Abstract
Purpose
Given the lack of a reference standard diagnostic tool for carpal tunnel syndrome (CTS), we conducted a population-level analysis of patients undergoing carpal tunnel release to characterize utilization of preoperative electrodiagnostic studies (EDS). Secondarily, we sought to determine the impact of EDS utilization on timeliness of surgery, number of preoperative physician visits, and costs.
Methods
The 2009 to 2013 Truven MarketScan Databases were used to identify a national cohort of adult patients undergoing carpal tunnel release. Three multivariable regression models were designed to evaluate the relationship between preoperative EDS use and timing of surgical release, the number of preoperative physician visits, and total costs for CTS-related visits, while controlling for socio-demographic variables, insurance type, comorbid conditions, and treatment characteristics.
Results
The final study cohort included 62,894 patients who underwent carpal tunnel release, of whom 58% had preoperative EDS. Patients undergoing EDS waited 36% longer for surgical release than patients without EDS. The mean time between diagnosis and surgery was predicted to be 183 days for patients who underwent preoperative EDS and 135 days for patients who did not. Patients having EDS experienced 1 additional visit, $996 greater total costs, and $112 additional out-of-pocket costs on average. Occupational therapy consultation and steroid injection were also associated with increased time to surgery, but with one-fourth and one-third the added cost of EDS, respectively.
Conclusions
Based on national practice trends, providers do not consistently agree with the practice of performing EDS prior to carpal tunnel release. Given the uncertain utility of routine EDS prior to carpal tunnel release and its association with delays to surgery and increased costs, further evaluation of EDS in relation to patient preferences and value of care is warranted.
Level of Evidence
Level II (retrospective prognostic study)
Keywords: carpal tunnel syndrome, carpal tunnel release, electrodiagnostic studies, practice guidelines, practice patterns
INTRODUCTION
General practitioners and surgeons commonly obtain electrodiagnostic studies (EDS) as diagnostic support for cases of suspected carpal tunnel syndrome (CTS) (1, 2). The severity of nerve compression, graded by a combination of preoperative clinical examination and EDS testing, is closely associated with anticipated improvement in symptoms after carpal tunnel release (3–5). Given the application of EDS in diagnosis and grading severity of nerve compression (6), past American Academy of Orthopaedic Surgeons (AAOS) practice guidelines for diagnosis of CTS recommended that providers obtain electrodiagnostic tests if surgical intervention was under consideration (5). However, previous guidelines have acknowledged the low quality of evidence supporting widespread use of EDS in the diagnosis of CTS and fair quality of evidence in using EDS to predict symptom relief after surgery.
Although EDS may be viewed as a low-risk confirmatory test, it is unpleasant for patients. Furthermore, the delay in time to surgery and the financial burden of additional diagnostic testing may reduce the overall value of care to patients and their satisfaction. The rationale for obtaining EDS for patients being considered for surgery is debated in practice. Some authors believe EDS is unnecessary for classic presentation of CTS (7, 8), whereas other providers use EDS to screen all patients prior to scheduling an initial evaluation (9, 10). Adherence to a practice of obtaining EDS on patients considered for surgery is unknown at a population level. In addition, the manner in which EDS utilization affects time to surgery, the overall cost of care, and out of pocket expenses for patients with CTS is also unclear.
We sought to evaluate national practice patterns of EDS utilization for patients undergoing carpal tunnel release. Specifically, we aimed to conduct a population-level analysis of patients undergoing carpal tunnel release to characterize overall utilization of preoperative EDS. Secondarily, we sought to determine the impact of EDS utilization on (1) the timeliness of treatment between diagnosis and surgery; (2) the number of CTS-related outpatient physician visits between diagnosis and surgery; and (3) the overall cost of care and out of pocket expenses. We hypothesize that providers do not uniformly perform EDS for all patients having carpal tunnel release and that use of EDS is associated with prolonged time to surgery and increased costs of care.
MATERIALS AND METHODS
Data Source and Study Cohort
This study qualified for exempt status by the Institutional Review Board. The 2009 to 2013 Truven MarketScan Commercial Claims and Encounters and Medicare Supplement/Coordination of Benefits (MarketScan) databases were used to identify a national sample of patients undergoing carpal tunnel release. The MarketScan databases include a national convenience sample from large employers, health plans, government, and public organizations for over 55 million enrollees per year (11). The dataset contains individual encounters from inpatient, outpatient, and pharmacy domains, and allows for longitudinal evaluation of patients across providers as long as they remain enrolled in the health plan. In addition, procedures are more specifically identified with the utilization of Current Procedural Terminology (CPT) codes.
