Abstract
The elastomeric proteins elastin and resilin have been used extensively in the fabrication of biomaterials for tissue engineering applications due to their unique mechanical and biological properties. Tropoelastin is the soluble monomer component of elastin. Tropoelastin and resilin are both highly elastic with high resilience, substantial extensibility, high durability and low energy loss, which makes them excellent candidates for the fabrication of elastic tissues that demand regular and repetitive movement like the skin, lung, blood vessels, muscles and vocal folds. Combinations of these proteins with silk fibroin further enhance their biomechanical and biological properties leading to a new class of protein alloy materials with versatile properties. In this review, the properties of tropoelastin- and resilin-based biomaterials with and without silk are described in concert with examples of their applications in tissue engineering.
Graphical abstract
Introduction
An elastomer is a rubber-like material with high resilience, extensibility and effective energy storage[1,2]. Elastomeric materials undergo large elastic deformations, and energy used for their deformation can be recovered after removal of the applied force [2]. There are multiple elastomeric proteins in nature including elastin, resilin, wheat gluten, abductin and flagelliform silk but of these elastin is the only mammalian elastomer. These materials have been reviewed previously [1,3,4]. Pure elastin comprises assembled tropoelastin monomers. In this review we focus on tropoelastin and resilin as elastomeric proteins that have been extensively investigated for the fabrication of materials for tissue engineering applications. Tropoelastin-silk and resilin-silk composite materials are also reviewed as an emerging, versatile class of biomaterials with tunable mechanical and biological properties for engineering of multiple tissue types.
Tropoelastin
Tropoelastin is the soluble protein precursor of elastin. Elastin, a highly insoluble, durable and long lasting protein polymer, is responsible for the elasticity of different tissues in the body including, skin, blood vessels and lung [5-7]. Elastin's monomer tropoelastin is a biologically active molecule secreted by elastogenic cells after which it self-assembles and organizes in association with a fibrillin-rich microfibrillar structure to form elastin [8,9]. Synthetic elastin does not require the microfibrillar component to form as it is fabricated. Tropoelastin is composed of alternating hydrophobic and hydrophilic domains. At low temperatures tropoelastin is a soluble monomer. With increasing temperature, the hydrophobic domains interact such that the molecules begin to aggregate into a non-soluble viscoelastic phase termed a coacervate. The coacervate is an early step towards elastin fibre formation [6,7]. These coacervated tropoelastin molecules then align with each other. Covalent crosslinking of hydrophilic domains, particularly those rich in Lys and Ala ensues resulting in the formation of a stabilized mature elastic protein polymer network [6,8,10]. The rubber-like properties of elastin are due to repetitive modules such as VPGXG[3,11]. With a Young's modulus of ∼3 kPa, the tropoelastin molecule is significantly more elastic than other extracellular molecules[9,12]. Naturally cross-linked tropoelastin has a Young's modulus of 300-600 kPa [9]. Tropoelastin can be extended to 8 times of its resting length in a fully reversible process[12] due to the dynamic nature of the hydrophobic domains and the reversible organization of solvent molecules around these domains[13]. This interaction with water confers an entropic mechanism of elasticity, where stretching elastin decreases bulk entropy due to the increased organization of water around these domains; thus the maximum entropic level is restored when the protein recoils back to its original state [7,12]. The glass transition temperature (Tg) of elastin depends on its hydration. Dehydrated elastin has Tg of 200°C whereas 30% hydration decreases Tg to 30°C[14].
Tropoelastin is produced by bacterial overexpression [15]. Recombinant tropoelastin is used to generate materials that restore the function of damaged or diseased elastic tissues. The fabrication of elastin-based biomaterials generally involves coacervation and crosslinking of the precursor molecule. For example, tropoelastin proceeds to gel-sol transition under alkaline conditions, where tropoelastin forms a stable hydrogel without the need for further chemical crosslinking[16]. There are multiple alternative paths to stabilize tropoelastin hydrogels including chemical, physical, pH-based, photochemical and enzymatic crosslinking, to ensure its stability, durability and utility as a biomaterial for tissue engineering. Tropoelastin assemblies have been used in the fabrication of a range of biomaterials in diverse forms and shapes including sponges, hydrogels, electrospun mats and tubes [17-24].
