Abstract
Chlorophylls are essential for photosynthesis and also one of the most abundant pigments on earth. Using the optofluidic ring resonator of extremely high Q-factors (>107), we investigated unique characteristics and the underlying mechanism of chlorophyll lasers. The chlorophyll lasers with dual lasing bands at 680 nm and 730 nm were observed for the first time in isolated chlorophyll a (Chla). Particularly, laser at the 730 nm band was realized in 0.1 mM Chla with a lasing threshold of only 8 μJ/mm2. Additionally, we observed lasing competition between the two lasing bands. The presence of the laser emission at the 680 nm band can lead to quenching or significant reduction of laser emission at the 730 nm band, effectively increasing the lasing threshold for the 730 nm band. Further concentration dependent studies, along with the theoretical analysis, elucidated the mechanism that determines when and why the laser emission band appears at one of the two bands, or concomitantly at both bands. Finally, Chla was exploited as the donor in fluorescence resonance energy transfer to extend the laser emission into the near infrared regime with an unprecedented wavelength shift as large as 380 nm. Our work will open a door to the development of novel biocompatible and biodegradable chlorophyll based lasers for various applications such as miniaturized tunable coherent light sources and in-vitro/in-vivo biosensing. It will also provide important insight into the chlorophyll fluorescence and photosynthesis processes inside plants.
Keywords: Chlorophyll, lasers, ring resonator, FRET
Introduction
The optofluidic laser has recently emerged as a new technology to integrate microfluidics, optical microcavity, and gain medium in the liquid environment1-9. The broad applications of the optofluidic laser include wavelength-tunable coherent light sources on chip10-13 and highly sensitive in-vitro/in-vivo biochemical analysis using the intra-cavity detection method1,14-21. To date, many gain media have been employed in the optofluidic laser, such as organic dyes2,23, water-soluble quantum dots24-26, and biomaterials (fluorescent proteins27,28, vitamins29, and luciferins30, etc.), and products of enzyme-substrate reaction31).
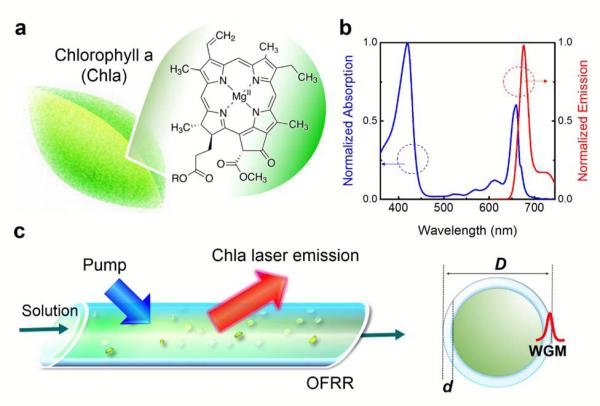
Chlorophylls (Fig. 1a) are one of the most abundant and common pigments on earth32. They are not only essential for photosynthesis and genetic engineering, but also particularly important for regulating metabolic functions in living organisms33,34. Chlorophyll fluorescence has been extensively studied for fundamental understanding of energy transfer mechanisms inside chloroplasts35,36. Two bands of emission corresponding to the (0,0) and (0,1) vibronic regions of the S1→S0 transition are usually observed37,38 (see also Fig. 1b and Fig. S1). The quantum yield of most chlorophylls is approximately 30%39,40, similar to that of other organic dyes such as Cy5, Cy5.5, and Alexa Fluor 647. The strong fluorescent capability of chlorophylls suggest that they can possibly be used as the laser gain medium. In turn, stimulated emission of chlorophylls provides insight into the energy transfer processes during photosynthesis. It can also be used to better understand the S1 electronic states and interpret fluorescence results (such as intensity dependent fluorescence lifetime and quantum yield)41.
Figure 1.
(a) Molecular structure of chlorophyll a (Chla) extracted from spinach leaves. (b) Normalized absorption and emission spectrum of Chla in ethanol. (c) Schematic diagram of the chlorophyll laser using a high Q-factor optofluidic ring resonator (OFRR). Green particles represent the chlorophyll molecules dissolved in ethanol. The laser was excited by a pulsed optical parametric oscillator (OPO) (pulse width=5 ns; wavelength=430 nm). The diagram on the right side shows the profile of the OFRR and the whispering gallery mode (WGM) circulating along the OFRR circumference. D=80 μm; d=2 μm.
In comparison with many other laser gain media, chlorophylls are biocompatible and biodegradable, making them very attractive in in-vitro and in-vivo sensing applications. Meanwhile, the unique dual-absorption bands in the visible spectrum (Fig. 1b) with extremely high extinction coefficients (~105 M−1cm−1 at 430 nm) render chlorophylls excellent light harvesting capability to achieve lower lasing thresholds. This characteristic, together with the large wavelength shift between absorption and emission, suggests that chlorophylls can serve as an excellent donor in a fluorescence resonance energy transfer (FRET) laser, which allows implementation of a laser in the red or near infrared (NIR) spectrum with blue or ultra-violet (UV) excitation.
