Abstract
Background
Dialysis-requiring acute kidney injury (AKI-D) is a documented complication of hospitalization and procedures. Temporal incidence of AKI-D and related hospital mortality in the U.S. population has not been recently characterized. We describe the epidemiology of AKI-D as well as associated in-hospital mortality in the U.S.
Methods
Retrospective cohort of a national discharge data (n = 86,949,550) from the Healthcare Cost and Utilization Project’s National Inpatient Sample, 2001 to 2011 of patients hospitalization with dialysis-requiring acute kidney injury. Primary outcomes were dialysis-requiring acute kidney injury and in-hospital mortality. We determined the annual incidence rate of AKI-D in the U.S. from 2001 to 2011. We estimated odds ratios for AKI-D and in-hospital mortality for each successive year compared to 2001 using multiple logistic regression models, adjusted for patient and hospital characteristics, and stratified the analyses by sex and age. We also calculated population-attributable risk of in-hospital mortality associated with AKI-D.
Results
The adjusted odds of AKI-D increased by a factor of 1.03 (95% CI: 1.02–1.04) each year. The number of AKI-D-related (19,886 to 34,195) in-hospital deaths increased almost twofold, although in-hospital mortality associated with AKI-D (28.0% to 19.7%) declined significantly from 2001 to 2011. Over the same period, the adjusted odds of mortality for AKI-D patients were 0.60 (95% CI: 0.56–0.67). Population-attributable risk of mortality associated with AKI-D increased (2.1% to 4.2%) over the study period.
Conclusions
The incidence rate of AKI-D has increased considerably in the U.S. since 2001. However, in-hospital mortality associated with AKI-D hospital admissions has decreased significantly.
Keywords: acute kidney injury, acute renal failure, albuminuria, glomerular filtration rate, mortality, renal failure
INTRODUCTION
Acute kidney injury (AKI) is a serious condition characterized by a sudden decline in kidney function, leading to inadequate excretion of nitrogenous waste from the body and deficient volume and electrolyte regulation. Common life-threatening complications of AKI include volume overload, hyperkalemia, acidosis, and uremia. [1] Acute tubular necrosis (ATN) caused by ischemia, exposure to nephrotoxic agents (e.g. medications and contrast media), or sepsis is the leading cause of AKI among hospitalized patients in the U.S. [2] Patients at risk for AKI include those with advanced age, diabetes mellitus, heart failure (HF), liver failure, chronic kidney disease, hypotension, and sepsis. Patients who undergo cardiac/vascular surgery, organ transplantation, mechanical ventilation or who are exposed to contrast media, nonsteroidal-inflammatory drugs (NSAIDs), anti-microbial drugs or chemotherapeutic agents commonly experience AKI as a complicating condition. [3]
According to various reports, AKI occurs in anywhere from five to twenty percent of hospitalizations in the U.S. [4, 5] Patients that experience AKI are at increased risk for adverse health outcomes such as end stage renal disease (ESRD), pulmonary complications, and cardiovascular events, [6–9] all-cause mortality, increased length of stay, and an additional hospital cost of approximately $7,500. [7, 10] AKI severity is positively associated with patient morbidity and mortality, and dialysis-requiring AKI (AKI-D) is associated with the highest rates of morbidity and mortality among patients who develop AKI. [11, 12]
The patient characteristics, procedures, and nephrotoxic agents associated with increased risk of AKI are on the rise in the United States. For example, the U.S. population is aging and has experienced stark increases in the incidences of diabetes, HF and sepsis over the last few decades. [13–16] Additionally, the utilization of procedures requiring nephrotoxic contrast media, such as cardiac catheterization, computerized tomography scans, and peripheral angiograms, as well as the use of anti-microbial drugs has grown tremendously over the same time period. [2, 17–22] Recently, Hsu and colleagues reported an increasing trend in incidence of AKI-D from 2000–2009.[23] However, these trends were restricted to evaluating the trends in AKI-D itself and not AKI-D in-hospital mortality. Since patients developing AKI requiring dialysis have an increased risk of in-hospital and long-term mortality, we have sought to evaluate the national trends in AKI-D and hospital mortality. [24–26]
Due to the large increase in the prevalence of risk factors for AKI, we hypothesized that the incidence of in-hospital mortality during an AKI-D hospitalization has correspondingly increased. Using a nationally representative sample, we determined the incidence and associated in-hospital mortality of AKI-D among hospitalized patients in the U.S. from 2001 to 2011.
METHODS
Study Population
We extracted discharge data for the years 2001 to 2011 from the Healthcare Cost and Utilization Project’s (HCUP) National Inpatient Sample (NIS). The NIS is a nationally representative, stratified sample of approximately 20 percent of community hospitals in the U.S. each year. It is the largest de-identified U.S. all-payer inpatient database publically available for research purposes. In 2001, 986 hospitals in 33 states contributed over 7.4 million discharge records to the NIS. By 2011, the NIS grew to 1,049 hospitals in 46 states and over 8 million discharge records. The committee for the Protection of Human Subjects at Dartmouth College waived required approval for this study under an existing exemption approval including NIS data.
We identified cases of AKI using the following ICD-9-CM codes for acute renal failure: 584.5 (with tubular necrosis), 584.6 (with lesion of renal cortical necrosis), 584.7 (with lesion of renal medullary necrosis), 584.8 (other specified pathologic lesion in kidney) or 584.9 (unspecified). We defined a case of AKI-D as an AKI diagnosis code plus a co-listed code for hemodialysis, hemofiltration, or peritoneal dialysis (V45.1, V56.0, 39.95, V56.1, 54.98). Records with hemodialysis, hemofiltration or peritoneal dialysis procedure codes but without an AKI diagnosis code were excluded from the analysis. [27] We also excluded patients with arteriovenous fistula creation or revision procedure codes (39.27, 39.42, 39.43, 39.93) from the analysis to increase sensitivity and specificity for acute dialysis procedures. [23] The final cohort included 86,949,550 admissions. Table 1 reports annual comorbid conditions for the hospital admission.
TABLE 1.
Characteristics of All Patients in the HCUP sample, 2001 to 2011.