Tropoelastin-based hydrogels can be prepared by chemical crosslinking using bis-sulfosuccinimidyl suberate (BS3) and glutaraldehyde [18,25,26] that benefit from the availability of 35 juxtaposed lysine residues across the molecule. Physical crosslinking has been used to produce elastic hydrogels. For example, methacrylated tropoelastin is formed through photo-initiated cross-linking. Using this approach, a highly stretchable and porous hydrogel with a high extensibility of 400% and tensile modulus of 2.8±0.6-14.8±1.9 kPa is produced [19]. This method allows for the fabrication of a range of biomaterials with different pore size, swelling and mechanical performance based on tropoelastin concentration and degree of methacrylation [19]. Methacrylated tropoelastin has also been used to generate micropatterned elastin-based hydrogels suitable for engineering of tissues like cardiac tissues where elasticity and organization of cells plays a central role [27]. In contrast, the solubility of tropoelastin in organic solvents like hexafluoroisopropanol facilitates the development of 3-dimensional elastomeric biomaterials using electrospinning techniques. Electrospun elastic mats and tubes made of tropoelastin have been stabilized chemically by crosslinkers selected from hexamethylene diisocyanate [20-22], glutaraldehyde [18,23] and disuccinimidyl suberate [24].
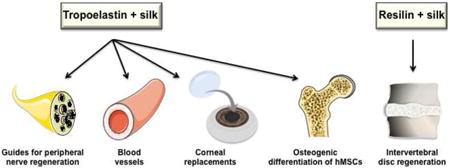
These tropoelastin-based constructs not only provide mechanical integrity and elasticity to multiple tissue types but also confers biological interactions including cell binding and signalling cues [5,17]. They can recruit and support the growth of diverse attachment cell types including dermal fibroblasts[18], stem cells[28] and cardiomyocytes [27]. On this basis, tropoelastin-based constructs have been investigated for engineering of vascular grafts, wound dressing materials, skin replacement and stem cell delivery for wound healing [18,20-24]. Tropoelastin and elastin-based materials have been recognised as non-thrombogenic and non-inflammatory biomaterials [29]. Bio-functionalization of metallic coronary stents with tropoelastin decreases thrombogenic responses to implanted stents [30].
Resilin and resilin-like polypeptides (RLPs)
Resilin, first described in locusts by Weis-Fogh, is present in the tissues of insects that require rapid elastic responses [3,31,32]. Thus resilin is important for insect jumping [33] and flight [32] and in organs that demand highly repetitive movement such as their specialized sound-producing organs. For this reason, resilin responds elastically at higher frequencies than elastin; in contrast, elastin typically operates around the heartbeat frequencies of mammals. The flexibility of resilin is due to the presence of repetitive amino acid sequences rich in proline and glycine. Natural resilin is cross-linked by the formation of di-and tri-tyrosine between tyrosine residues connecting resilin polypeptides [13,34,35].
Resilin is a highly elastomeric, rubber-like protein with high elasticity and resilience[13,31]. The cross-linked protein can be extended up to 3-4 times its size and can return to its original state [36]. Natural resilin has an elastic modulus of 600-2000 kPa [32,37,38]. Like elastin, resilin is brittle and stiff when dry but after hydration, it becomes soft and elastic. The elasticity of resilin is directly proportional to the level of hydration [39]. Resilin is stable when heated up to 125°C and can withstand vibrations with the frequency of 4 kHz [3,32,40]. It is insoluble in water and nonpolar solvents [32].