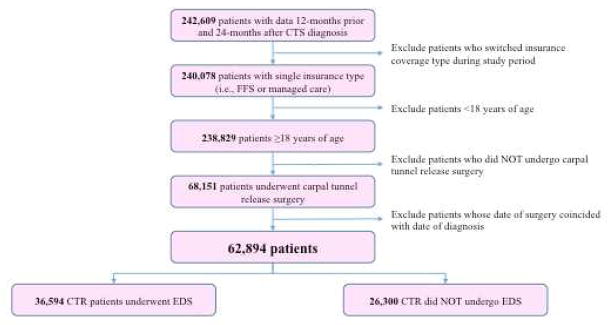
The study cohort included patients age 18 and older with a primary diagnosis of CTS who underwent carpal tunnel release during the observation period. ICD-9 diagnosis codes and CPT codes (see Table, Supplemental Digital Content) were used to identify patients with CTS and patients undergoing carpal tunnel release, respectively. In order to allow time to observe preoperative EDS and associated comorbidities, patients were excluded from analysis if they were not enrolled for at least 12 months before initial diagnosis of CTS. Patients were also excluded if they were not enrolled for at least 24 months after diagnosis. A small group of patients underwent carpal tunnel release without a prior encounter with CTS as the primary diagnosis. These patients were excluded from the analysis owing to inadequate time of observation prior to diagnosis and surgery and the possibility that previous evaluations for CTS were unreliably captured if the patient was evaluated for multiple complaints with CTS as a secondary diagnosis. Thus, all patients in the cohort had at least one encounter prior to surgery in which CTS was a primary diagnosis. Patients were also excluded if they changed insurance plan type (fee-for-service versus managed care) during the observation period in order to allow comparison between insurance plan types. The full inclusion and exclusion algorithm is outlined in Figure 1.
Figure 1.
Study cohort selection of patients undergoing carpal tunnel release using MarketScan Databases, 2009–2013. EDS: electrodiagnostic studies
Predictor and Outcome Variables
Preoperative EDS utilization was recorded among the patients undergoing carpal tunnel release (see Table, Supplemental Digital Content for CPT codes). Three outcomes of interest were recorded, including the timing of surgical release, the number of preoperative CTS-related encounters, and the cost for CTS-related healthcare visits. The first outpatient encounter with CTS as the primary diagnosis was recorded as the day of initial diagnosis. The timing of surgical release was calculated as the number of days between initial diagnosis and surgical release. The number of preoperative CTS-related physician visits was counted. The total cost, including insurance payment and out-of-pocket expenses were calculated for all CTS-related outpatient services from diagnosis to 12 months after surgical release. Insurance payment includes reimbursement for provider and facility fees. The out-of-pocket spending was measured by adding the amount of coinsurance, copayment, and deductible attributed to CTS-related encounters. All payment values were adjusted to 2013 U.S. dollars using year-specific consumer price index (CPI) (12).
Covariates
Additional patient variables were recorded, including socio-demographic, insurance plan type, comorbid conditions, and treatment characteristics. Socio-demographic factors included age, gender, and patient geographic region. Insurance plan type was designated as fee-for-service (FFS) or managed care. ICD-9 diagnosis codes were used to identify presence of comorbid conditions, including rheumatoid arthritis/collagen vascular disease, wrist fracture, obesity, and diabetes. Treatment characteristics, identified by CPT codes, included use of preoperative occupational therapy, use of preoperative steroid injection, and anesthesia type during carpal tunnel release (see Table, Supplemental Digital Content). We categorized anesthesia type into four groups: local only, regional block only, anesthesia provider without regional block, and anesthesia provider with regional block. We were unable to discern between sedation versus general anesthetic utilization given lack of specific CPT codes. Thus, the anesthesia provider category includes use of sedation or general anesthetic.