| NIS 2001–2011 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All years | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | ||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| N | 86,949,550 | 7,448,092 | 7,846,919 | 7,943,251 | 7,976,107 | 7,966,141 | 8,051,077 | 8,018,493 | 8,132,304 | 7,785,677 | 7,777,442 | 8,004,047 | |||||||||||||
| Female | 51,046,977 | 58.7 | 4,409,806 | 59.1 | 4,655,811 | 59.3 | 4,705,525 | 59.2 | 4,712,898 | 59.1 | 4,691,223 | 58.9 | 4,720,130 | 58.6 | 4,724,352 | 58.8 | 4,762,344 | 58.5 | 4,533,641 | 58.2 | 4,488,471 | 57.7 | 4,642,776 | 58.0 | |
| Congestive heart failure | 4,981,781 | 5.7 | 61 | 0.0 | 436,410 | 5.6 | 442,162 | 5.6 | 490,495 | 6.2 | 509,344 | 6.4 | 511,439 | 6.4 | 499,822 | 6.3 | 510,462 | 6.3 | 497,101 | 6.4 | 510,321 | 6.6 | 574,164 | 7.2 | |
| Pulmonary circulation disorder | 771,196 | 0.9 | 10 | 0.0 | 34,675 | 0.4 | 35,940 | 0.5 | 39,056 | 0.5 | 44,651 | 0.6 | 52,160 | 0.7 | 80,457 | 1.0 | 104,198 | 1.3 | 112,293 | 1.4 | 121,932 | 1.6 | 145,824 | 1.8 | |
| Peripheral vascular disorder | 3,039,267 | 3.5 | 19 | 0.0 | 234,933 | 3.0 | 242,648 | 3.1 | 263,056 | 3.3 | 268,030 | 3.4 | 294,483 | 3.7 | 309,193 | 3.9 | 348,828 | 4.3 | 341,623 | 4.4 | 337,853 | 4.3 | 398,601 | 5.0 | |
| Hypertension, uncomplicated | 27,628,768 | 31.8 | 259 | 0.0 | 1,999,451 | 25.5 | 2,322,231 | 29.2 | 2,541,431 | 31.9 | 2,628,701 | 33.0 | 2,815,192 | 35.0 | 2,814,819 | 35.3 | 3,065,651 | 37.8 | 3,02,143 | 38.9 | 3,083,526 | 39.7 | 3,335,364 | 41.7 | |
| Other neurological disorders | 4,096,061 | 4.7 | 18 | 0.0 | 328,096 | 4.2 | 322,220 | 4.1 | 356,467 | 4.5 | 363,055 | 4.6 | 389,941 | 4.9 | 425,352 | 5.3 | 461,373 | 5.7 | 456,786 | 5.9 | 474,913 | 6.1 | 517,840 | 6.5 | |
| Chronic pulmonary disease | 10,960,305 | 12.6 | 180 | 0.0 | 897,733 | 11.4 | 945,919 | 12.0 | 1,036,509 | 13.0 | 1,095,356 | 13.8 | 1,145,344 | 14.3 | 1,136,210 | 14.3 | 1,154,771 | 14.3 | 1,140,870 | 14.7 | 1,156,657 | 14.8 | 1,250,756 | 15.6 | |
| Diabetes | 10,800,947 | 12.4 | 101 | 0.0 | 872,033 | 11.1 | 923,765 | 11.6 | 994,750 | 12.5 | 1,016,959 | 12.8 | 1,087,401 | 13.5 | 1,093,436 | 13.7 | 1,174,187 | 14.5 | 1,173,183 | 15.1 | 1,188,056 | 15.3 | 1,277,076 | 16.0 | |
| Diabetes (chronic complications) | 2,346,404 | 2.7 | 14 | 0.0 | 187,279 | 2.4 | 194,314 | 2.5 | 206,401 | 2.6 | 215,013 | 2.7 | 229,420 | 2.9 | 238,707 | 3.0 | 256,388 | 3.2 | 251,937 | 3.2 | 260,427 | 3.3 | 306,504 | 3.9 | |
| Renal failure | 5,002,682 | 5.8 | 5 | 0.0 | 250,712 | 3.2 | 258,087 | 3.2 | 286,323 | 3.6 | 344,107 | 4.3 | 527,495 | 6.6 | 573,608 | 7.2 | 616,916 | 7.6 | 653,404 | 8.4 | 691,150 | 8.9 | 800,875 | 10.0 | |
| CKD Stage | |||||||||||||||||||||||||
| I | 13,370 | . | . | . | . | . | . | . | . | 421 | 0.0 | 1,628 | 0.0 | 1,192 | 0.0 | 1,883 | 0.0 | 2,106 | 0.0 | 3,253 | 0.0 | 2,887 | 0.0 | ||
| II | 84,056 | . | . | . | . | . | . | . | . | 1,228 | 0.0 | 6,620 | 0.1 | 8,965 | 0.1 | 12,302 | 0.2 | 15,500 | 0.2 | 18,221 | 0.2 | 21,220 | 0.3 | ||
| III | 521,378 | . | . | . | . | . | . | . | . | 3,250 | 0.0 | 24,751 | 0.3 | 42,858 | 0.5 | 66,435 | 0.8 | 94,037 | 1.2 | 122,852 | 1.6 | 167,195 | 2.1 | ||
| IV | 294,297 | . | . | . | . | . | . | . | . | 2,976 | 0.0 | 18,365 | 0.2 | 28,624 | 0.4 | 45,402 | 0.6 | 56,225 | 0.7 | 64,995 | 0.8 | 77,710 | 1.0 | ||
| V | 68,970 | . | . | . | . | . | . | . | . | 1,857 | 0.0 | 10,118 | 0.1 | 11,118 | 0.1 | 12,315 | 0.2 | 11,250 | 0.1 | 10,800 | 0.1 | 11,512 | 0.1 | ||
| ESRD | 1,072,420 | . | . | . | . | . | . | . | . | 32,965 | 0.4 | 157,303 | 2.0 | 166,993 | 2.1 | 170,648 | 2.1 | 170,793 | 2.2 | 176,963 | 2.3 | 196,755 | 2.5 | ||
| Unspecified | 1,518,302 | . | . | . | . | . | . | . | . | 62,613 | 0.8 | 185,590 | 2.3 | 323,465 | 4.0 | 259,743 | 3.2 | 239,440 | 3.1 | 226,703 | 2.9 | 220,748 | 2.8 | ||
| Obesity | 4,692,560 | 5.4 | 25 | 0.0 | 270,561 | 3.4 | 296,014 | 3.7 | 333,602 | 4.2 | 367,071 | 4.6 | 409,821 | 5.1 | 463,090 | 5.8 | 570,935 | 7.0 | 600,759 | 7.7 | 630,343 | 8.1 | 750,339 | 9.4 | |
| Fluid and electrolyte disorders | 12,095,531 | 13.9 | 149 | 0.0 | 873,915 | 11.1 | 939,742 | 11.8 | 1,046,696 | 13.1 | 1,130,041 | 14.2 | 1,208,336 | 15.0 | 1,246,372 | 15.6 | 1,339,623 | 16.5 | 1,332,703 | 17.1 | 1,405,723 | 18.1 | 1,572,231 | 19.6 | |
