Abstract
BACKGROUND
Migraine is common in children and adolescents and can be disabling. Being able to predict which patients will respond to triptans based on their clinical phenotype would be helpful. Adult data suggest cranial autonomic symptoms (CAS) and aura predict triptan response. This study examined clinical predictors of triptan response in pediatric migraineurs.
METHODS
This retrospective chart review study included all patients <18 years old with migraine who were seen at the University of California, San Francisco Headache Center in 2014. Univariate Chi-squared analyses were performed, followed by multivariate logistic regression modeling.
RESULTS
Of 127 pediatric migraineurs, 70 (55%) had chronic migraine and 24 (19%) had aura. The majority (55%) had at least one CAS. Of 65 with triptan outcome data, 47 (73%) benefitted from a triptan. In univariate analyses, triptan benefit was seen in: 65% with chronic migraine vs. 88% with episodic migraine (p=0.048), 67% with aura vs. 74% without (p=0.66), and 70% of those with CAS vs. 74% without (p=0.76). In a multivariate logistic regression model, chronic migraine (CM), aura, and CAS were not statistically significant predictors of triptan benefit: CM: 0.25 (0.06–1.04)); aura: 0.65 (0.09–4.45); CAS: 0.75 (0.22–2.52).
CONCLUSIONS
In univariate analysis, those with chronic migraine were less likely to benefit from triptans. In contrast to what has been seen in adults, CAS and aura did not predict triptan response, though small sample size limited power. Larger pediatric studies are needed and future pediatric triptan trials should provide response rates stratified by clinical variables such as aura.
Keywords: Migraine, Pediatrics, Triptan, Aura, Cranial Autonomic Symptoms
Introduction
Migraine is both common and disabling in children and adolescents. Prevalence of migraine is approximately 5% by age 10 and increases across adolescence, approaching adult prevalence rates by late adolescence1. Chronic migraine, meaning migraine affecting ≥15 days per month for at least the last three months2, is also common in the pediatric population, affecting 0.6% of 5–12 year olds and 0.8–1.8% of 12–17 year olds3,4. Over half of pediatric patients with chronic migraine are severely disabled by their headaches4 and many miss or perform poorly in school3. Ten percent of children and adolescents with migraine missed at least one day of school over a two-week period5. Therefore, with the goal of achieving rapid return to normal function, it is important to diagnose migraine accurately and to treat attacks in children and adolescents.
Being able to predict which acute migraine treatments will be effective for a given child based on their clinical phenotype would be helpful. In adult studies, presence of unilateral cranial autonomic symptoms (CAS) predicts a better response to triptans compared to those without CAS6,7. In addition, there is recent evidence in adults that the presence of aura predicts a lower triptan response rate compared to those who have migraine without aura8. This study aims to evaluate the impact of CAS and aura on triptan efficacy in pediatric migraine.
Methods
The University of California, San Francisco (UCSF) Committee for Human Research approved this retrospective chart review study (CHR 15–15938). The study population consisted of patients 1) <18 years of age, 2) who were seen at the UCSF Headache Center between January and December 2014, and 3) who met International Classification of Headache Disorders (ICHD) third edition (beta) criteria9 for migraine. Analysis of triptan benefit was performed on that subset that had had at least one adequate triptan trial.
Definition of an adequate triptan trial
For a trial to be considered adequate, the patient had to have tried the triptan, at an appropriate dose for weight (see Table 1), ≥3 times for headache. We selected this definition as it can be difficult to assess efficacy after just one or two trials of a medication, and as under-dosing may impair efficacy. For the two triptans that were FDA-labeled for pediatric use at the time of the study design, we considered the labeled doses to be appropriate: i.e. rizatriptan oral or melt, 5 mg for <40 kg, 10 mg for ≥40 kg, and almotriptan 12.5 mg orally for adolescents 12–17 years (presumably over 40 kg). For the others, appropriate doses were determined through a combination of reviewing published data of studied dosing in this age group and consensus expert opinion of the authors.
Table 1.