Statistical Analysis
Three multivariable regression models were designed to evaluate the relationship between preoperative EDS utilization and each outcome of interest, while controlling for patient socio-demographic characteristics, insurance type, comorbid conditions, and treatment characteristics. Poisson and negative binomial regression models, both often used to model count data, were created to evaluate the relationship between preoperative EDS utilization and the number of days between diagnosis and surgical release. Ultimately the Poisson model was chosen as the best fit to explain variance in the outcome. A separate Poisson regression model was used to evaluate the relationship between use of preoperative EDS and number of preoperative physician visits. A log-linear regression model, often used for positively skewed outcomes such as cost, was created to evaluate the relationship between preoperative EDS utilization and total CTS-related costs. The type of anesthesia was used as a control variable in the cost model only. Post-estimation marginal effects were calculated to compare adjusted mean outcome predictions among patients in the EDS and non-EDS groups, while still controlling for patient variables in the model. A multivariable Poisson regression model reports incidence rate ratios (IRR), which represent the predicted proportional increase or decrease in outcome compared to the reference group. The post-estimation marginal calculation is helpful because it allows comparison of the groups of interest in a measure that is easier to understand compared to the IRRs, while still controlling for patient variables in the model.
RESULTS
Of all patients with CTS, 242,609 patients were followed in the dataset for at least 12 months prior to diagnosis and 24 months after diagnosis of CTS (Figure 1). The final study cohort included 62,894 patients who underwent carpal tunnel release, of which 58% received preoperative EDS prior to surgical release. Mean demographic, insurance, and enrollment characteristics for the study cohort are outlined in Table 1. The cohort maintained stable enrollment in the data source, with average patient enrollment in the MarketScan database for nearly 5 years.
Table 1.
Patient Characteristics
| Number | Percent (%) | |
|---|---|---|
| N= 62,894 | 62,894 | |
|
| ||
| Demographic Data | ||
| Female | 40,173 | 64 |
| Age, mean (SD) | 57 (13) | |
|
| ||
| Insurance Type | ||
| Fee-for-service | 55,992 | 89 |
| Managed care | 6,902 | 11 |
|
| ||
| Longitudinal Enrollment Time (Months) | ||
| Enrollment before diagnosis, mean (SD) | 22 (7) | |
| Enrollment after diagnosis, mean (SD) | 35 (7) | |
|
| ||
| Comorbid Conditions | ||
| Rheumatoid arthritis | 2,179 | 4 |
| Wrist fracture | 1,616 | 3 |
| Obesity | 9,012 | 13 |
| Diabetes | 14,959 | 24 |
|
| ||
| Region | ||
| Northeast | 12,348 | 20 |
| North central | 18,520 | 30 |
| South | 21,773 | 35 |
| West | 8,968 | 14 |
| Unspecified | 1,285 | 2 |
|
| ||
| Treatment Characteristics | ||
| Steroid injection prior to surgery | 7,601 | 12 |
| Occupational therapy prior to surgery | 12,328 | 20 |
|
| ||
| Use of Electrodiagnostic Studies | 36,594 | 58 |
|
| ||
| Anesthesia Type | ||
| Local only | 12,348 | 20 |
| Regional block only | 666 | 1 |
| Anesthesia provider* | 48,682 | 77 |
| Anesthesia provider* + regional block | 1,198 | 2 |
Category includes use of sedation or general anesthesia administered by an anesthesia provider.
Patients who underwent preoperative EDS waited an average of 184 days (SD=249 days) from diagnosis to surgery, whereas patients who did not undergo EDS waited an average of 134 days (SD=215 days). After adjusting for control variables, patients undergoing EDS had an estimated 36% longer wait for surgical release than patients without EDS (IRR=1.36, P<0.001) (Table 2). The model predicted that the mean time between diagnosis and surgery in the controlled analysis among patients in the EDS and non-EDS groups was similar in magnitude to the unadjusted estimates (183 days and 135 days respectively, P<0.001). Of note, managed care insurance (IRR=1.10, P<0.001), preoperative occupational therapy (IRR=1.61, P<0.001) and preoperative steroid injection (IRR=1.98, P<0.001) were also associated with significantly increased time to surgery in the multivariable regression model (Table 2).
Table 2.