| Blood loss anemia | 1,626,858 | 1.9 | 17 | 0.0 | 137,791 | 1.7 | 145,512 | 1.8 | 163,034 | 2.1 | 163,629 | 2.1 | 171,350 | 2.1 | 184,288 | 2.3 | 175,774 | 2.2 | 158,840 | 2.0 | 160,048 | 2.1 | 166,575 | 2.1 | |
| Deficiency anemias | 8,729,212 | 10.0 | 86 | 0.0 | 600,275 | 7.6 | 637,778 | 8.1 | 700,801 | 8.8 | 740,584 | 9.3 | 806,970 | 10.0 | 897,327 | 11.2 | 1,036,122 | 12.7 | 1,039,662 | 13.3 | 1,063,252 | 13.7 | 1,206,355 | 15.1 | |
| PCI only | 211,931 | 0.2 | 22,344 | 0.3 | 25,284 | 0.3 | 23,354 | 0.3 | 22,130 | 0.3 | 19,754 | 0.3 | 31,114 | 0.4 | 18,073 | 0.2 | 17,608 | 0.2 | 14,203 | 0.2 | 9,140 | 0.1 | 8,927 | 0.1 | |
| PCI total | 1,665,863 | 1.9 | 153,933 | 2.1 | 157,866 | 2.0 | 168,816 | 2.1 | 162,401 | 2.1 | 163,986 | 2.1 | 187,454 | 2.3 | 146,346 | 1.8 | 155,695 | 1.9 | 138,801 | 1.8 | 112,082 | 1.5 | 118,483 | 1.5 | |
| Cath only | 1,967,899 | 2.3 | 204,933 | 2.8 | 206,739 | 2.6 | 210,776 | 2.6 | 187,289 | 2.4 | 177,910 | 2.2 | 184,304 | 2.3 | 166,384 | 2.1 | 167,873 | 2.1 | 164,169 | 2.1 | 147,297 | 1.9 | 150,225 | 1.9 | |
| Cath total | 3,421,831 | 3.9 | 336,522 | 4.6 | 339,321 | 4.3 | 356,238 | 4.5 | 327,560 | 4.1 | 327,560 | 4.0 | 340,644 | 4.2 | 294,657 | 3.7 | 305,960 | 3.8 | 288,767 | 3.7 | 250,239 | 3.2 | 259,781 | 3.2 | |
| Cath and PCI | 1,453,932 | 1.7 | 131,589 | 1.8 | 132,582 | 1.7 | 145,462 | 1.8 | 140,271 | 1.8 | 144,232 | 1.8 | 156,340 | 1.9 | 128,273 | 1.6 | 138,087 | 1.7 | 124,598 | 1.6 | 102,942 | 1.3 | 109,556 | 1.4 | |
| Number of stents | |||||||||||||||||||||||||
| 0 | 86,038,304 | 99.0 | 7,448,092 | 100.0 | 7,846,919 | 100.0 | 7,943,251 | 100.0 | 7,976,107 | 100.0 | 7,926,098 | 99.5 | 7,869,688 | 97.8 | 7,868,719 | 98.1 | 7,973,613 | 98.1 | 7,643,139 | 98.2 | 7,663,081 | 98.5 | 7,879,597 | 98.5 | |
| 1 | 127,618 | 0.7 | . | . | . | . | . | . | . | . | 24,683 | 0.3 | 113,101 | 1.4 | 96,716 | 1.2 | 100,534 | 1.2 | 90,846 | 1.2 | 73,569 | 1.0 | 80,136 | 1.0 | |
| 2 | 53,967 | 0.3 | . | . | . | . | . | . | . | . | 10,070 | 0.1 | 46,131 | 0.6 | 36,873 | 0.5 | 39,780 | 0.5 | 35,338 | 0.5 | 28,298 | 0.4 | 30,683 | 0.4 | |
| 3 | 19,183 | 0.1 | . | . | . | . | . | . | . | . | 3,591 | 0.1 | 15,093 | 0.2 | 11,190 | 0.1 | 12,552 | 0.2 | 11,379 | 0.1 | 8,749 | 0.1 | 9,565 | 0.1 | |
| 4 | 9,273 | 0.0 | . | . | . | . | . | . | . | . | 1,699 | 0.0 | 7,064 | 0.1 | 4,995 | 0.1 | 5,825 | 0.1 | 4,975 | 0.1 | 3,745 | 0.1 | 4,066 | 0.1 | |
| Sepsis | 2,989,617 | 3.4 | 187,786 | 2.5 | 206,989 | 2.6 | 225,735 | 2.8 | 228,998 | 2.9 | 246,062 | 3.1 | 264,699 | 3.3 | 278,709 | 3.5 | 313,941 | 3.9 | 315,925 | 4.1 | 339,627 | 4.4 | 381,146 | 4.8 | |
| Died | 1,797,676 | 2.1 | 172,707 | 2.3 | 175,030 | 2.2 | 177,211 | 2.2 | 170,654 | 2.1 | 167,083 | 2.1 | 164,585 | 2.1 | 154,869 | 1.9 | 165,464 | 2.0 | 149,820 | 1.9 | 148,097 | 1.9 | 152,156 | 1.9 | |
| Age group | |||||||||||||||||||||||||
| 0–19 | 15,779,993 | 18.1 | 1,390,452 | 18.6 | 1,471,994 | 18.6 | 1,473,459 | 18.5 | 1,502,200 | 18.8 | 1,543,695 | 19.4 | 1,467,397 | 18.2 | 1,512,297 | 18.7 | 1,421,211 | 17.4 | 1,363,582 | 17.4 | 1,351,422 | 17.4 | 1,282,284 | 16.0 | |
| 20–44 | 21,189,803 | 24.3 | 1,858,645 | 24.9 | 1,986,061 | 25.3 | 1,985,904 | 24.9 | 2,017,593 | 25.3 | 1,933,654 | 24.2 | 1,972,082 | 24.4 | 1,982,992 | 24.7 | 1,933,329 | 23.7 | 1,849,868 | 23.7 | 1,823,524 | 23.5 | 1,846,151 | 23.0 | |
| 45–64 | 19,954,133 | 23.0 | 1,532,916 | 20.6 | 1,662,087 | 21.2 | 1,734,159 | 21.8 | 1,762,240 | 22.1 | 1,765,090 | 22.1 | 1,860,074 | 23.1 | 1,846,256 | 23.1 | 1,937,463 | 23.9 | 1,901,216 | 24.4 | 1,942,261 | 25.0 | 2,010,371 | 25.1 | |
| 65–74 | 11,390,882 | 13.1 | 1,008,899 | 13.6 | 1,030,893 | 13.2 | 1,034,203 | 13.1 | 1,015,547 | 12.8 | 1,003,875 | 12.6 | 1,027,570 | 12.7 | 1,008,323 | 12.6 | 1,074,535 | 13.3 | 1,037,398 | 13.4 | 1,034,476 | 13.3 | 1,115,163 | 14.0 | |
| 75+ | 18,625,850 | 21.5 | 1,656,750 | 22.2 | 1,694,995 | 21.7 | 1,715,125 | 21.7 | 1,677,520 | 21.1 | 1,718,841 | 21.6 | 1,723,368 | 21.5 | 1,667,770 | 20.9 | 1,765,013 | 21.8 | 1,632,799 | 21.1 | 1,624,504 | 20.8 | 1,749,165 | 21.9 | |
NB: Patient counts were taken from the sample population, whereas percentages were calculated using weights. CKD stage percentages are unweighted.
Note: Rows represent co-morbid conditions for hospital admission.