The laser of chlorophylls was briefly studied nearly 40 years ago using a low quality optical cavity based on a 1-cm long cuvette41, whose Q-factor (Q) and finesse (F) were only 1.3×105 and 6, respectively. However, while laser emission at the 680 nm band was observed, which corresponds to the (0,1) vibronic region, no laser emission was found around 730 nm and the mechanism behind the missing 730 nm band was not fully understood. Here we investigated both experimentally and theoretically the optofluidic laser using chlorophyll a (Chla) as the gain medium and a thin-walled glass capillary based optofluidic ring resonator (OFRR, Fig. 1c) as the laser microcavity. The purposes of this work were two-fold. First, we were aimed to develop novel chlorophyll based optofluidic lasers using chlorophyll as the outstanding gain medium and the donor. Second, we took advantage of the extremely high Q-factor (>107) and finesse (F~104) of the OFRR to study unique characteristics and reveal the unresolved underlying mechanism of chlorophyll lasers.
Due to the high Q-factor, a new laser emission band around 730 nm with a lasing threshold as low as 8 μJ/mm2 was achieved for 0.1 mM Chla. The second laser emission band around 680 nm was realized with much higher excitation. In addition, competition between the lasing at the 680 nm and 730 nm band was observed. It is found that lasing at the 680 nm band can quench or significantly reduce the laser emission at 730 nm band, effectively increasing the lasing threshold for the 730 nm band. Further concentration dependent studies, along with the detailed theoretical analysis, elucidated the mechanism that determines when and why the laser emission band appears at 680 nm or 730 nm, or concomitantly at both wavelengths. Finally, Chla was exploited as the donor in FRET to extend the optofluidic laser emission into the near infrared regime with an unprecedented wavelength shift as large as 380 nm, respectively and lasing threshold lower than 0.5 μJ/mm2. Our work will open a door to the development of novel biocompatible and biodegradable chlorophyll based lasers for various applications such as on-chip tunable coherent light sources and in-vitro/in-vivo biosensing. It will also provide important insight into the chlorophyll fluorescence and photosynthesis processes inside plants and help resolve critical issues in plant biology.
Experimental
The experimental setup of the chlorophyll laser based on the OFRR is illustrated in Fig. 1c (see details in Fig. S2). Here Chla was chosen as the gain medium due to its primary role in photosynthesis. Chlorophyll a was purchased from Sigma-Aldrich (Product #C5753). It is known that Chla is dissolved in organic solvents, such as ethanol and acetone, not in water41,42. Therefore in our experiments, we chose ethanol as the solvent. Both AF680 and AF700 dyes were purchased from Thermo-Fisher (Product #A37574 & Product #A-20110). Each dye (powder) was first dissolved in ethanol (99.9%, Sigma-Aldrich) to form a 10 mM solution, and then diluted with ethanol to lower concentrations. For FRET lasing experiments, the compound solutions were prepared by mixing 10 mM Chla with 10 mM AF680 solutions (and 10 mM AF700 solutions) to achieve the desired Chla-AF680 and Chla-AF680-AF700 concentrations.
In our experiments, Chla dissolved in ethanol was flowed through the OFRR. The whispering gallery modes (WGMs) circulate along the circumference of the OFRR circular cross section (Fig. 1c, right). The evanescent field of the WGM in the OFRR core interacts with the gain medium (Chla) and provides the optical feedback for lasing43. Fabrication of the OFRR have been well documented elsewhere43-45. Briefly, a fused silica capillary preform (Polymicro Technologies TSP700850) was first etched with diluted hydrofluoric acid and then rapidly stretched under CO2 laser irradiation. The resulting OFRR capillary was slightly bulged with a diameter of 80 μm at the center and of a few microns smaller at the two necking points approximately 1 mm apart. The wall thickness of the OFRR was approximately 2-4 μm and the Q-factor is approximately 107 43,46. A typical confocal setup was used to excite the sample and collect emission light from the OFRR (Fig. S2). The pump intensity was adjusted by a continuously variable neutral density filter. The emission light was collected through the same lens and sent to a spectrometer (Horiba iHR550) for analysis.
Results and discussion
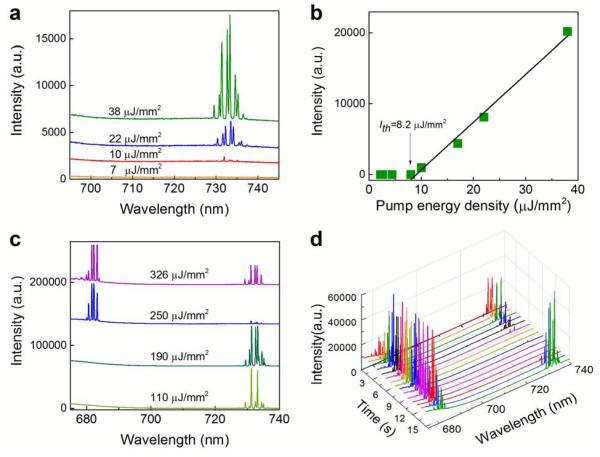
Fig. 2a demonstrates the lasing emission spectra of Chla at a concentration of 0.1 mM under low pump energy densities. Initially, a number of periodic lasing peaks emerge around 730 nm. The multiple lasing peaks are the result of multi-mode nature of the WGMs. With the increased pump energy density, those lasing peaks grow accordingly, but remain within the same spectral region around 730. Fig. 2b plots the total laser emission in the 730 nm band as a function of the pump energy density, from which the lasing threshold is derived to be approximately 8.2 μJ/mm2. Such a low lasing threshold at a low Chla concentration results from the extremely high Q-factor of the OFRR (>107)43,46,47. Lasing emission gradually levels off as the pump intensity is beyond 110 μJ/mm2. During experiments, the Chla laser at the 730 nm band exhibits high photostability when the pump energy density is below 100 μJ/mm2, suggesting practical use of the Chla laser. However, as the pump energy density reaches 250 μJ/mm2, the lasing emission at the 730 nm band suddenly vanishes and meanwhile the lasing emission at shorter wavelengths starts to emerge around 680 nm. This extraordinary phenomenon can clearly be seen in Fig. 2c that plots the Chla lasing emission spectra under various high pump energy densities. Similar to the lasing profile at the 730 nm band, periodic lasing peaks grow but remain consistently within the same spectral region around 680 nm with the increased pump energy density. Interestingly, at very high pump energy densities, the lasing peaks at the 730 nm band start to re-emerge and compete with those at the 680 nm band. No other lasing emission bands are observed between the two competing bands. In order to better illustrate the competition between the two lasing bands, Fig. 2d shows the lasing profile recorded over a time interval of 15 seconds. Due to conservation of the Chla in the excited states, competition in emission between two laser bands can be clearly observed under a constant pump energy density. According to our experiments, the corresponding lasing threshold is approximately 230 μJ/mm2 for the laser emission at the 680 nm band.