Multivariable Poisson Regression Models for Timing of Surgery and Number of Physician Visits Prior to Surgery, N=62,894
| Days between Diagnosis and Surgery | Number of Physician Visits Prior to Surgery | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| IRR | 95% CI | P | IRR | 95% CI | P | |
| Female | 1.06 | 1.06–1.07 | <0.001 | 1.56 | 1.01–1.03 | <0.001 |
|
| ||||||
| Age | ||||||
| 18–34 | Reference | Reference | ||||
| 35–44 | 1.01 | 1.01–1.01 | <0.001 | 0.96 | 0.93–0.98 | <0.001 |
| 45–54 | 0.99 | 0.99–1.00 | <0.001 | 0.92 | 0.92–0.97 | <0.001 |
| 55–64 | 0.90 | 0.90–0.91 | <0.001 | 0.89 | 0.89–0.93 | <0.001 |
| 65+ | 0.99 | 0.97–0.97 | <0.001 | 0.92 | 0.93–0.97 | <0.001 |
|
| ||||||
| Insurance Type | ||||||
| Managed care | 1.10 | 1.10–1.10 | <0.001 | 1.06 | 1.04–1.08 | <0.001 |
|
| ||||||
| Comorbid Conditions | ||||||
| Rheumatoid arthritis | 0.97 | 0.96–0.97 | <0.001 | 1.01 | 0.98–1.04 | 0.572 |
| Wrist fracture | 0.85 | 0.85–0.86 | <0.001 | 0.91 | 0.88–9.40 | <0.001 |
| Obesity | 1.03 | 1.03–1.03 | <0.001 | 1.01 | 1.00–1.03 | 0.078 |
| Diabetes | 1.02 | 1.02–1.02 | <0.001 | 1.00 | 0.99–1.01 | 0.576 |
|
| ||||||
| Region | ||||||
| Northeast | 1.09 | 1.09–1.09 | <0.001 | 1.04 | 1.03–1.05 | <0.001 |
| North central | 1.06 | 1.06–1.06 | <0.001 | 0.97 | 0.96–0.99 | <0.001 |
| South | Reference | Reference | ||||
| West | 1.18 | 1.18–1.18 | <0.001 | 1.08 | 1.06–1.10 | <0.001 |
| Unspecified | 1.05 | 1.04–1.05 | <0.001 | 1.02 | 0.98–1.06 | 0.274 |
|
| ||||||
| Treatment Options | ||||||
| Steroid injection prior to surgery | 1.98 | 1.99–1.99 | <0.001 | 1.55 | 1.53–1.57 | <0.001 |
| Occupational therapy prior to surgery | 1.61 | 1.61–1.61 | <0.001 | 0.96 | 0.95–0.97 | <0.001 |
|
| ||||||
| Use of Electrodiagnostic Studies | 1.36 | 1.36–1.36 | <0.001 | 1.56 | 1.54–1.57 | <0.001 |
Patients who underwent EDS had an average of 2.9 preoperative physician visits between diagnosis and surgical release, whereas patients who did not undergo EDS had an average of 1.9 preoperative physician visits. This difference in the number of preoperative visits was similar in the multivariable analysis, in which utilization of EDS was associated with a significant increase in the number of preoperative physician visits (IRR=1.56, P<0.001) (Table 2). Among other control variables, preoperative steroid injection was associated with a significant increase in the number of preoperative physician visits (IRR=1.55, P<0.001) on a similar magnitude as seen with utilization of preoperative EDS (Table 2).
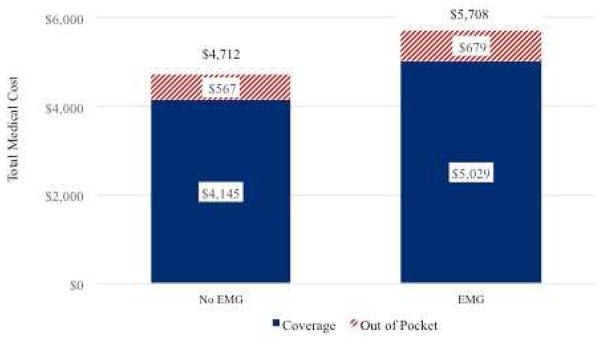
The unadjusted mean cost for CTS-related encounters for patients receiving EDS was $5,708 (SD=$5,456), whereas cost for those not undergoing EDS was $4,712 (SD=$4865). The preoperative EDS group had an increased out of pocket expense of $112 (Figure 2). In the controlled analysis, EDS utilization was associated with 25% greater costs (P<0.001) (Table 3). Anesthesia type was the only treatment factor to influence cost at a similar magnitude as seen with EDS utilization. For example, use of local anesthetic only was associated with a 27% decrease in total costs (P<0.001). Prior nonoperative treatments were associated with 6 – 8% added cost (P <0.001; Table 3).