The resilin precursor CG15920 in Drosophila melanogaster consists of a signal peptide sequence and three main exons[41]; after removal of the signal peptide, the pro-resilin is secreted to the extracellular space. Pro-resilin is uncross-linked and is composed of three main domains, N-terminal and C-terminal elastic domains encoded by exon 1 and exon 3 respectively and the chitin-binding domain encoded by exon 2 [34,41,42]. Recombinant production of resilin regions in bacteria yields 15 to 450 mg of protein [34]. Proteins produced by exon 1 showed higher elasticity than those produced from exon 3 and better mimic the elastic properties of the natural resilin [42]. Rec1-resilin (N-terminal domain of resilin) encoded by exon 1 of D. melanogaster is a water-soluble protein that has been produced in Escherichia coli. This protein can be cross-linked by formation of di-tyrosine crosslinking either chemically (peroxidase) or physically (ruthenium-mediated photo-crosslinking) resulting in stable elastomeric biomaterials. The cross-linked material is resilient and can be stretched up to 300% of its original length[13,39]. A photo-Fenton reaction is also used to crosslink the proteins produced by exon 1 or exon 3 of the CG15920 gene, which results in a rubber like and highly adhesive materials [42].
In order to improve the biological properties of resilin, RLPs are produced by the addition of other sequences to the resilin consensus sequences. Charati et al. has reported RLP12, which contains 12 repeats of resilin consensus sequences from D. melanogaster, additional lysine residues outside the resilin repeat as crosslinking sites, the cell binding ligand RGDSP, heparin-binding domain and metalloproteinase sensitive domain to promote the proteolytic degradation [36,43,44]. Crosslinking of additional lysine residues in RLP12 by [tris (hydroxymethyl) phosphino] propionic acid via Mannich-type reaction results in hydrogels with elastic moduli of ∼ 15-60 kPa, an extensibility of 180%-335% and high-frequency responsiveness properties [36,43,45]. Renner et al. also have designed RLPs consists of 10 repeats of resilin consensus sequences from Anopheles gambiae (mosquito) and RGD as a bioactive domain (RZ10-RGD) [46].
Resilin-based materials have potential in tissue engineering applications like cardiovascular tissues[47], cartilage[46] muscles[48] and vocal fold[45] however, their biocompatibility and frequency response requirements needs to be assessed due the demand of adapting this elastic protein to the biology and mechanics of mammalian tissues.
Composite biomaterials
Both resilin and elastin are highly resilient and extensible proteins but lack the mechanical strength and stiffness range required for engineering most tissues. In order to improve their mechanical properties, these proteins have been used in combination with other natural or synthetic polymers to produce materials with a range of different mechanical, physical and biological properties to match different tissues types. Blended tropoelastin materials have been reviewed recently [26]. Here we focus on tropoelastin/silk- and resilin/silk- based hybrid materials.
Silk fibroins are fibrous proteins synthesized by the arthropods silkworms and spiders. The long history of silkworm silk means it is available abundantly through adapted textile technologies [49]. Silk fibroin lacks the resilience of elastin and resilin and the cell interaction benefits of tropoelastin but has high strength. Aqueous solutions of silk stabilize to an insoluble form by facilitated interactions between hydrophobic domains to form beta-sheet crystals. Beta-sheet formation is induced by a variety of techniques including autoclaving, vortexing, gelation, water annealing, methanol, heat, low pH or sonication [49-52].
Protein alloys: Tropoelastin- and silk-blended biomaterials
Composite materials made of tropoelastin and silk offer a new generation of materials with desired elasticity and strength. Since both silk fibroin and tropoelastin are composed of alternating hydrophobic and hydrophilic domains, where silk is negative and tropoelastin is positive respectively, they interact with each other through electrostatic and hydrophobic-hydrophilic interactions to form a novel family of stable protein alloys [52]. These strong interactions between the two types of protein chains, combined with physically induced beta-sheet crystallization, results in the formation of stable composite materials without the need for chemical crosslinking, and with properties that combine the benefits of each material [51-54]. In contrast, protein based materials like elastin and resilin typically require crosslinking through chemical, photochemical or enzyme for stabilization.