Figure 2.
(a) Lasing spectra of 0.1 mM Chla in ethanol with relatively low pump energy densities. Curves are vertically shifted for clarity. (b) Spectrally integrated laser output as a function of pump energy density extracted from the spectra in (a). The solid line is the linear fit above the lasing threshold, showing a laser threshold of ~8.2 μJ/mm2. Spectral integration takes place between 728 and 738 nm. (c) Lasing spectra of 0.1 mM Chla in ethanol with relatively high pump energy densities. Curves are vertically shifted for clarity. (d) Competition emission between two laser bands under a constant pump energy density of 326 μJ/mm2. Different colors represent the measured lasing spectra at different timing (seconds).
The above results reveal important characteristics of chlorophyll lasers. First, there exist two separate lasing bands at 680 nm and 730 nm in Chla lasers, which correspond to the (0,0) and (0,1) vibronic regions in the S1→S0 transition (Fig. S1). Second, lasing emission emerges at the 730 nm band first. With the increased pump energy density, lasing emission at the 680 band becomes dominant with concomitant significant reduction in lasing emission at the 730 nm band. Third, at very high pump energy densities, lasing emission at the 730 nm band re-emerges and competes with that in the 680 nm band. The above phenomenon is in sharp contrast to the previous observation four decades ago41. In that study while the 680 nm lasing band was observed, stimulated emission at the 730 nm band was never achieved, even at high chlorophyll concentration (2 mM). Although not completely understood, the disappearance of the 730 nm band was attributed to the formation of non-fluorescent Chla dimers at high concentrations. However, as shown in Fig. S3 the 730 nm lasing band can still be observed even with 5 mM Chla in our experiment.
To resolve the contradictory experimental observations and elucidate the underlying lasing mechanisms of Chla lasers, we carried out the following theoretical analysis. At the lasing threshold, we have48
| (1) |
where nT is the total concentration of Chla and n1 is the concentration of the Chla in the excited state (S1).σe (λL) and σ a (λL) are the Chla emission and absorption cross sections at the lasing wavelength (λL), η is the fraction of the light in the evanescent field of the OFRR, Q0 is the OFRR empty-cavity Q-factor, and m is the effective refractive index of the WGM (m~1.4 for the OFRR). Rewriting Eq. (1) we obtain the fractional Chla at the excited state, γth:
| (2) |
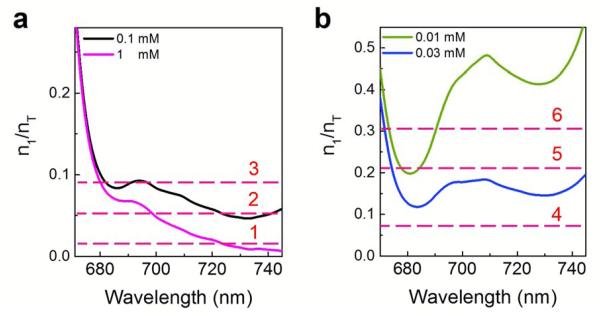
Figure 3 plots γth values for various representative concentrations of Chla based on Eq. (2), in which the absorption and emission cross sections were measured in Section III (Fig. S4) of the Supporting Information, under the assumption of a high Q-factor (ηQ0=1×106)46. At relatively high Chla concentrations (Fig. 3a), two γth minima around 680 nm and 730 nm are found, but γth at the 730 nm band is much lower than that at the 680 nm band, indicating that the laser emission emerges around 730 nm first. Below we use the 0.1 mM Chla in Fig. 3a as an example to elucidate the Chla lasing mechanism. When the excitation is low (Curve 1 in Fig. 3a), no laser emission can be observed. With the increased excitation (Curve 2), the 730 nm band is the first to reach the lasing threshold, and consequently, lasing emission emerges at this band. When the excitation continues to increase (Curve 3), the 680 nm band reaches the lasing threshold and starts to lase. Since the number of the excited states is fixed at a given pump energy density, lasing action at the 680 nm band results in quenching or significant reduction in lasing emission at the 730 nm, effectively increasing the lasing threshold for the 730 nm band. Finally, at very high pump energy density, the laser emission at the 680 nm band continues to grow and meanwhile the 730 band laser emission re-emerges and competes with the 680 nm band. The above simulation agrees well with the experimental observations in Figs. 2a and 2c. It is interesting to note that laser emission at the 680 nm band can easily overtake that at 730 nm, despite the fact that γth is lower at 730 nm than at 680 nm. This unconventional phenomenon is due to the fact that the (0,1) vibronic band at 680 nm in Chla, which is populated first before further relaxation down to the (0,0) vibronic band at 730 nm. Once the lasing threshold at the 680 nm band is reached, very rapid population depletion occurs due to stimulated emission between S1 and S0, thus making it much more difficult for the 730 nm band to achieve population inversion.