Figure 2.
Differential cost of care for carpal tunnel release patients with and without preoperative electrodiagnostic studies (EDS). Cost of care includes insurance reimbursement and out-of-pocket costs for all CTS-related visits from time of diagnosis to 12 months after surgery.
Table 3.
Multivariable Log-Linear Regression Model for Cost of CTS-related Encounters, N=62,894
| Coeff. | 95% CI | P | |
|---|---|---|---|
| Female | −0.009 | −0.02–0.00 | 0.106 |
|
| |||
| Age | |||
| 18–34 | Reference | ||
| 35–44 | −0.009 | −0.04–0.02 | 0.518 |
| 45–54 | −0.021 | −0.05–0.01 | 0.112 |
| 55–64 | −0.057 | −0.08–−0.03 | <0.001 |
| 65+ | −0.359 | −0.39–−0.33 | <0.001 |
|
| |||
| Insurance Type | |||
| Managed care | −0.004 | −0.02–0.01 | 0.654 |
|
| |||
| Comorbid Conditions | |||
| Rheumatoid arthritis | 0.034 | 0.01–0.06 | 0.017 |
| Wrist fracture | −0.132 | −0.16–−0.10 | <0.001 |
| Obesity | 0.028 | 0.01–0.04 | <0.001 |
| Diabetes | 0.006 | −0.01–0.02 | 0.322 |
|
| |||
| Region | |||
| Northeast | 0.026 | 0.01–0.04 | 0.001 |
| North central | −0.010 | −0.02–0.00 | 0.119 |
| South | Reference | ||
| West | 0.095 | 0.08–0.11 | <0.001 |
| Unspecified | 0.027 | −0.01–0.06 | 0.148 |
|
| |||
| Treatment Options | |||
| Steroid injection prior to surgery | 0.079 | 0.06–0.10 | <0.001 |
| Occupational therapy prior to surgery | 0.061 | 0.05–0.07 | <0.001 |
|
| |||
| Anesthesia Type | |||
| Local only | −0.274 | −0.29–−0.26 | <0.001 |
| Regional block only | −0.381 | −0.43–−0.33 | <0.001 |
| Anesthesia provider* | Reference | ||
| Anesthesia provider * + regional block | 0.19 | 0.15–0.23 | <0.001 |
|
| |||
| Use of Electrodiagnostic Studies | 0.243 | 0.23–0.25 | <0.001 |
Category includes use of sedation or general anesthesia administered by an anesthesia provider.
DISCUSSION
Despite previous AAOS guidelines at the time of this study that recommended preoperative EDS in patients being considered for surgical management of CTS, 42% of patients in this national cohort underwent carpal tunnel release without preoperative EDS. Patients having preoperative EDS experienced a nearly two-month longer delay in time to surgery, one additional office visit, nearly $1000 greater total cost, and an additional $110 in out-of-pocket costs, compared to patients not having EDS. These utilization differences may partly explain why providers were not putting the AAOS recommendation at the time into practice for all patients having carpal tunnel release. Prior steroid injection and consultation with occupational therapy were also associated with longer time to surgery. However, the added costs associated with these nonoperative modalities were associated with one-fourth to one-third of the added cost associated with EDS. Thus, noting symptomatic improvement after less costly services, such as splinting or steroid injection, may provide a lower cost alternative to EDS if an additional indicator beyond the history of physical exam is needed to confirm the diagnosis of CTS. The bigger question remains as to whether a confirmatory test is warranted for all patients. The push toward bundled payment systems that reward physicians for providing efficient care should encourage all stakeholders in healthcare delivery to closely examine current practices regarding preoperative EDS use, their role in clinical decision-making, impact on treatment timing, and influence on value of care.
Our findings support previous research suggesting that EDS is utilized less often than recommended in previous guidelines from the AAOS (5), the American Association of Electrodiagnostic Medicine (AAEM) (13), and the National Guideline Clearinghouse (14). For example, Storm et al found that 21% of Medicare beneficiaries undergoing carpal tunnel release in Washington State from 1998 to 1999 did not undergo preoperative EDS, as was recommended by the AAEM at the time (15). A 2014 survey of 705 American Society for Surgery of the Hand (ASSH) members found that only 55% of survey respondents believed that EDS was usually a necessary supplement to the history and physical exam to aid decision-making for surgery. Of the surveyed ASSH members more likely to order preoperative EDS, 57% reported that they do so to avoid potential medicolegal consequences (6). The proportion of providers that obtain EDS but believe it to be unnecessary is unknown in the present study.