These protein alloys are primarily defined by their proportional blend of silk fibroin and tropoelastin, and so define a broad spectrum of materials with different mechanical, physical and biological properties [51-54]. Mechanical properties including stiffness and elasticity of produced materials are controlled by varying the ratio of the two proteins. The elastic moduli of these tropoelastin-silk protein alloys can be further tuned between 0.54±0.09 to 68.5±4.3 MPa by their protein content and the choice of crosslinking method [50,52]. These hybrid biomaterials can be made into multiple shapes and produced with controllable surface roughness [50,53]. The net charge of the protein alloy materials is also tuned by varying the proportion of the constituent proteins, which is important in controlling neural cell responses [51,52].
Composite materials with higher silk content offer materials with low surface roughness and high stiffness. These materials have been shown to induce the myogenic differentiation of C2C12 muscle cells. The osteogenic differentiation of human bone marrow stem cells is enhanced with a higher tropoelastin content and high surface roughness [50]. Increasing the tropoelastin content in tropoelastin-silk protein alloy films improves neural cell growth and neurite extension [51]; in contrast, pure silk and tropoelastin biomaterials do not support the attachment and growth of neural cells indicating the value in adjusting charge density of these materials in obtaining functional neurons [51,52,54]. These constructs offer considerable promise in protein-based promotion of neuronal performance, as evidenced by protein alloy films that show at least a doubling in neurite extension over poly-D-lysine as the in vitro standard for neuron growth [51,54]. Combined materials also support the growth of two main corneal cell types, epithelial and endothelial cells (B. Aghaei and A. S. Weiss, unpublished data). This family of protein alloys defines a new generation of biologically compatible materials with controllable mechanical, electrical and chemical properties [52,55].
Resilin/silk-based biomaterials
The construction and use of resilin-silk composite materials is a nascent field. Recently, Whittaker et al. developed a photocrosslinkable hydrogel composed of Rec1-resilin and regenerated silk fibroin [56,57]. This combination of Rec1 with high elasticity and water uptake with silk with high strength provides an opportunity to design materials with a range of properties that could emulate tropoelastin-silk successes but with an entirely arthropod-protein based solution. The incorporation of silk increases the storage modulus (E′) of these material from 0.002 MPa in a Rec1 hydrogel to ∼ 6.56 MPa in composite hydrogel [39,56]. There are indications that Rec1-silk materials may have potential applications in intervertebral disc regeneration due to their high water content and mechanical strength [56].
Conclusions
The elastomeric proteins tropoelastin and resilin have unique elastic properties. They are found in different tissues in mammals and insects respectively where high elasticity and repetitive movements are required, but with different frequency requirements. Tropoelastin-based materials benefit from cell attachment interactions and have been used to fabricate skin replacements, wound healing materials and blood vessels, while resilin-based materials have been investigated for the mechanical regeneration of cardiovascular tissues, muscles and vocal folds. Combinations of tropoelastin with silk fibroin has lead to a new class of protein alloy biomaterials with tunable mechanical and biological properties. Resilin combinations with silk are less explored. These protein alloys define a new class of biomaterials with adjustable mechanical, cell interactive, electrical and repair performance to match specific tissue needs.
Highlights.