Definition of adequate pediatric and adolescent dosing of triptans
| <40 kg | ≥40 kg | |
|---|---|---|
| Sumatriptan NS10–12 | ≥5 mg | 20 mg |
| Sumatriptan PO13,14 | ≥25 mg | ≥50 mg |
| Sumatriptan SC15,16 | ≥0.1mg/kg | ≥4 mg |
| Zolmitriptan PO17 | ≥2.5 mg | 5 mg |
| Zomitriptan NS18 | N/A | 5 mg |
| Naratriptan PO19 | ≥1 mg | 2.5 mg |
| Frovatriptan PO | 2.5 mg | 2.5 mg |
| Eletriptan PO | 20 mg | 40 mg |
| Rizatriptan PO, MLT20,21 | 5 mg | 10 mg |
| Almotriptan PO22 | ≥6.25 mg | 12.5 mg |
PO= per os, MLT= melt, NS=nasal spray, SC=subcutaneous
As we generally counsel patients to take triptans with an NSAID for best efficacy14,23,24 (unless there is an NSAID contra-indication), we did not differentiate whether the triptan was taken in isolation or along with an NSAID. However, all patients who were prescribed a triptan had had inadequate relief with NSAIDs alone (or NSAIDs were contra-indicated).
Definition of outcomes
Benefit was defined as any degree of improvement as noted in the medical chart. If there was no comment on triptan efficacy in the chart, the patient was not included in the efficacy analysis.
Determination of migraine subtype diagnosis
Definition of migraine with aura and migraine without aura was made based on ICHD third edition (beta) criteria2, as was determination of episodic vs. chronic migraine. As per criteria, if a patient was experiencing more than 14 days of headache per month, but that pattern had not yet been present for at least three months, they were still considered to have episodic migraine. Some patients fluctuated between episodic and chronic migraine throughout their treatment; for the analysis, the diagnosis during the 2014 examination year was used. NSAID overuse was defined as NSAID use ≥ 15 days per month. Triptan overuse was defined as triptan use ≥ 10 days per month.
Determination of cranial autonomic symptoms (CAS)
Patients were interviewed with at least one parent present as part of our standard semi-structured interview for all new patients. They were explicitly asked whether they ever experience each of the following symptoms with their headaches:
Conjunctival injection
Lacrimation
Sense of grittiness or scratchiness in the eye
Nasal congestion
Rhinorrhea
Eyelid edema
Ptosis
Sense of ear fullness or pressure
Facial sweating/flushing
If at least one symptom was present with at least some attacks, they were categorized as having CAS. The frequency of each of these symptoms was not recorded.
Data collection
Data were collected from the medical records onto a standardized abstraction form and then entered into a secure web-based electronic REDCap (Research Electronic Data Capture)25 database.
Data Analysis
Data were analyzed using STATA v.13 (College Station, TX). Descriptive statistics were calculated, including demographics and clinical features.
The primary predictors of interest were presence of CAS and aura. Effect of chronic migraine status, sex, and age were also analyzed given their general clinical importance and a possible effect of age on triptan response in adults26. Age was examined as a binary variable: preadolescent (≤ 11 years) vs. adolescent (12–17 years). Given the relative infrequency of medication overuse in the sample, the absence of any opioid or barbiturate overuse, and the relatively small study sample size which limits the number of predictors that can be studied, we did not examine the effect of medication overuse on triptan benefit. The primary outcome measure was headache benefit. This was a binary outcome measured as any benefit vs. no benefit.
First, univariate analysis was performed using Chi-Squared or Fisher’s Exact, as appropriate. Subsequently multivariate logistic regression modeling was performed. Age and sex were not retained in the multivariate regression model given the relatively small number of outcomes, and that they were not significant in univariate analyses. Chronic migraine was retained as it was significant in univariate analysis, as were aura and CAS as they were the primary predictors of interest.
Results
There were 127 pediatric migraine patients during the study period. Their demographics and clinical variables are shown in Table 2.
Table 2.