We used adjusted analyses to account for comorbid conditions, age, sex, and hospital utilization practices, such as annual median length of stay and the proportion of patients discharged to skilled nursing facilities (SNF). Heart failure, pulmonary circulation disorders, peripheral vascular disease, hypertension, neurological disorders, chronic pulmonary disease, diabetes without chronic complications, diabetes with chronic complications, obesity, fluid and electrolyte disorders, blood loss anemia, and deficiency anemias were identified using NIS comorbidity indicators. Specific ICD-9-CM codes used to identify comorbid conditions are available for review in the online supplement. [28]
Statistical Analysis
We calculated population incidence rates for AKI-D in the U.S. from 2001 to 2011 by dividing the number of discharges with AKI-D by the U.S. population in each year, using estimates from the U.S. Census Bureau. [23, 29] Incidence estimates were stratified by sex and age for subgroup investigation. With data from 2001 and then for each successive year, we used multiple logistic regression models to determine the odds of AKI-D and corresponding in-hospital mortality for each year included in the study. Models were adjusted for age, sex, race, HF, pulmonary circulation disorders, peripheral vascular disease, hypertension, other neurological disorders, chronic pulmonary disease, diabetes without chronic complications, diabetes with chronic complications, obesity, fluid and electrolyte disorders, blood loss anemia, deficiency anemias, sepsis, and cardiac catheterization. Population attributable risk of in-hospital death associated AKI-D was calculated to approximate the proportion of in-patient deaths that could have been prevented if AKI-D were avoided. All data was analyzed using Stata 11.2 (StataCorp, College Station, TX) and weighted at the discharge-level to account for the NIS sampling scheme. [23, 29]
RESULTS
We identified 282,212 hospitalizations associated with AKI-D diagnoses from 2001 to 2011. Over this period, significant differences were found in AKI-D patients in all characteristics except for sex, age, HF, chronic pulmonary disease, and length of stay. The most notable increases were in the proportion of AKI-D patients with hypertension (14.2% to 53.0%), obesity (3.3% to 18.7%), electrolyte disorders (51.5% to 70.9%), deficiency anemias (25.5% to 52.5%) and sepsis (25.0% to 35.6%) during the study period. Complete annual characteristics for patients are displayed in Table 1 – patient counts are taken directly from the sample, whereas proportions are reported using weights.