Elastin and resilin are highly elastic proteins containing both mechanically and biologically active properties
Tropoelastin is assembled to make elastin. Tropoelastin- and resilin-based materials offer remarkable physical, mechanical and biological properties for tissue engineering applications
Combinations of these elastic proteins with silk generate a new class of biomaterials with tunable mechanical and biological properties
Acknowledgments
B.A. is the recipient of an Endeavour Scholarship Award. A.S.W. received grant support from the Australian Research Council and the National Health & Medical Research Council. Part of this research was funded by NIH EB014283 (A.S.W.). A.S.W. is the Scientific Founder of Elastagen Pty Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Tatham AS, Shewry PR. Comparative structures and properties of elastic proteins. Philos Trans R Soc Lond B Biol Sci. 2002;357:229–234. doi: 10.1098/rstb.2001.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, Xu J, Parker T. Elastin: a representative ideal protein elastomer. Philos Trans R Soc Lond B Biol Sci. 2002;357:169–184. doi: 10.1098/rstb.2001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Charati MB, Kiick KL. Elastomeric polypeptide-based biomaterials. J Polym Sci A Polym Chem. 2010;1:1160–1170. doi: 10.1039/b9py00346k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatham AS, Shewry PR. Elastomeric proteins: biological roles, structures and mechanisms. Trends Biochem Sci. 2000;25:567–571. doi: 10.1016/s0968-0004(00)01670-4. [DOI] [PubMed] [Google Scholar]
- 5.Werkmeister JA, Ramshaw JA. Recombinant protein scaffolds for tissue engineering. Biomed Mater. 2012;7:012002. doi: 10.1088/1748-6041/7/1/012002. [DOI] [PubMed] [Google Scholar]
- 6.Vrhovski B, Jensen S, Weiss AS. Coacervation characteristics of recombinant human tropoelastin. Eur J Biochem. 1997;250:92–98. doi: 10.1111/j.1432-1033.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 7.Vrhovski B, Weiss AS. Biochemistry of tropoelastin. Eur J Biochem. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- 8.Bellingham CM, Keeley FW. Self-ordered polymerization of elastin-based biomaterials. Current Opinion in Solid State and Materials Science. 2004;8:135–139. [Google Scholar]
- 9.Mithieux SM, Wise SG, Weiss AS. Tropoelastin--a multifaceted naturally smart material. Adv Drug Deliv Rev. 2013;65:421–428. doi: 10.1016/j.addr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Annabi N, Mithieux SM, Camci-Unal G, Dokmeci MR, Weiss AS, Khademhosseini A. Elastomeric Recombinant Protein-based Biomaterials. Biochem Eng J. 2013;77:110–118. doi: 10.1016/j.bej.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indik Z, Yeh H, Ornstein-Goldstein N, Sheppard P, Anderson N, Rosenbloom JC, Peltonen L, Rosenbloom J. Alternative splicing of human elastin mRNA indicated by sequence analysis of cloned genomic and complementary DNA. Proc Natl Acad Sci U S A. 1987;84:5680–5684. doi: 10.1073/pnas.84.16.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldock C, Oberhauser AF, Ma L, Lammie D, Siegler V, Mithieux SM, Tu Y, Chow JY, Suleman F, Malfois M, et al. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc Natl Acad Sci U S A. 2011;108:4322–4327. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 14.Samouillan V, Andre C, Dandurand J, Lacabanne C. Effect of water on the molecular mobility of elastin. Biomacromolecules. 2004;5:958–964. doi: 10.1021/bm034436t. [DOI] [PubMed] [Google Scholar]
- 15.Martin SL, Vrhovski B, Weiss AS. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin. Gene. 1995;154:159–166. doi: 10.1016/0378-1119(94)00848-m. [DOI] [PubMed] [Google Scholar]
- 16.Mithieux SM, Tu Y, Korkmaz E, Braet F, Weiss AS. In situ polymerization of tropoelastin in the absence of chemical cross-linking. Biomaterials. 2009;30:431–435. doi: 10.1016/j.biomaterials.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Mithieux SM, Rasko JE, Weiss AS. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25:4921–4927. doi: 10.1016/j.biomaterials.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Rnjak J, Li Z, Maitz PK, Wise SG, Weiss AS. Primary human dermal fibroblast interactions with open weave three-dimensional scaffolds prepared from synthetic human elastin. Biomaterials. 2009;30:6469–6477. doi: 10.1016/j.biomaterials.