Figure 3.
(a) and (b) Fraction of Chla molecules in the excited states needed at the laser threshold for various representative Chla concentrations based on Eq. (2) with η=0.1 and Q0=107. Curves 1-6 corresponds to various excitation levels of Chla molecules. Curve 1: At a very low pump energy density, the Chla excitation is quite low. No laser emission is expected to emerge for 0.1 mM Chla. Curve 2: At an intermediate pump energy density, the lasing emission at the 730 nm band emerges for 0.1 mM Chla. Curve 3: At a relatively high pump energy density, both 680 nm and 730 nm bands emerge and compete with each other. Curve 4: At a low pump energy density, the Chla excitation is low. No laser emission is expected to emerge for either 0.03 mM or 0.01 mM Chla. Curve 5: At an intermediate pump energy density, two lasing bands are expected to appear around 680 nm and 730 nm for 0.03 mM Chla, and only one lasing band is expected to appear around 680 nm. Curve 6: With further increased pump energy density, the lasing emission increases in comparison with the case for Curve 5, but no laser emission is expected to appear at the 730 nm band for 0.01 mM Chla due to the requirement for extremely high Chla excitation.
To further understand the lasing mechanism in Chla, in Fig. 3b we plot γth for two very low Chla concentrations. Both curves show two minima at 680 nm and 730 nm, respectively. In contrast to Fig. 3a, γth is lower at the 680 nm than at the 730 nm, indicating that the laser emission should occur around 680 nm first and play a dominant role in overall lasing action. At low excitation (Curve 4 in Fig. 3b), no lasing emission is expected for either 0.03 mM or 0.01 mM Chla. With the increased excitation, 0.03 mM Chla should start to lase at the 680 nm band. When the excitation continues to increase (Curve 5), laser emission should be observed at both the 680 nm and 730 nm band for 0.03 mM Chla, whereas only laser emission at the 680 nm band can be observed for 0.01 mM Chla. Finally, at very high excitation (Curve 6), laser emission from 0.03 mM Chla continues to increase at both the 680 nm and 730 nm band, whereas only laser emission at the 680 nm band can be observed for 0.01 mM Chla.
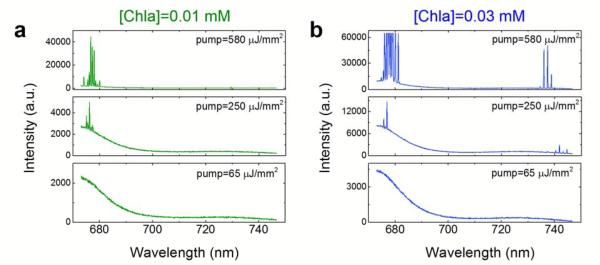
To validate the above theoretical analysis and simulation results at low Chla concentrations, the measured lasing spectra of 0.01 mM and 0.03 mM under different pump energy densities are exemplified in Figs. 4a and b, respectively. We chose three pump energy densities (65 μJ/mm2, 250 μJ/mm2, and 580 μJ/mm2) to represent the low, intermediate, and high Chla excitation described by Curves 4-6 in Fig. 3b. Note that Curves 4-6 plotted in Fig. 3b are only representatives of different excitation levels and not equivalent to the exact excitation shown in Fig. 4. Initially, no lasing emission is observed for either 0.01 mM or 0.03 mM Chla, as shown by 65 μJ/mm2 in Figs. 4a and b. When the pump energy density reaches 250 μJ/mm2, two lasing bands emerge for 0.03 mM Chla; however, only one lasing band around 680 nm appears for 0.01 mM Chla. At 580 μJ/mm2, laser emission around 680 nm and 730 nm continues to grow for 0.03 mM Chla. In contrast, for 0.01 mM Chla despite intensity increase in the 680 nm band, no laser emission is observed at 730 nm, as it requires much higher pump energy density. The experimental observation in Fig. 4 matches perfectly the phenomena described by Curves 4-6 in Fig. 3b.
Figure 4.
Lasing spectra for 0.01 mM (a) and 0.03 mM (b) Chla under the pump energy density of 65 μJ/mm2, 250 μJ/mm2, and 580 μJ/mm2. The chosen three pump energy densities correspond to the low, intermediate, and high Chla excitation described by Curves 4-6 in Fig. 3.