Dialysis-Requiring Acute Kidney Injury
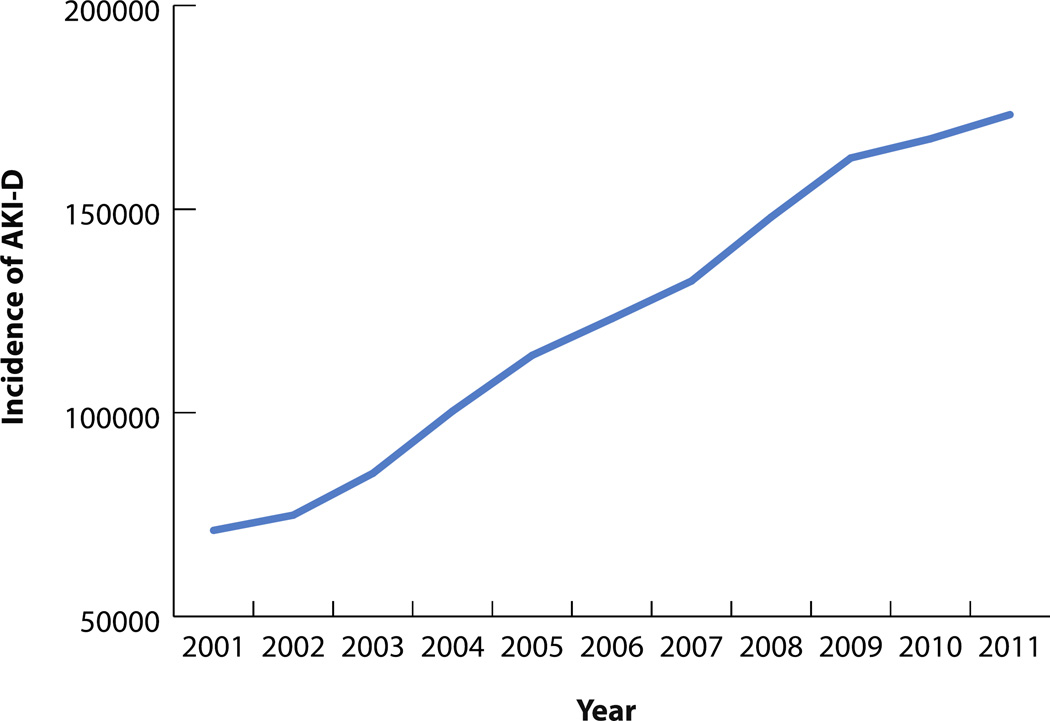
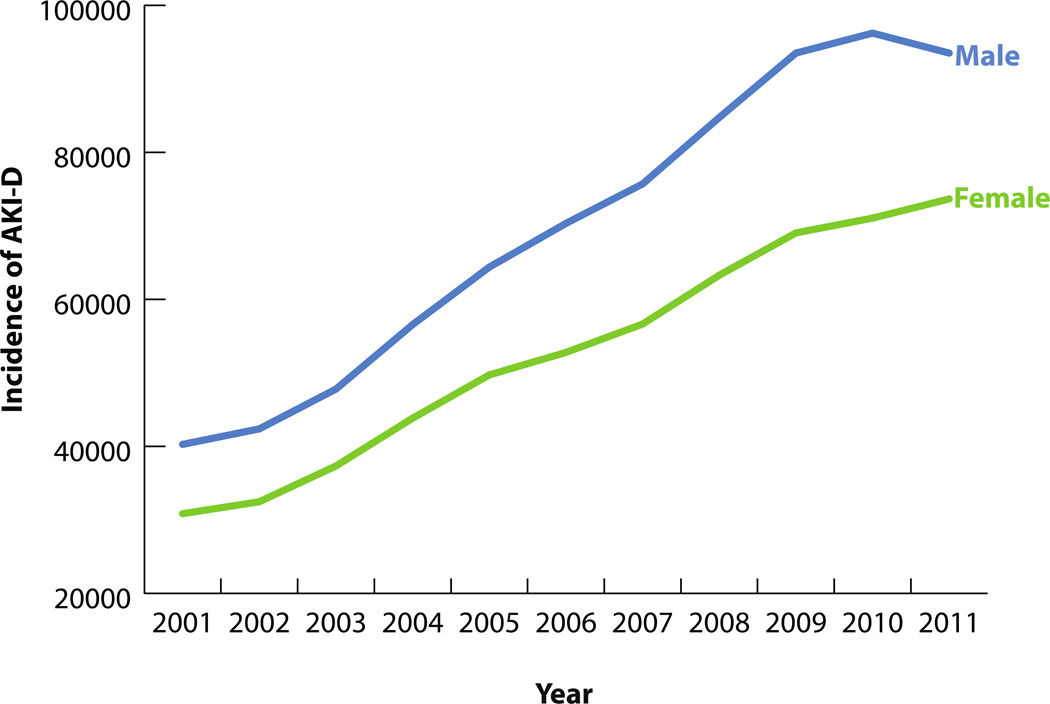
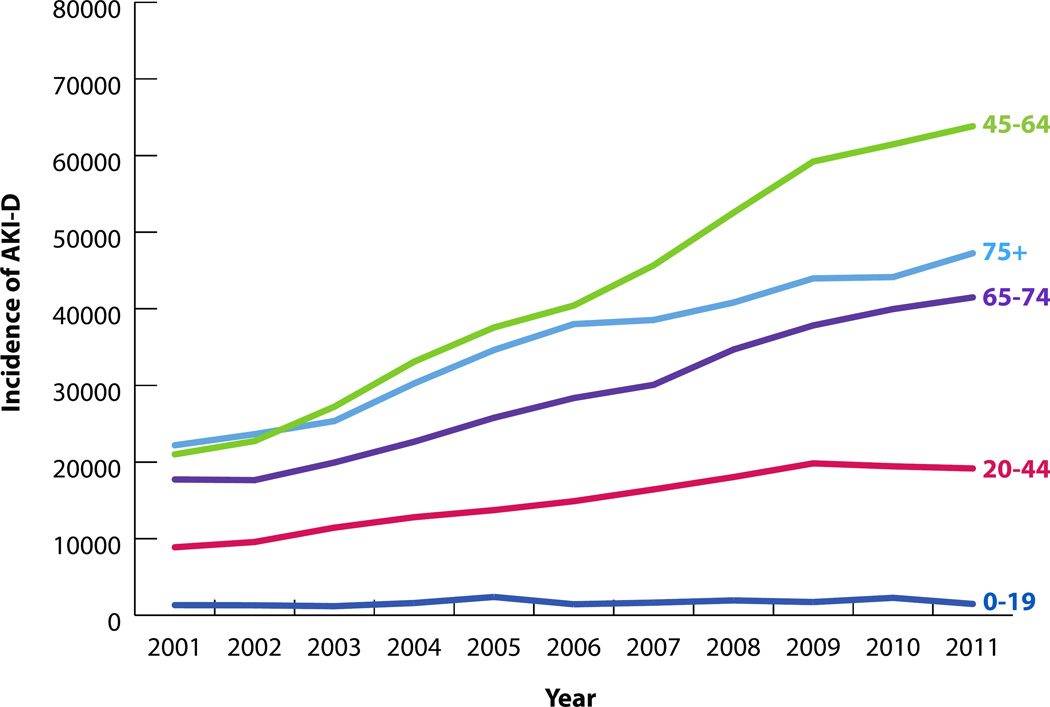
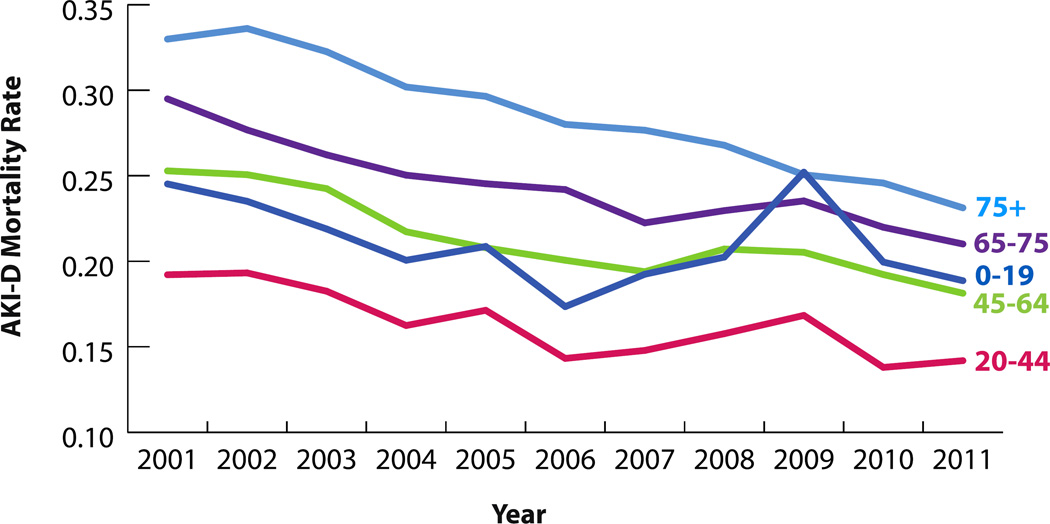
282,212 hospitalizations were associated with AKI-D over the study period. The incidence of AKI-D increased more than twofold over the study period, from 71,119 in 2001 to 173,205 cases in 2011 (Figure 1). The unadjusted odds of AKI-D increased each year by a factor of 1.10 (95% CI: 1.09–1.10), while the adjusted odds increased by a factor of 1.03 (95% CI: 1.02–1.04) (Table 3). Similar patterns of AKI-D incidence were observed between men and women, as well as among different age groups (Figures 2 and 3). Patients 45 to 64 years of age experienced the largest increase in AKI-D incidence over the study period (Figure 3).
Figure 1.
TABLE 3.
Crude and Adjusted Odds Ratios for Mortality Amongst Dialysis-Requiring Acute Kidney Injury in the United States, 2011 Compared to 2001.
| Odds of Mortality – AKI-D | ||||
|---|---|---|---|---|
| Year | Crude OR | 95 % CI | Adjusted OR | 95% CI |
| 2001 | referent | |||
| 2002 | 0.99 | (0.91 – 1.07) | 0.98 | (0.90 – 1.07) |
| 2003 | 0.92 | (0.84 – 1.00) | 0.91 | (0.83 – 1.00) |
| 2004 | 0.82 | (0.75 – 0.90) | 0.77 | (0.71 – 0.85) |
| 2005 | 0.81 | (0.74 – 0.88) | 0.74 | (0.67 – 0.81) |
| 2006 | 0.76 | (0.70 – 0.82) | 0.70 | (0.65 – 0.76) |
| 2007 | 0.72 | (0.66 – 0.79) | 0.66 | (0.60 – 0.76) |
| 2008 | 0.73 | (0.67 – 0.80) | 0.67 | (0.62 – 0.73) |
| 2009 | 0.72 | (0.67 – 0.79) | 0.67 | (0.61 – 0.72) |
| 2010 | 0.67 | (0.62 – 0.73) | 0.61 | (0.56 – 0.67) |
| 2011 | 0.65 | (0.62 – 0.73) | 0.60 | (0.56 – 0.67) |
Odds Ratios are calculated for the effect from each successive year. Adjusted analyses account for comorbid conditions (including congestive heart failure, pulmonary circulation disorders, peripheral vascular disease, hypertension, neurological disorders, chronic pulmonary disease, diabetes without chronic complications, diabetes with chronic complications, obesity, fluid and electrolyte disorders, blood loss anemia, and deficiency anemias), age, sex, and hospital utilization practices (including annual median length of stay and the proportion of patients discharged to skilled nursing facilities).
Figure 2.
Figure 3.