Previous authors have suggested reasons for not ordering EDS studies prior to surgical release that include inconvenience of the test, delay to operation, conclusive history and physical exam, and unnecessary distress caused to the patient (15). The economic consequences of additional office visits and prolonged time to definitive treatment associated with EDS use could not be estimated in the current study. However, EDS may have added negative impact beyond treatment delay and direct medical costs in patients who need to take time off of work. Thus, the added costs associated with preoperative EDS use is likely underestimated in this study. Additionally, differing practices may be driven by a lack of consistent, high-quality research supporting the use of EDS in CTS diagnosis. For example, in a prospective blinded study by Graham, EDS did not change the probability of CTS diagnosis relative to pretest probability estimates for the majority of patients considered to have CTS based on history and physical alone (8). Furthermore, the probability of CTS was lowered after EDS for most patients with low pretest probability of CTS (<0.5) based on exam findings alone. A latent class analysis comparison of the Carpal Tunnel Syndrome 6 (CTS-6) clinical diagnostic tool, ultrasound, and nerve conduction studies by Fowler et al found similar sensitivities between the three diagnostic tests (95%, 91%, and 91% respectively). Whereas, ultrasound and the CTS-6 questionnaire had greater specificity (94% and 91%) compared to nerve conduction studies (83%) (16). Lack of consensus regarding the clinical protocol following a negative EDS tests also calls into the question the usefulness of preoperative EDS as a routine practice to confirm the diagnosis of CTS. For example, 59% of surveyed ASSH members would at least sometimes still perform surgical release in patients with normal EDS if patients had experienced complete resolution of symptoms following steroid injection (6). EDS may influence management in some patients, including patients with an atypical presentation of CTS or patients seeking secondary gain. However, in many patients with classic presentation of CTS, EDS may not change the probability of diagnosing CTS beyond what can be estimated in the patient consultation. Furthermore, EDS may not influence the treatment plan for patients with high or low likelihood of CTS based on exam findings or patients with negative EDS who respond to steroid injection. The continued evidence citing the lack of a reference standard diagnostic tool for CTS prompted removal of the previous recommendation of obtaining EDS in patients considered for surgery in the 2016 AAOS Management of Carpal Tunnel Clinical Practice Guideline (draft version at the time of writing).
This study had several limitations. The MarketScan dataset provides a snapshot in time of patients enrolled as employees or beneficiaries in large employer-based health plans between 2009 and 2013. Patients were required to have a primary diagnosis of CTS documented for inclusion in the study to attempt to eliminate patients with additional upper extremity conditions. As a result, some patients evaluated for other comorbidities by a primary care physician may not have CTS documented as a primary diagnosis, which may influence the noted time of diagnosis or inclusion in the study. MarketScan lacks granularity regarding the clinical aspects of each patient encounter and the provider’s motivation for ordering the preoperative test. For example, MarketScan does not provide data related to compression severity as diagnosed by history and physical exam, which may influence a provider’s decision to order preoperative EDS. Similarly, we cannot glean from the data whether the providers ordered EDS to confirm diagnosis, to screen patients prior to scheduling an initial evaluation, to guard against potential medicolegal issues, or whether they were influenced by clinical practice guidelines at the time. Identification of underlying provider beliefs and motivations would further delineate added costs and inefficiency associated with use of the test when providers view the test to be unnecessary for diagnosis or treatment decisions. In addition, we are unable to discern whether the referring provider or the surgeon ordered the EDS. Limitations of the dataset prevented us from associating preoperative EDS results with the decision to pursue surgical intervention, postoperative outcomes, and patient satisfaction. Lastly, the dataset does not have the ability to document the specific services provided by consultation with an occupational therapist. Thus, we are unable to discern whether providers use therapy visits to provide recommended nonoperative treatments, such as splinting, or whether modalities with poor to inconclusive evidence are being provided for the added cost. These differences in occupational therapy use and patient-driven factors that delay treatment likely lead to considerable variation seen in cost and time to surgery. However, we expect that these differences will similarly impact patients with and without preoperative EDS for comparison in this study. Despite these limitations, our study highlights the national patterns of EDS utilization in the several-year period prior to surgery and can inform providers of the impact on timing of definitive care, costs to the insurer, and out-of-pocket costs for the patient.