Based on the above theoretical analysis and experimental observation, it becomes clear that there exist two lasing band around 680 nm and 730 nm for Chla. Depending on the Q-factor and the Chla concentration, either 680 nm or 730 nm band can start to lase first. In addition, there exists competition between the lasing at the 680 nm band and 730 nm band. Lasing at the 680 nm band can quench or significantly reduce the laser emission at 730 nm band, effectively raising the lasing threshold for the 730 nm band. The reason that the 680 nm overtakes the 730 nm is due to the fact that the 680 nm is populated first during the relaxation process in chlorophylls upon photo-excitation. Capability to control which band to lase first and observation of the competition between the two bands will have significant impact on optofluidic laser development and provide vital information to better understand the fluorescence and photosynthesis processes in plants.
Previous work showed lasing emission around 680 nm, but failed to achieve lasing emission at the 730 nm band. This was actually due to the low Q-factor cavity (1-cm long cuvette) and the competition between the 680 nm and the 730 nm band. Fig. S5 plots γth for the Chla concentration ranging from 0.1 mM to 2 mM with a low Q-factor. It can be seen easily at the 680 nm band dominates for all concentrations of interest, in particular, at relatively low concentrations (0.1 mM – 0.5 mM). At a high concentration of 2 mM, γth at the 730 nm is close to that at the 680 nm band and lasing at 730 nm is seemingly possible. However, due to the competition of the 680 nm band lasing, the lasing threshold for the 730 nm band is actually much higher than theoretically predicted in all concentrations. Furthermore, the penetration depth was only about 200 μm at the excitation wavelength of 337.1 nm for 2 mM Chla41, which made the 730 nm lasing emission even more difficult to realize for Chla solution in a 1-cm long cuvette. Previous studies attributed the disappearance of the 730 nm band lasing to the formation of non-fluorescent Chla dimers at 2 mM, which we believe does not reflect the actual underlying mechanism. As a control experiment, lasing at the 730 nm band from 5 mM Chla can easily be achieved in Fig. S3 with relatively low pump energy density using our high Q-factor OFRR (over two orders of magnitude lower than the maximal pump energy density used in Ref. 41).
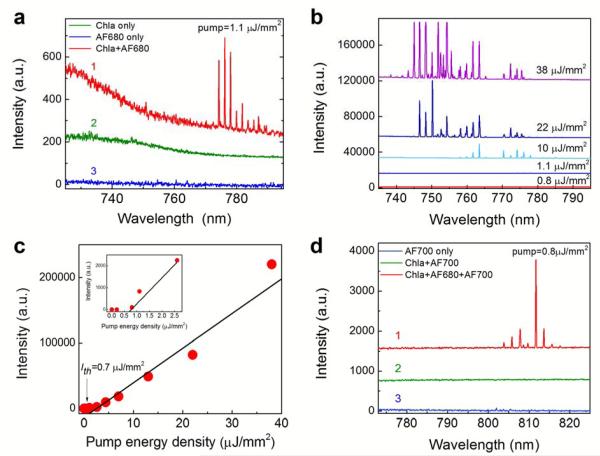
In order to further explore the unique properties of chlorophylls such as high absorption at blue and UV spectrum and large wavelength shift between absorption and emission, we developed and studied an optofluidic chlorophyll FRET laser using chlorophyll and dye as the donor and acceptor, respectively. In our experiments, Chla and Alexa Fluor 680 dye (AF680) were chosen to be the FRET pair (see Fig. S6 for spectral overlap between Chla and AF680). Curve 1 in Fig. 5a shows the FRET lasing emission around 780 nm under a very low pump energy density of 1.1 μJ/mm2 at 430 nm. Meanwhile, only featureless emission spectra are observed for Chla and AF680, as exemplified in the Curve 2 and Curve 3, respectively. In fact, the detailed control experiment performed in Fig. S7 shows that AF680 alone has a lasing threshold of 31 μJ/mm2. Therefore, the lasing emission at 780 nm at 1.1 μJ/mm2 is from AF680 under FRET excitation, which extends the lasing emission to the NIR. We further investigated the FRET laser emission spectra at different pump energy densities, as shown in Fig. 5b. A significant blue-shift of the FRET lasing peaks is observed as the pump intensity increases, which is typical for dye lasers46. Note that at 38 μJ/mm2, the AF680 alone can lase through direct excitation at 430 nm (see Fig. S7). However, such laser emission (around 780 nm based on Fig. S7a) can be easily distinguished from that via FRET (around 750 nm according to Fig. 5b). The spectrally integrated FRET emission versus pump energy density for Chla-AF680 is plotted in Fig. 5c, in which the lasing threshold is derived to be as low as 0.7 μJ/mm2 from the inset in Fig. 5c, which is 40 times lower than that of AF680 alone (Fig. S7b).
Figure 5.
(a) Comparison of the lasing spectra of mixture of Chla-AF680 in ethanol (Curve 1), Chla alone in ethanol (Curve 2), and Alexa Fluor 680 (AF680) alone in ethanol (Curve 3) under the same pump energy density of 1.1 μJ/mm2. (b) Lasing spectra of AF680 in ethanol via energy transfer from Chla under various pump energy densities. In (a) and (b), [Chla]=5 mM and [AF680]=5 mM for all curves. Excitation wavelength=430 nm. Curves are vertically shifted for clarity. (c) Spectrally integrated (745 nm – 790 nm) FRET laser output as a function of pump energy density extracted from (b). The inset presents the enlarged portion for the pump energy density below 3.0 μJ/mm2. Solid lines are the linear fit, showing a lasing threshold of approximately 0.7 μJ/mm2. (d) Emission spectra of mixture of Chla-AF680-AF700 (Curve 1), mixture of Chla-AF700 (Curve 2), and Alexa Fluor (AF700) dye alone (Curve 3) under the same pump energy density of 0.8 μJ/mm2. [Chla]=5 mM, [AF680]=5 mM, and [AF700]=5 mM for all curves. Excitation wavelength=430 nm. Curves are vertically shifted for clarity.