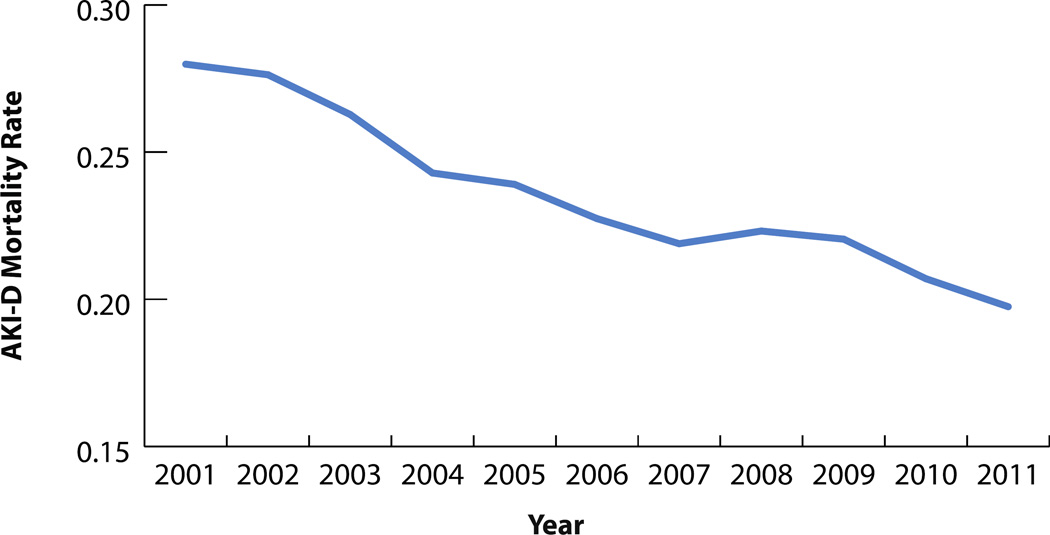
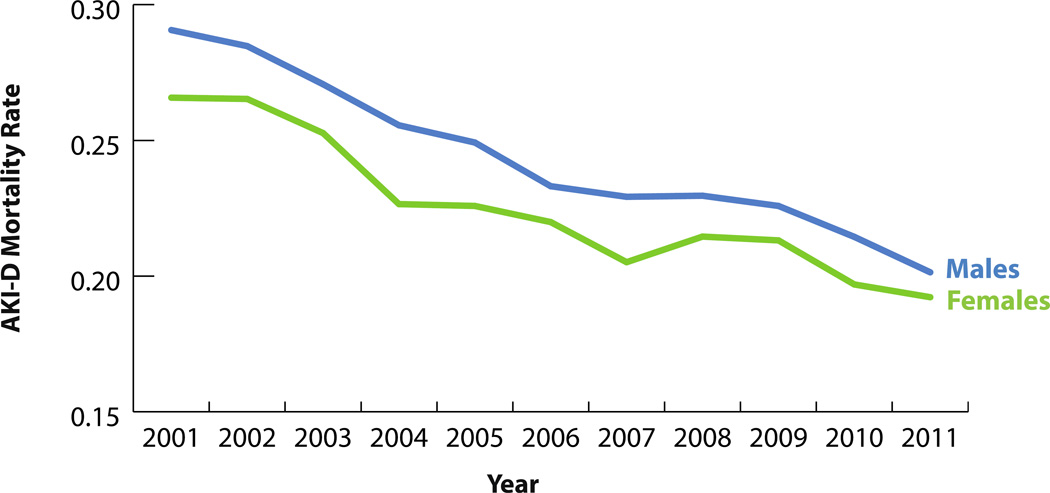
While the annual raw number of AKI-D patients who died in the hospital increased almost twofold (3,979 to 7,146), in-hospital mortality for AKI-D patients decreased over the study period, from 28.0% to 19.7% (Figure 4). Similar trends were observed for men and women, and age categories (Figures 5 and 6). Compared to 2001, the unadjusted odds of mortality for AKI-D patients in 2011 were 0.65 (95% CI: 0.62–0.73), while the adjusted odds were 0.60 (95% CI: 0.56–0.67). Approximately 5% of all in-hospital deaths were associated with AKI-D in 2011, demonstrating a 1.3% increase since 2001. The population-attributable risk of mortality associated with AKI-D increased during the study period, from 2.1% in 2001 to 4.2% in 2011 (Table 4).
Figure 4.
Figure 5.
Figure 6.
TABLE 4.
Population Attributable Risk of Mortality Associated with Dialysis-Requiring Acute Kidney Injury in the United States, 2001 through 2011.
| Year | Population Attributable Risk of Mortality AKI-D (%) |
|---|---|
| 2001 | 2.1 |
| 2002 | 2.4 |
| 2003 | 2.6 |
| 2004 | 2.7 |
| 2005 | 3.0 |
| 2006 | 3.2 |
| 2007 | 3.4 |
| 2008 | 3.7 |
| 2009 | 4.3 |
| 2010 | 4.3 |
| 2011 | 4.2 |
DISCUSSION
With this nationally representative study of hospitalizations in the U.S., we are the first to show that while AKI-D has increased twofold, hospital mortality rates for AKI-D have decreased significantly from 2001 to 2011. Even though we found a downward trend in the proportion of AKI-D patients with hospital mortality, the raw number of deaths and population-attributable risk of death for AKI-D increased significantly from 2001 to 2011. Approximately 5% of all in-hospital deaths were associated with AKI-D in 2011, demonstrating a 1.3% increase since 2001. Our findings demonstrate both the clinical and public health significance of AKI-D in the U.S., and the opportunity that exists to prevent and manage its life-threatening complications.
Our foremost contribution is to report on the growing incidence of AKI-D in the United States from 2001 to 2011 and to demonstrate that while AKI-D admissions are on the rise, hospital mortality for AKI-D has declined significantly. We hypothesize the reduction in in-hospital mortality may be due to improved dialysis and ICU care, although we acknowledge that given these data are based on administrative data with its attendant limitations, we cannot confirm or refute this hypothesis. Our study confirms recent findings from Hsu and colleagues that the incidence of AKI-D rose by 10% each year between 2000 and 2009. [23] However, our study builds upon theirs in two important ways: we have provided robust hospital mortality analysis for AKI-D from 2001 to 2011, and we have updated the analysis of AKI-D trends to include 2010 and 2011 data. Compared to Hsu and colleagues, [23] we found a similar yearly increase in the unadjusted odds of AKI-D (OR: 1.10 95% CI: 1.09–1.10). However, in adjusted analyses our expanded covariates list explained all but three percent of the observed increase in the odds of AKI-D in 2011 compared to 2001.
The early 2000s saw the first investigations into the incidence of AKI and associated in-hospital mortality. These studies expressed their findings in terms of either cases per hospitalization or intensive care unit days, making the results difficult to interpret and generalize across providers as the number of admissions and length of stay vary between hospitals. [2, 11, 30] More recent studies have taken a more population-based approach, estimating the population incidence of AKI-D to be 222–533 per million people per year. [23, 31, 32] To date, only Hsu and colleagues have investigated the temporal incidence of any kind of AKI in the U.S. (in their case, AKI-D specifically). [23, 33] Our analysis now provides the most up-to-date and comprehensive description of AKI-D and related hospital mortality epidemiology in the U.S. The observed increases in the incidences of AKI-D in our study were likely driven by the increasing commonality of patient risk factors for AKI in the U.S. population, as well as the more frequent use of procedures and medications known to cause kidney damage.