There is a need to examine whether costly diagnostic testing is needed to confirm the diagnosis of CTS for all patients prior to carpal tunnel release. Previous guidelines recommended that patients being considered for surgical release undergo EDS. Just over one-half of providers actually performed preoperative EDS in our national cohort. Substantial savings for the health care system may occur if providers reserve EDS for situations in which results will influence treatment decisions rather than to routinely use EDS for all patients considered for surgery. Given the increased time to surgery and added costs, providers should understand the impact of routine EDS use for all patients considered for surgical release, especially if the test results may not alter the treatment plan.
Supplementary Material
Acknowledgments
Support for this study was provided in part by a Midcareer Investigator Award in Patient-Oriented Research (K24-AR053120-06) (to Dr. Kevin C. Chung).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Makinde OA, Ezomike CF, Lehmann HP, Ibanga IJ. Lessons learned in the deployment of a HIV counseling and testing management information system on a new project. AIDS. 2011;25(18):2289–2293. doi: 10.1097/QAD.0b013e32834d6ad2. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Claussen GC, Oh S. Clinical nerve conduction and needle electromyography studies. J Am Acad Orthop Surg. 2004;12(4):276–287. doi: 10.5435/00124635-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Boniface SJ, Morris I, Macleod A. How does neurophysiological assessment influence the management and outcome of patients with carpal tunnel syndrome? Br J Rheumatol. 1994;33(12):1169–1170. doi: 10.1093/rheumatology/33.12.1169. [DOI] [PubMed] [Google Scholar]
- 4.Prick JJ, Blaauw G, Vredeveld JW, Oosterloo SJ. Results of carpal tunnel release. Eur J Neurol. 2003;10(6):733–736. doi: 10.1046/j.1468-1331.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Orthopaedic S. Clinical Practice Guideline on the Diagnosis of Carpal Tunnel Syndrome. 2007 [Google Scholar]
- 6.Lane LB, Starecki M, Olson A, Kohn N. Carpal tunnel syndrome diagnosis and treatment: a survey of members of the American Society For Surgery of the Hand. J Hand Surg Am. 2014;39(11):2181–2187. e2184. doi: 10.1016/j.jhsa.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Concannon MJ, Gainor B, Petroski GF, Puckett CL. The predictive value of electrodiagnostic studies in carpal tunnel syndrome. Plast Reconstr Surg. 1997;100(6):1452–1458. doi: 10.1097/00006534-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2008;90(12):2587–2593. doi: 10.2106/JBJS.G.01362. [DOI] [PubMed] [Google Scholar]
- 9.Buch-Jaeger N, Foucher G. Correlation of clinical signs with nerve conduction tests in the diagnosis of carpal tunnel syndrome. J Hand Surg Br. 1994;19(6):720–724. doi: 10.1016/0266-7681(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 10.de Krom MC, Knipschild PG, Kester AD, Spaans F. Efficacy of provocative tests for diagnosis of carpal tunnel syndrome. Lancet. 1990;335(8686):393–395. doi: 10.1016/0140-6736(90)90218-t. [DOI] [PubMed] [Google Scholar]
- 11.Danielson E. Health Research Data for the Real World: The MarketScan Databases (White Paper) Truven Health Analytics. 2014 [Google Scholar]
- 12.United States Department of Labor: Bureau of Labor Statistics. [Accessed October 1, 2015];CPI Inflation Calculator. http://www.bls.gov/data/inflation_calculator.htm.
- 13.American Association of Electrodiagnostic Medicine AAoN, American Academy of Physical M, Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25(6):918–922. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 14.National Guideline Clearinghouse. [Accessed October 19, 2015];Carpal tunnel syndrome. http://www.guideline.gov/content.aspx?id=34436.
- 15.Storm S, Beaver SK, Giardino N, et al. Compliance with electrodiagnostic guidelines for patients undergoing carpal tunnel release. Arch Phys Med Rehabil. 2005;86(1):8–11. doi: 10.1016/j.apmr.2004.02.027. quiz 180. [DOI] [PubMed] [Google Scholar]
- 16.Fowler JR, Cipolli W, Hanson T. Comparison of three diagnstic tests for carpal tunnel syndrome using latent class analysis. J Bone Joint Surg Am. 2015;97:1958–1961. doi: 10.2106/JBJS.O.00476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.