In order to extend the laser emission further into the NIR, a third dye (Alexa Fluor 700 - AF700) was used in conjunction with Chla and AF680 (see Fig. S6 for the spectral overlap among Chla, AF680, and AF700) to form a cascade FRET laser, in which the pump energy is first absorbed by Chla, subsequently transferred to AF680, and finally to AF700. Curve 1 in Fig. 5d shows the cascade FRET laser emission from AF700 at a pump energy density of 0.8 μJ/mm2. Multiple lasing peaks around 810 nm, which represent a spectral shift over 380 nm, are obtained. Further pump energy density dependent experiment in Fig. S8a shows that the lasing threshold is approximately 0.5 μJ/mm2. To confirm the laser emission was indeed from AF700 via cascade FRET, two control experiments were carried out. Curve 2 in Fig. 5d shows no laser emission from AF700 in the absence of AF680 due to insufficient energy transfer between Chla and AF700 caused by small spectral overlap. Curve 3 further shows that no laser emission from AF700 alone at 0.8 μJ/mm2, since its lasing threshold is 1.8 μJ/mm2 (Fig. S8b), 3.6 times higher than that obtained in Curve 1. Finally, to further demonstrate the versatility of the cascade FRET laser, in Fig. S9 we replaced AF700 with DyLight 700 (Dyl700) and achieved Dyl700 laser emission around 810 nm.
Conclusions
We have investigated the chlorophyll based optofluidic laser, in which two competing lasing bands at the 680 nm and 730 nm were observed for the first time. The lasing threshold for the 730 nm as low as 8 μJ/mm2 was achieved for 0.1 mM Chla. Furthermore, we found that the lasing emission at the 680 nm band results in the quenching or significant reduction in the lasing emission at the 730 nm, effectively increasing its lasing threshold. The theoretical analysis and experimental measurement revealed the detailed mechanism that determines when and why the laser emission band appears at 680 nm or 730 nm, or concomitantly at both wavelengths. In addition, using Chla as the donor, we have achieved FRET laser at the NIR with a wavelength shift as large as 380 nm.
We envision that our work lead to the development of novel biocompatible optofluidic devices and optofluidic FRET lasers with low lasing thresholds and large wavelength shifts. The ability to control the laser emission band will enable us to engineer and optimize the optofluidic lasers for various applications. Our work can further be applied to energy harvesting, solar lighting49,50, and many other optofluidic applications51,52. Our work will also help resolve critical issues in plant biology, such as the role of stimulated emission in chlorophyll fluorescence and photosynthesis.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge support from the National Institutes of Health (NIBIB-1R21EB016783).
Footnotes
AUTHOR CONTRIBUTIONS. Y.C. and X.F. conceived the research; Y.C., Q.C., and X.F. designed the experiments; Y.C. and Q.C. performed the experiments; Y.C., Q.C., and X.F. analyzed data; and Y.C., Q.C., and X.F. wrote the paper.
References
- 1.Sun Y, Shopova SI, Wu C-S, Arnold S, Fan X. Bioinspired optofluidic FRET lasers via DNA scaffolds. Proc. Natl. Acad. Sci. USA. 2010;107:16039–16042. doi: 10.1073/pnas.1003581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Lee W, Fan X. Bio-switchable Optofluidic Lasers Based on DNA Holliday Iunctions. Lab Chip. 2012;12:3673–3675. doi: 10.1039/c2lc40183e. [DOI] [PubMed] [Google Scholar]
- 3.Suter JD, Lee W, Howard DJ, Hoppmann E, White IM, Fan X. Demonstration of the coupling of optofluidic ring resonator lasers with liquid waveguides. Opt. Lett. 2010;35:2997–2999. doi: 10.1364/OL.35.002997. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Ritt M, Sivaramakrishnan S, Sun Y, Fan X. Optofluidic lasers with a single molecular layer of gain. Lab Chip. 2014;14:4590–4595. doi: 10.1039/c4lc00872c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan X, Yun S-H. The potential of optofluidic biolasers. Nature Methods. 2014;11:141–147. doi: 10.1038/nmeth.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Zhou C, Zhang T, Chen J, Liu S, Fan X. Optofluidic laser array based on stable high-Q Fabry-Perot microcavities. Lab Chip. 2015;15:3862–3869. doi: 10.1039/c5lc00847f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aas M, Jonáš A, Kiraz A. Lasing in optically manipulated, dye-doped emulsion microdroplets. Opt. Commun. 2013;290:183–187. [Google Scholar]