Demographic and clinical variables of 127 pediatric migraine patients
| Demographics | |
|---|---|
| Female, n (%) | 85 (67) |
| Age, years (mean; SD) | 13.3 (3.1) |
| Clinical Variables | |
| Chronic migraine, n (%) | 70 (55) |
| Headache frequency, days per month (mean; SD) |
Overall: 18.4 (11.5) CM: 27.1 (5.1) EM: 7.3 (6.8) |
| Aura, n (%) | 24 (19) |
| CAS n (%) | 67 (55)* |
SD=standard deviation, CM=chronic migraine, EM=episodic migraine, CAS=cranial autonomic symptoms
missing data on 5; 67/122 = 55%
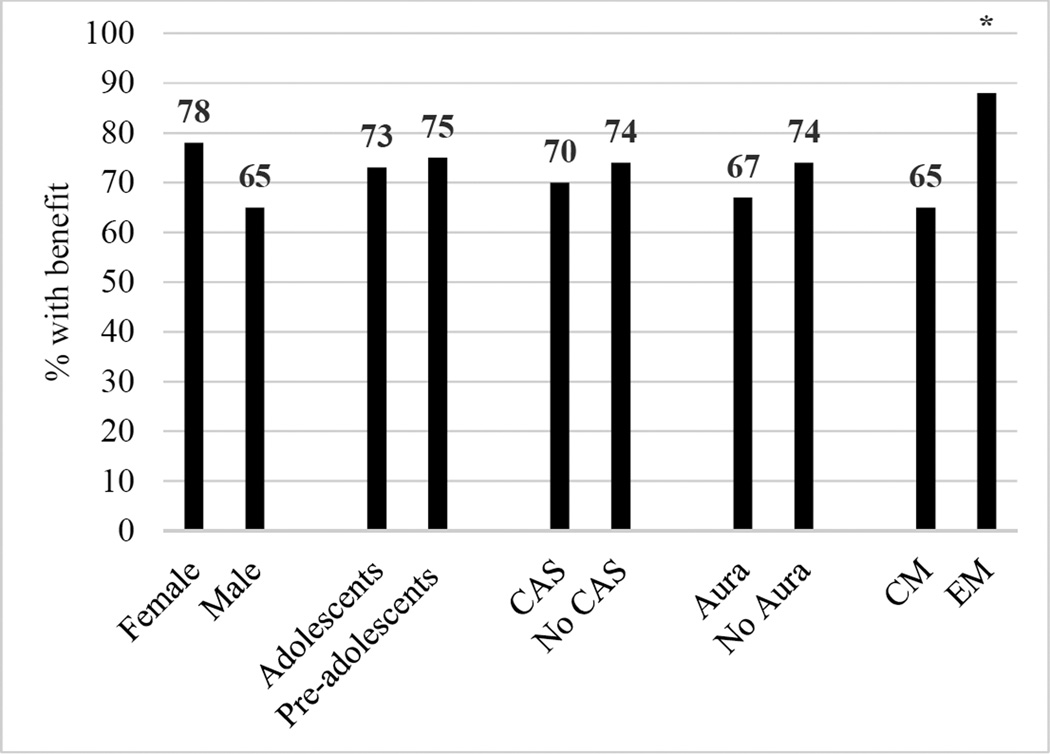
Of the 127 patients with migraine, 65 (51%) had at least one documented adequate triptan trial. The majority of these patients had one adequate triptan trial (51), while 9 had two and 5 had three. Of 64 with triptan outcome data, 47 (73%) benefitted from a triptan. Results of univariate analyses are shown in Figure 1. Having episodic migraine was the only statistically significant predictor of triptan benefit (p=0.048, Fisher’s exact).
Figure 1. Percentage of pediatric migraine patients who benefitted from triptans, stratified by clinical predictors.
CAS=cranial autonomic symptoms, CM=chronic migraine, EM=episodic migraine
In a multivariate logistic regression model, chronic migraine, aura, and CAS were not statistically significant predictors of triptan benefit (see Table 3).
Table 3.
Multivariate logistic regression model examining predictors of triptan benefit in pediatric migraineurs
| Predictor Variable | Odds Ratio | 95% CI |
|---|---|---|
| Chronic migraine | 0.25 | 0.06 – 1.04 |
| Aura | 0.65 | 0.09 – 4.45 |
| CAS | 0.75 | 0.22 – 2.52 |
CI=confidence interval, CAS=cranial autonomic symptoms
Discussion
The majority (73%) of pediatric migraine patients attending a subspecialty headache clinic benefitted from triptans. Those with chronic migraine were less likely to benefit compared to those with episodic migraine (65% vs. 88%) in univariate analysis, though the majority of chronic migraineurs still benefitted from triptans and would therefore reasonably warrant a triptan trial. While the presence of aura was associated with lower likelihood of triptan benefit, this was not statistically significant. In adults, aura is associated with only an 8% lower response rate8. Given our small sample size, we were likely underpowered to detect this relatively small effect size. Cranial autonomic symptoms were not associated with a higher triptan response rate, which is different from what has been seen in adults6,7.
One explanation for the lower triptan response rate seen in chronic migraineurs compared to episodic migraineurs is that those with chronic migraine may spend less time in the mild headache intensity phase, which is when triptans are most effective27. Because of this decrease in triptan response rate, it is essential for pediatric patients with chronic migraine to receive effective prophylactic treatment options to reduce the number of headache days per month, thereby diminishing reliance on triptans.