Other studies have confirmed the increase in AKI using administrative data. Grams and colleagues reported that AKI increased from 10% to 24% over a similar time interval as our study with high and consistent specificity.[35–38] Though the increase in administrative coding likely accounts for some of the increase in AKI, other investigations that have used creatinine-based definitions for AKI have also suggested a temporal increase in the incidence of AKI.[39] Other studies have also demonstrated that mortality due AKI-D has decreased in the U.S.[23, 27, 43] Khera and colleagues noted an adjusted odds of 0.74 of mortality among elderly patients undergoing angioplasty who developed AKI-D in 2010 compared to 2002, which translated into a 26% reduction in mortality for patients with AKI-D.[43]
Changing dialysis treatment patterns over the time period of the study may account for reduced AKI-D mortality. That is, despite lack of randomized controlled data to support this contention, perhaps earlier and higher dose and more frequent dialysis may be beneficial. An alternative explanation however may be that less sick patients are being initiated on dialysis, which would also translate into improved survival on dialysis. Siddiqui and colleagues noted that median time to dialysis after major elective surgery decreased from 5 to 2 days, however mortality did not change over time.[40]
Increased utilization of hospice referrals may also account for reduced inpatient AKI-D mortality. That is, increased use of hospice[41] may influence the decision to initiate dialysis, but also to withdraw from dialysis and potentially die outside the hospital. Cohen and colleagues reported that a hospice intervention led to discontinuation of dialysis of 15.9% compared to 6.0% in the controls.[41] We acknowledge however that an administrative-based investigation is unable to evaluate these issues in more detail. [42]
The implications of our results are as follows. First, they reinforce the recognition of an increasing incidence of AKI requiring dialysis therapy and that efforts should continue to minimize risk of AKI and prevent the complications associated with AKI. Second, dialysis mortality due to AKI is decreasing, which is encouraging, but it is important to understand which of changes we have made in care over the last decade have resulted in this improvement. It is interesting to note that mortality in chronic maintenance dialysis patients has also declined over the last decade despite the paucity of randomized trials which have shown a benefit of any specific intervention; therefore it is possible that multiple changes/improvements over time have combined to improve outcomes.[44, 45]
Treatments on the horizon for AKI include Renal Guard Therapy, alpha-melanocyte-stimulation hormone, alkaline phosphates, siRNAs, THR-184, and mesenchymal stem cell therapy.[46] The creation of AKI improvement collaboratives and initiatives may help reduce AKI incidence and associated mortality, as described elsewhere. Efforts should be focused on minimizing causes of AKI, increasing awareness of the importance of serial measurements of serum creatinine in high risk patients, and documenting urine volume in acutely ill people to achieve early diagnosis. For instance, implementing educational programs and using simple tools, such as the Donut bundle (first-line interventions for AKI management – Dehydration, Obstruction, Nephrotoxins, Urine, Think Sepsis), has been found to improve management of AKI patients.[47] Recommendations to improve care pathways for AKI patients are also available in the 2009 National Confidential Enquiry into Patient Outcome and Death report38 for care teams to review and implement into routine practice.
The strengths of our study include the use of a large nationally representative sample of hospitalizations in the U.S. reporting on the incidence of AKI-D and hospital mortality. In addition, our use of population attributable risk of mortality estimates allows readers to understand both the clinical and public health significance of AKI-D in the U.S. We utilized a combination of ICD-9-CM codes plus a companion dialysis code to identify cases of AKI-D with high specificity. Prior analyses have not utilized ICD-9-CM codes alone to identify cases of AKI due to concerns of validity. [23, 48, 47, 38] Therefore, we did not extend our analysis to included AKI without dialysis even though some evidence has shown a high specificity (97.7%) and low sensitivity (35.4%) of ICD-9-CM codes in identifying cases of AKI without dialysis.
However, our study has some important limitations. While administrative billing codes have been validated in the literature [23, 27], clinical or laboratory data, such as serum creatinine levels, would have provided the opportunity to expand our cohort to include AKI without requiring dialysis. [48, 47, 38] Second, we cannot rule out the possibility that the lower in-hospital mortality with AKI-D is due to initiating dialysis in less sick patients at an earlier stage or discharging patients earlier to outpatient hospice and thereby reducing in-hospital mortality. Third, the NIS only collects a 20% representative sample of national data. Although we apply population weights to estimate the national rates, it is possible we may have over or under estimated the true national rates. Fourth, we do not have data on outcomes after hospital discharge. Therefore it is possible that patients are discharged earlier in the current environment but have higher out of hospital mortality or higher mortality on subsequent admissions. The NIS is a completely de-identified data set, making it impossible to track specific patients over time.
Although our study suggests the problem and greater comorbidity burden over time, additional research is needed to understand the drivers of increases of AKI-D incidence in the U.S. and the interventions resulting in lower mortality. More research is also needed confirm our results in other observational studies not relying on administrative data and to understand geographic and subgroup variation, as well as which prevention and management strategies result in the greatest decline and control of AKI-D among hospitalized patients.
In summary, our study demonstrates that the incidence of AKI-D increased significantly in the U.S. between 2001 and 2011. The percentage of all in-hospital deaths occurring in combination with AKI-D however has decreased over the study period. AKI-D represents a growing clinical and public health problem for Americans.
Supplementary Material
TABLE 2.
Crude and Adjusted Odds Ratios for Dialysis-Requiring Acute Kidney Injury in the United States, 2011 Compared to 2001.
| AKI-D | ||||
|---|---|---|---|---|
| Population at Risk | Crude OR/yr | 95% CI | Adjusted OR/yr | 95% CI |
| Overall | 1.10 | (1.09 – 1.10) | 1.03 | (1.02 – 1.04) |
| Age Groups | ||||
| 0–19 | 1.06 | (1.01 – 1.10) | 1.00 | (0.96 – 1.03) |
| 20–44 | 1.10 | (1.09 – 1.11) | 1.02 | (1.01 – 1.03) |
| 45–64 | 1.10 | (1.09 – 1.11) | 1.05 | (1.04 – 1.06) |
| 65–74 | 1.09 | (1.08 – 1.10) | 1.05 | (1.04 – 1.05) |
| 75+ | 1.08 | (1.07 – 1.09) | 1.02 | (1.01 – 1.03) |
| Sex | ||||
| Male | 1.09 | (1.09–1.10) | 1.03 | (1.03 – 1.04) |
| Female | 1.09 | (1.09–1.10) | 1.03 | (1.02 – 1.04) |
Odds Ratios are calculated for the effect from each successive year. Adjusted analyses account for comorbid conditions (including congestive heart failure, pulmonary circulation disorders, peripheral vascular disease, hypertension, neurological disorders, chronic pulmonary disease, diabetes without chronic complications, diabetes with chronic complications, obesity, fluid and electrolyte disorders, blood loss anemia, and deficiency anemias), age, sex, and hospital utilization practices (including annual median length of stay and the proportion of patients discharged to skilled nursing facilities).
Acknowledgments
This project was supported in part by HS018443 (Brown) from the Agency for Healthcare Research and Quality, the Veterans Health Administration Health Services Research and Development (HSR&D) CDA-08-020 (Matheny), HSR&D IIR-11-292 (Matheny, Brown), and Dr. Sarnak were supported by DK078204 (Sarnak), from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors would like to thank Emily Marshall for constructing the final versions of the figures in this manuscript.