- 8.Chandrahalim H, Chen Q, Said AA, Dugan M, Fan X. Monolithic optofluidic ring resonator lasers created by femtosecond laser nanofabrication. Lab Chip. 2015;15:2335–2340. doi: 10.1039/c5lc00254k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubry G, Kou Q, Soto-Velasco J, Wang C, Meance S, He JJ, Haghiri-Gosnet AM. A Multicolor Microfluidic Droplet Dye Laser with Single Mode Emission. Appl. Phys. Lett. 2011;98:111111. [Google Scholar]
- 10.Li Z, Zhang Z, Scherer A, Psaltis D. Mechanically tunable optofluidic distributed feedback dye laser. Opt. Express. 2006;14:10494–10499. doi: 10.1364/oe.14.010494. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Liu AQ, Lei L, Chin LK, Ohl CD, Wang QJ, Yoon HS. A tunable 3D optofluidic waveguide dye laser via two centrifugal Dean flow streams. Lab Chip. 2011;11:3182–3187. doi: 10.1039/c1lc20435a. [DOI] [PubMed] [Google Scholar]
- 12.Lee W, Li H, Suter JD, Reddy K, Sun Y, Fan X. Tunable single mode lasing from an on-chip optofluidic ring resonator laser. Appl. Phys. Lett. 2011;98:061103. [Google Scholar]
- 13.Gersborg-Hansen M, Kristensen A. Tunability of optofluidic distributed feedback dye lasers. Opt. Express. 2007;15:137–142. doi: 10.1364/oe.15.000137. [DOI] [PubMed] [Google Scholar]
- 14.Gather MC, Yun SH. Single-cell biological lasers. Nature Photon. 2011;5:406–410. [Google Scholar]
- 15.Humar M, Yun SH. Intracellular microlasers. Nature Photon. 2015;9:572–576. doi: 10.1038/nphoton.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Ozdemir K, Zhu J, Kim W, Yang L. Detecting single viruses and nanoparticles using whispering gallery microlasers. Nature Nanotechnol. 2011;6:428–432. doi: 10.1038/nnano.2011.99. [DOI] [PubMed] [Google Scholar]
- 17.Bog U, Laue T, Grossmann T, Beck T, Wienhold T, Richter B, Hirtz M, Fuchs H, Kalt H, Mappes T. On-chip microlasers for biomolecular detection via highly localized deposition of a multifunctional phospholipid ink. Lab Chip. 2013;13:2701–2707. doi: 10.1039/c3lc50149c. [DOI] [PubMed] [Google Scholar]
- 18.Lu T, Lee H, Chen T, Herchak S, Kim JH, Fraser SE, Flagan RC, Vahala K. High sensitivity nanoparticle detection using optical microcavities. Proc. Natl. Sci. Acad. USA. 2011;108:5976–5979. doi: 10.1073/pnas.1017962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Khaing Oo MK, Reddy K, Chen Q, Sun Y, Fan X. Optofluidic laser for dual-mode sensitive biomolecular detection with a large dynamic range. Nature Commun. 2014;5:3779. doi: 10.1038/ncomms4779. [DOI] [PubMed] [Google Scholar]
- 20.Aas M, Chen Q, Jonáš A, Kiraz A, Fan X. Optofluidic FRET lasers and their applications in novel photonic devices and biochemical sensing. IEEE J. Sel. Top. Quantum Electron. 2015 DOI: 10.1109/JSTQE.2015.2477397. [Google Scholar]
- 21.Sun Y, Fan X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011;399:205–211. doi: 10.1007/s00216-010-4237-z. [DOI] [PubMed] [Google Scholar]
- 22.Intracavity DNA Melting Analysis with Optofluidic Lasers. Anal. Chem. 2012;84:9558–9563. doi: 10.1021/ac302416g. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Lee W, Fan X. Bio-switchable optofluidic lasers based on DNA Holliday junctions. Lab Chip. 2012;12:3673–3675. doi: 10.1039/c2lc40183e. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Leck KS, Ta VD, Chen R, Nalla V, Gao Y, He T, Demir HV, Sun H. Blue Liquid Lasers from Solution of CdZnS/ZnS Ternary Alloy Quantum Dots with Quasi-Continuous Pumping. Adv. Mater. 2015;27:169–175. doi: 10.1002/adma.201403237. [DOI] [PubMed] [Google Scholar]
- 25.Kiraz A, Chen Q, Fan X. Optofluidic Lasers with Aqueous Quantum Dots. ACS Photon. 2015;2:707–713. [Google Scholar]
- 26.Chen Q, Kiraz A, Fan X. Optofluidic FRET lasers using aqueous quantum dots as donors. Lab Chip. 2016;16:353–359. doi: 10.1039/c5lc01004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Zhang X, Sun Y, Ritt M, Sivaramakrishnan S, Fan X. Highly sensitive fluorescent protein FRET detection using optofluidic lasers. Lab Chip. 2013;13:2679–2681. doi: 10.1039/c3lc50207d. [DOI] [PubMed] [Google Scholar]
- 28.Gather MC, Yun SH. Bio-optimized energy transfer in densely packed fluorescent protein enables near-maximal luminescence and solid-state lasers. Nature Commun. 2014;5:5722. doi: 10.1038/ncomms6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nizamoglu S, Gather MC, Yun SH. All-biomaterial laser using vitamin and biopolymers. Adv. Mater. 2013;25:5943–5947. doi: 10.1002/adma201300818. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Chen Q, Sun Y, Fan X. Bio-inspired optofluidic lasers with luciferin. Appl. Phys. Lett. 2013;102:203706. [Google Scholar]