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Annabi N, Mithieux SM, Zorlutuna P, Camci-Unal G, Weiss AS, Khademhosseini A. Engineered cell-laden human protein-based elastomer. Biomaterials. 2013;34:5496–5505. doi: 10.1016/j.biomaterials.2013.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nivison-Smith L, Rnjak J, Weiss AS. Synthetic human elastin microfibers: stable cross-linked tropoelastin and cell interactive constructs for tissue engineering applications. Acta Biomater. 2010;6:354–359. doi: 10.1016/j.actbio.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Nivison-Smith L, Weiss AS. Alignment of human vascular smooth muscle cells on parallel electrospun synthetic elastin fibers. J Biomed Mater Res A. 2012;100:155–161. doi: 10.1002/jbm.a.33255. [DOI] [PubMed] [Google Scholar]
- 23.Machula H, Ensley B, Kellar R. Electrospun Tropoelastin for Delivery of Therapeutic Adipose-Derived Stem Cells to Full-Thickness Dermal Wounds. Adv Wound Care (New Rochelle) 2014;3:367–375. doi: 10.1089/wound.2013.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna KA, Hinds MT, Sarao RC, Wu PC, Maslen CL, Glanville RW, Babcock D, Gregory KW. Mechanical property characterization of electrospun recombinant human tropoelastin for vascular graft biomaterials. Acta Biomater. 2012;8:225–233. doi: 10.1016/j.actbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 26.Yeo GC, Aghaei-Ghareh-Bolagh B, Brackenreg EP, Hiob MA, Lee P, Weiss AS. Fabricated Elastin. Adv Healthc Mater. 2015 doi: 10.1002/adhm.201400781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater. 2013;23 doi: 10.1002/adfm.201300570. Demonstrated the fabrication of micropatterned elastin-based hydrogels using methacrylated tropoelastin. These highly elastic materials have the potential for engineering tissues like cardiac tissues where elasticity and organization of cells play a central role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozsvar J, Mithieux SM, Wang R, Weiss AS. Elastin-based biomaterials and mesenchymal stem cells. Biomater Sci. 2015;3:800–809. doi: 10.1039/C5BM00038F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waterhouse A, Wise SG, Ng MK, Weiss AS. Elastin as a nonthrombogenic biomaterial. Tissue Eng Part B Rev. 2011;17:93–99. doi: 10.1089/ten.TEB.2010.0432. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse A, Wise SG, Yin Y, Wu B, James B, Zreiqat H, McKenzie DR, Bao S, Weiss AS, Ng MKC, et al. In vivo biocompatibility of a plasma-activated, coronary stent coating. Biomaterials. 2012;33:7984–7992. doi: 10.1016/j.biomaterials.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 31.Weis-Fogh T. Molecular interpretation of the elasticity of resilin, a rubber-like protein. Journal of Molecular Biology. 1961;3:648–667. [Google Scholar]
- 32.Weis-Fogh T. A rubber-like protein in insect cuticle. Journal of Experimental Biology. 1960;37:889–907. [Google Scholar]
- 33.Burrows M. Biomechanics: Froghopper insects leap to new heights. Nature. 2003;424:509–509. doi: 10.1038/424509a. [DOI] [PubMed] [Google Scholar]
- 34.Su RS, Kim Y, Liu JC. Resilin: protein-based elastomeric biomaterials. Acta Biomater. 2014;10:1601–1611. doi: 10.1016/j.actbio.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Andersen SO. The cross-links in resilin identified as dityrosine and trityrosine. Biochim Biophys Acta. 1964;93:213–215. doi: 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- 36.Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL. Hydrophilic elastomeric biomaterials based on resilin-like polypeptides. Soft Matter. 2009;5:3412–3416. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weis-Fogh T. Thermodynamic properties of resilin, a rubber-like protein. Journal of Molecular Biology. 1961;3:520–531. [Google Scholar]
- 38.Gosline JM. The elastic properties of rubber-like proteins and highly extensible tissues. Symp Soc Exp Biol. 1980;34:332–357. [PubMed] [Google Scholar]
- 39.Truong MY, Dutta NK, Choudhury NR, Kim M, Elvin CM, Nairn KM, Hill AJ. The effect of hydration on molecular chain mobility and the viscoelastic behavior of resilin-mimetic protein-based hydrogels. Biomaterials. 2011;32:8462–8473. doi: 10.1016/j.biomaterials.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 40.Skals N, Surlykke A. Sound production by abdominal tymbal organs in two moth species: the green silver-line and the scarce silver-line (Noctuoidea: Nolidae: Chloephorinae) J Exp Biol. 1999;202:2937–2949. doi: 10.1242/jeb.202.21.2937. [DOI] [PubMed] [Google Scholar]