Footnotes
Conflict of Interest Statement
None of the authors have conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part.
Author contributions: Dr. Brown conceived of the study and developed the scope of work and analytic protocol. Dr. Brown and Mr. Rezaee outlined, drafted, and revised the manuscript. Mr. Hisey managed the manuscript revisions, formatting, tables, and figures. All authors contributed to the interpretation of results and revisions. The Dartmouth Institute’s Data Analytic Core conducted the NIS analysis and drafted the original tables and figures. Dr. Brown is the guarantor of the manuscript. All authors have approved the final manuscript.
REFERENCES
- 1.Okusa MD, Rosner MH. In: Overview of the management of acute kidney injury (acute renal failure) Palevsky PM, editor. UpToDate, Wolters Kluwer; 2014. 2014. [Google Scholar]
- 2.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: Inherent and modifiable risks. Current opinion in critical care. 2005;11:533–536. doi: 10.1097/01.ccx.0000183666.54717.3d. [DOI] [PubMed] [Google Scholar]
- 4.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. American journal of nephrology. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: A prospective study. The American journal of medicine. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 6.Faubel S. Pulmonary complications after acute kidney injury. Advances in chronic kidney disease. 2008;15:284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of esrd among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 11.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clinical journal of the American Society of Nephrology : CJASN. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the united states, 1988–1994 and 1999–2010. Annals of internal medicine. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A, Friedman B, Stranges E. Hcup statistical brief #122. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Septicemia in u.S. Hospitals, 2009. 2014. [PubMed] [Google Scholar]
- 15.Anderson GF, Hussey PS. Population aging: A comparison among industrialized countries. Health affairs (Project Hope) 2000;19:191–203. doi: 10.1377/hlthaff.19.3.191. [DOI] [PubMed] [Google Scholar]
- 16.Roger VL. Epidemiology of heart failure. Circulation research. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehran R, Nikolsky E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney international. 2006:S11–S15. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 18.Lang K, Huang H, Lee DW, Federico V, Menzin J. National trends in advanced outpatient diagnostic imaging utilization: An analysis of the medical expenditure panel survey, 2000–2009. BMC medical imaging. 2013;13:40. doi: 10.1186/1471-2342-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan A, Newman A, Ko DT, Rinfret S, Hirsch G, Ghali WA, Tu JV. Increasing rates of angioplasty versus bypass surgery in canada, 1994–2005. American heart journal. 2010;160:958–965. doi: 10.1016/j.ahj.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the united states, 2001–2008. JAMA : the journal of the American Medical Association. 2011;305:1769–1776. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general u.S. Population. Pharmacoepidemiology and drug safety. 2014;23:43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]
- 22.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in us academic health centers: 2002 to 2006. Archives of internal medicine. 2008;168:2254–2260. doi: 10.1001/archinte.168.20.2254. [DOI] [PubMed] [Google Scholar]
- 23.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring aki. J Am Soc Nephrol. 2013;24:37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. The Annals of thoracic surgery. 2010;90:1142–1148. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Cochran RP, Dacey LJ, Ross CS, Kunzelman KS, Dunton RF, Braxton JH, Charlesworth DC, Clough RA, Helm RE, Leavitt BJ, Mackenzie TA, O'Connor GT Northern New England Cardiovascular Disease Study G. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 26.Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, Thompson CA Dartmouth Dynamic Registry I. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: Insights from the dartmouth dynamic registry. Catheter Cardiovasc Interv. 2008;72:347–354. doi: 10.1002/ccd.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. The Annals of thoracic surgery. 2013;95:20–28. doi: 10.1016/j.athoracsur.2012.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healthcare Cost and Utilization Project. Hcup comorbidity software, Agency for Healthcare Research and Quality, 2013. 2014. [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. Population estimates 2013. 2014
- 30.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. Journal of the American Society of Nephrology : JASN. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 31.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: How big is the problem? Critical care medicine. 2008;36:S146–S151. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 32.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. Journal of the American Society of Nephrology : JASN. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 33.Hsu RK, McCulloch CE, Ku E, Dudley RA, Hsu CY. Regional variation in the incidence of dialysis-requiring aki in the united states. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1476–1481. doi: 10.2215/CJN.12611212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, Stolker JM, Parikh CR, Masoudi FA, Jones PG, Kosiborod M. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Archives of internal medicine. 2012;172:246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 35.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of aki. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang YJ, Shariff SZ, Gandhi S, Wald R, Clark E, Fleet JL, Garg AX. Validity of the international classification of diseases, tenth revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ open. 2012;2 doi: 10.1136/bmjopen-2012-001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James M, Pannu N. Methodological considerations for observational studies of acute kidney injury using existing data sources. Journal of nephrology. 2009;22:295–305. [PubMed] [Google Scholar]
- 38.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX. Validity of administrative database coding for kidney disease: A systematic review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:29–43. doi: 10.1053/j.ajkd.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Siew ED, Davenport A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney international. 2015;87:46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui NF, Coca SG, Devereaux PJ, Jain AK, Li L, Luo J, Parikh CR, Paterson M, Philbrook HT, Wald R, Walsh M, Whitlock R, Garg AX. Secular trends in acute dialysis after elective major surgery--1995 to 2009. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2012;184:1237–1245. doi: 10.1503/cmaj.110895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen LM, Ruthazer R, Germain MJ. Increasing hospice services for elderly patients maintained with hemodialysis. J Palliat Med. 2010;13:847–854. doi: 10.1089/jpm.2009.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghavan D, Holley JL. Conservative care of the elderly ckd patient: A practical guide. Adv Chronic Kidney Dis. 2016;23:51–56. doi: 10.1053/j.ackd.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Khera S, Kolte D, Aronow WS, Palaniswamy C, Mujib M, Ahmed A, Chugh SS, Balasubramaniyam N, Edupuganti M, Frishman WH, Fonarow GC. Trends in acute kidney injury and outcomes after early percutaneous coronary intervention in patients >/=75 years of age with acute myocardial infarction. The American journal of cardiology. 2013;112:1279–1286. doi: 10.1016/j.amjcard.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Ponce D, Berbel MN, Abrao JM, Goes CR, Balbi AL. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. International urology and nephrology. 2013;45:869–878. doi: 10.1007/s11255-012-0301-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang AY, Bellomo R, Ninomiya T, Lo S, Cass A, Jardine M, Gallagher M. Angiotensin-converting enzyme inhibitor usage and acute kidney injury: A secondary analysis of renal study outcomes. Nephrology (Carlton, Vic) 2014;19:617–622. doi: 10.1111/nep.12284. [DOI] [PubMed] [Google Scholar]
- 46.Kaushal GP, Shah SV. Challenges and advances in the treatment of aki. Journal of the American Society of Nephrology : JASN. 2014;25:877–883. doi: 10.1681/ASN.2013070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhagwanani A, Carpenter R, Yusuf A. Improving the management of acute kidney injury in a district general hospital: Introduction of the donut bundle. BMJ Qual Improv Report. 2014;2 doi: 10.1136/bmjquality.u202650.w1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. Journal of the American Society of Nephrology : JASN. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.