- 31.Wu X, Oo MKK, Reddy K, Chen Q, Sun Y, Fan X. Optofluidic laser for dual-mode sensitive biomolecular detection with a large dynamic range. Nature Commun. 2014;5:3779. doi: 10.1038/ncomms4779. [DOI] [PubMed] [Google Scholar]
- 32.Jacob-Wilk D, Holland D, Goldschmidt EE, Riov J, Eyal Y. Chlorophyll breakdown by chlorophyllase: isolation and functional expression of the Chlase1 gene from ethylene-treated Citrus fruit and its regulation during development. Plant J. 1999;20:653–661. doi: 10.1046/j.1365-313x.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Feng P, Xu X, Guo H, Ma J, Chi, et al. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011;2 doi: 10.1038/ncomms1486. [DOI] [PubMed] [Google Scholar]
- 34.P. L.-J. Jarvis E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013;14:787–802. doi: 10.1038/nrm3702. [DOI] [PubMed] [Google Scholar]
- 35.Azzi W. A. a. J. R. chlorophyll energy levels and electron flow in photosynthesis. Proc. Natl. Acad. Sci. 1968;61 doi: 10.1073/pnas.61.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhyaksa GWP, Prima EC, Lee DK, Ock I, Yatman S, Yuliarto B, Kang JK. A Light Harvesting Antenna Using Natural Extract Graminoids Coupled with Plasmonic Metal Nanoparticles for Bio-Photovoltaic Cells. Adv. Energy Matter. 2014;4 [Google Scholar]
- 37.Parusel AB, Grimme S. A theoretical study of the excited states of chlorophyll a and pheophytin a. J. Phys. Chem. 2000;104:5395–5398. [Google Scholar]
- 38.De Boni L, Correa DS, Pavinatto FJ, dos Santos DS, Mendonca CR. Excited state absorption spectrum of chlorophyll a obtained with white-light continuum. J. Chem. Phys. 2007;126:165102–165102. doi: 10.1063/1.2722755. [DOI] [PubMed] [Google Scholar]
- 39.Losev AP, Sagun EI, Kochubeev GA, Nichiporovich IN. Fluorescence quantum yields, lifetimes, and critical distances for energy transfer for chlorophyll α and its pheophytin in solutions. J. Appl. Spec. 1986;45:798–803. [Google Scholar]
- 40.Leupold D, Struck A, Stiel H, Teuchner K, Oberländer S, Scheer H. Excited-state properties of 20-chloro-chlorophyll a. Chem. Phys. Lett. 1990;170:478–484. [Google Scholar]
- 41.Hindman JC, Kugel R, Svirmickas A, Katz JJ. Chlorophyll lasers: Stimulated light emission by chlorophylls and Mg-free chlorophyll derivatives. Proc. Natl. Acad. Sci. 1977;74:5–9. doi: 10.1073/pnas.74.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackinney G. Absorption of light by chlorophyll solutions. J. biol. Chem. 1941;140:315–322. [Google Scholar]
- 43.Shopova SI, Zhu H, Fan X, Zhang P. Optofluidic ring resonator based dye laser. Appl. Phys. Lett. 2007;90:221101. [Google Scholar]
- 44.White IM, Oveys H, Fan X. Liquid Core Optical Ring Resonator Sensors. Opt. Lett. 2006;31:1319–1321. doi: 10.1364/ol.31.001319. [DOI] [PubMed] [Google Scholar]
- 45.Han K, Kim KH, Kim J, Lee W, Liu J, Fan X, Carmon T, Bahl G. Fabrication and Testing of Microfluidic Optomechanical Oscillators. J. Vis. Exp. 2014:e51497. doi: 10.3791/51497. doi:10.3791/51497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacey S, White IM, Sun Y, Shopova SI, Cupps JM, Zhang P, Fan X. Versatile microfluidic lasers based on opto-fluidic ring resonators. Opt. Express. 2007;15:15523–15530. doi: 10.1364/oe.15.015523. [DOI] [PubMed] [Google Scholar]
- 47.Shopova SI, Cupps JM, Zhang P, Henderson EP, Lacey S, Fan X. Opto-fluidic ring resonator lasers based on highly efficient resonant energy transfer. Opt. Express. 2007;15:12735–12742. doi: 10.1364/oe.15.012735. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Fan X. Distinguishing DNA by Analog-to-Digital-like Conversion by Using Optofluidic Lasers. Angew. Chem. Int. Ed. 2012;51:1236–1239. doi: 10.1002/anie.201107381. [DOI] [PubMed] [Google Scholar]
- 49.Erickson D, Sinton D, Psaltis D. Optofluidics for energy applications. Nature Photon. 2011;5:583–590. [Google Scholar]
- 50.Song W, Psaltis D. Electrically tunable optofluidic light switch for reconfigurable solar lighting. Lab Chip. 2013;13:2708–2713. doi: 10.1039/c3lc50204j. [DOI] [PubMed] [Google Scholar]
- 51.Seow YC, Lim SP, Lee HP. Micro-light distribution system via optofluidic cascading prisms. Microfluid Nanofluid. 2011;11:451–458. [Google Scholar]
- 52.Yang Y, Liu AQ, Chin LK, Zhang XM, Tsai DP, Lin CL, Lu C, Wang GP, Zheludev NI. Optofluidic waveguide as a transformation optics device for lightwave bending and manipulation. Nature Commun. 2012;3651 doi: 10.1038/ncomms1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.