- 41.Ardell DH, Andersen SO. Tentative identification of a resilin gene in Drosophila melanogaster. Insect Biochem Mol Biol. 2001;31:965–970. doi: 10.1016/s0965-1748(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 42.Qin G, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL. Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials. 2011;32:9231–9243. doi: 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Kiick KL. Transient dynamic mechanical properties of resilin-based elastomeric hydrogels. Front Chem. 2014;2:21. doi: 10.3389/fchem.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Li L, Tong Z, Jia X, Kiick KL. Resilin-Like Polypeptide Hydrogels Engineered for Versatile Biological Functions. Soft Matter. 2013;9:665–673. doi: 10.1039/C2SM26812D. Demonstrated the production of new resilin-like polypeptides composed of 12 repeats of the putative resilin consensus sequence and a single biologically active domain. This allows for the fabrication of a range of materials with tailored mechanical and biological properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Teller S, Clifton RJ, Jia X, Kiick KL. Tunable mechanical stability and deformation response of a resilin-based elastomer. Biomacromolecules. 2011;12:2302–2310. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renner JN, Cherry KM, Su RS, Liu JC. Characterization of resilin-based materials for tissue engineering applications. Biomacromolecules. 2012;13:3678–3685. doi: 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- 47.McGann CL, Levenson EA, Kiick KL. Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering. Macromolecules. 2013;214:203–213. doi: 10.1002/macp.201200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li H. Designed biomaterials to mimic the mechanical properties of muscles. Nature. 2010;465:69–73. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- 49.Hu X, Cebe P, Weiss AS, Omenetto F, Kaplan DL. Protein-based composite materials. Materials Today. 2012;15:208–215. [Google Scholar]
- 50.Hu X, Park SH, Gil ES, Xia XX, Weiss AS, Kaplan DL. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials. 2011;32:8979–8989. doi: 10.1016/j.biomaterials.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **51.White JD, Wang S, Weiss AS, Kaplan DL. Silk-tropoelastin protein films for nerve guidance. Acta Biomater. 2015;14:1–10. doi: 10.1016/j.actbio.2014.11.045. Bio degradable thin film materials made of tropoelastin- silk blend were developed as promising candidates for peripheral nerve repair. Increasing the tropoelastin content improved Schwann cell growth and neurite extension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Hu X, Tang-Schomer MD, Huang W, Xia XX, Weiss AS, Kaplan DL. Charge-Tunable Silk-Tropoelastin Protein Alloys That Control Neuron Cell Responses. Adv Funct Mater. 2013;23:3875–3884. doi: 10.1002/adfm.201202685. Demonstrated the fabrication of a new group of biomaterials composed of tropoelastin and silk with tuned mechanical, biological and net charge properties. These materials can be utilized as a biomaterial platform to control neuron cell response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31:8121–8131. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghezzi CE, Rnjak-Kovacina J, Weiss AS, Kaplan DL. Multifunctional silk-tropoelastin biomaterial systems. Isr J Chem. 2013;53:777–786. doi: 10.1002/ijch.201300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, Wise SG, Rnjak-Kovacina J, Kaplan DL, Bilek MM, Weiss AS, Fei J, Bao S. Biocompatibility of silk-tropoelastin protein polymers. Biomaterials. 2014;35:5138–5147. doi: 10.1016/j.biomaterials.2014.03.024. [DOI] [PubMed] [Google Scholar]
- *56.Whittaker J, Dutta N, Elvin C, Choudhury N. Fabrication of highly elastic resilin/silk fibroin based hydrogel by rapid photo-crosslinking reaction. Journal of Materials Chemistry B. 2015 doi: 10.1039/c5tb00970g. The first study that uses resilin and silk in combination to fabricate elastomers with tailored mechanical and biological properties. [DOI] [PubMed] [Google Scholar]
- 57.Whittaker JL, Choudhury NR, Dutta NK, Zannettino A. Facile and rapid ruthenium mediated hoto-crosslinking of Bombyx mori silk fibroin. Journal of Materials Chemistry B. 2014;2:6259–6270. doi: 10.1039/c4tb00698d. [DOI] [PubMed] [Google Scholar]


