L, Sampath AP. Coordinated control of sensitivity by two splice variants of Galpha(o) in retinal ON bipolar cells. J Gen Physiol. 2010;136:443–454. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Araki F, Chaya T, Kajimura N, Irie S, Terada K, Muranishi Y, Tsujii T, Ueno S, Koyasu T, Tamaki Y, Kondo M, Amano S, Furukawa T. Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J Neurosci. 2012;32:6126–6137. doi: 10.1523/JNEUROSCI.0322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG, Martemyanov KA. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012;197:711–719. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillers DA. Dystrophin and the retina. Mol Genet Metab. 1999;68:304–309. doi: 10.1006/mgme.1999.2929. [DOI] [PubMed] [Google Scholar]
- Pillers DA, Fitzgerald KM, Duncan NM, Rash SM, White RA, Dwinnell SJ, Powell BR, Schnur RE, Ray PN, Cibis GW, Weleber RG. Duchenne/Becker muscular dystrophy: correlation of phenotype by electroretinography with sites of dystrophin mutations. Hum Genet. 1999;105:2–9. doi: 10.1007/s004399900111. [DOI] [PubMed] [Google Scholar]
- Pillers DA, Weleber RG, Woodward WR, Green DG, Chapman VM, Ray PN. mdxCv3 mouse is a model for electroretinography of Duchenne/Becker muscular dystrophy. Invest Ophthalmol Vis Sci. 1995;36:462–466. [PubMed] [Google Scholar]
- Ramakrishnan H, Dhingra A, Tummala SR, Fina ME, Li JJ, Lyubarsky A, Vardi N. Differential function of Ggamma13 in rod bipolar and ON cone bipolar cells. J Physiol. 2015;593:1531–1550. doi: 10.1113/jphysiol.2014.281196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007;27:14199–14204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Bongini RE, Code KA, Zelinka C, Petersen-Jones S, Fischer AJ. The pattern of expression of guanine nucleotide-binding protein beta3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169:1376–1391. doi: 10.1016/j.neuroscience.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, Dessauer CW, Ivanina T, Dascal N. Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GalphaiGDP and Gbetagamma. J Physiol. 2009;587:3473–3491. doi: 10.1113/jphysiol.2009.173229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R, Riker MJ, Hernandez J, Weiss RM, Anderson MG, Mullins RF, Moore SA, Stone EM, Campbell KP. Visual impairment in the absence of dystroglycan. J Neurosci. 2009;29:13136–13146. doi: 10.1523/JNEUROSCI.0474-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shim H, Wang CT, Chen YL, Chau VQ, Fu KG, Yang J, McQuiston AR, Fisher RA, Chen CK. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J Biol Chem. 2012;287:14873–14879. doi: 10.1074/jbc.M112.345751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman P, Xu Y, Fina ME, Tummala SR, Ramakrishnan H, Dhingra A, Vardi N. Kir2.4 surface expression and basal current are affected by heterotrimeric G-proteins. J Biol Chem. 2013;288:7420–7429. doi: 10.1074/jbc.M112.412791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Omi N. Effects of mGluR6-deficiency on photoreceptor ribbon synapse formation: comparison of electron microscopic analysis of serial sections with random sections. Vis Neurosci. 2014;31:39–46. doi: 10.1017/S0952523813000473. [DOI] [PubMed] [Google Scholar]
- Tummala SR, Neinstein A, Fina ME, Dhingra A, Vardi N. Localization of Cacna1s to ON bipolar dendritic tips requires mGluR6-related cascade elements. Invest Ophthalmol Vis Sci. 2014;55:1483–1492. doi: 10.1167/iovs.13-13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Matesic DF, Manning DR, Liebman PA, Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci. 1993;10:473–478. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- Wersinger E, Bordais A, Schwab Y, Sene A, Benard R, Alunni V, Sahel JA, Rendon A, Roux MJ. Reevaluation of dystrophin localization in the mouse retina. Invest Ophthalmol Vis Sci. 2011;52:7901–7908. doi: 10.1167/iovs.11-7519. [DOI] [PubMed] [Google Scholar]
- Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T, Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012;107:948–957. doi: 10.1152/jn.00933.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sulaiman P, Feddersen RM, Liu J, Smith RG, Vardi N. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J Neurosci. 2008;28:8873–8884. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jeffrey BG, Morgans CW, Burke NS, Haley TL, Duvoisin RM, Brown RL. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010;51:1121–1129. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.