While we were underpowered to detect the effect size of decreased triptan responsiveness that has been seen in adults with aura28, this study provides preliminary data to help support and plan a larger prospective study on the effect of aura on triptan efficacy in pediatric migraineurs. Differences in the pathophysiology of migraine with aura vs. migraine without aura may lead to differences in effective treatment strategies. For example, inhibiting cortical spreading depression with tonabersat prevents attacks of migraine with aura but has no effect on non-aura attacks29. Because of differences in pathophysiology, patients who have migraine with aura may respond differently to acute therapies30 and this is important to investigate in both pediatric and adult migraineurs.
CAS were not associated with a higher triptan response rate in pediatric migraineurs. In contrast, in adults triptans were more likely to lead to pain freedom in patients with unilateral CAS7. Perhaps this difference can be partially explained by the developmental aspects of migraine in the pediatric age group. For example, in children and adolescents with migraine, CAS are most often bilateral31, just as migraine headache in this age group is most often bilateral2. Given the involvement of the trigeminal autonomic reflex32 in migraineurs with CAS, these migraineurs may more intensely activate the trigeminal afferent system, resulting in greater engagement of peripheral 5-HT1B/1D receptors, which are the receptors targeted by triptans. Perhaps unilateral CAS more intensely activate the trigeminal autonomic reflex than bilateral CAS and thus recruit more of these receptors targeted by triptans.
The main limitations of this study are the small sample size and the retrospective design. In some cases medical record data on triptan trials was insufficiently detailed to determine adequacy, which further limited the sample size. Retrospective assessment of triptan benefit at the clinical follow-up visit also allowed for possible recall bias in the patients’ recollection of triptan efficacy.
Being able to predict triptan response based on clinical variables would be quite useful in pediatric migraine. This would allow early initiation of the most effective treatments, thereby decreasing disability and avoiding missed school. Given the developmental differences in migraine phenotype between children and adults, there may be age-related differences in treatment responses. Larger, prospective pediatric studies are needed to further study predictors of triptan response in pediatric migraineurs. In addition, future pediatric triptan trials should provide response rates stratified by clinical variables such as aura.
Acknowledgments
Peter J. Goadsby: Dr. Goadsby reports grants and personal fees from Allergan, grants and personal fees from eNeura, personal fees from Autonomic Technologies Inc, grants and personal fees from Amgen, personal fees from AlderBio, personal fees from Pfizer, personal fees from DrReddy, personal fees from Zosano, personal fees from Colucid, personal fees from Eli-Lilly, personal fees from Avanir, personal fees from Gore, personal fees from Heptares, personal fees from Nupathe, personal fees from Teva, personal fees from Cipla, personal fees from Ajinomoto, personal fees from Akita, personal fees from Wells Fargo, personal fees from Ethicon, personal fees from EMKinetics, personal fees from MedicoLegal work in headache, personal fees from Journal Watch, personal fees from Up-to-Date, outside the submitted work; In addition, Dr. Goadsby has a patent Magnetic stimulation for headache pending.
Amy A. Gelfand: Dr. Gelfand has received research funding from Allergan, eNeura and EMKinetics, as well as the Migraine Research Foundation and salary support from NIH/NCATS (8KL2TR000143-09). She has consulted for Eli Lilly and received personal compensation for medical-legal consulting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Hannah Johnson: no disclosures.
References
- 1.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia : an international journal of headache. 2010;30(9):1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- 2.The International Classificatio of Headache Disorders, 3rd edition (beta version) Cephalalgia : an international journal of headache. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 3.Arruda MA, Bigal ME. Migraine and migraine subtypes in preadolescent children: association with school performance. Neurology. 2012;79(18):1881–1888. doi: 10.1212/WNL.0b013e318271f812. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Manack A, Ricci JA, Chee E, Turkel CC, Winner P. Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in adolescents study (C-dAS) Headache. 2011;51(5):693–706. doi: 10.1111/j.1526-4610.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- 5.Stang PE, Osterhaus JT. Impact of migraine in the United States: data from the National Health Interview Survey. Headache. 1993;33(1):29–35. doi: 10.1111/j.1526-4610.1993.hed3301029.x. [DOI] [PubMed] [Google Scholar]
- 6.Barbanti P, Fabbrini G, Vanacore N, Pesare M, Buzzi MG. Sumatriptan in migraine with unilateral cranial autonomic symptoms: an open study. Headache. 2003;43(4):400–403. doi: 10.1046/j.1526-4610.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 7.Barbanti P, Fofi L, Dall'Armi V, et al. Rizatriptan in migraineurs with unilateral cranial autonomic symptoms: a double-blind trial. J Headache Pain. 2012;13(5):407–414. doi: 10.1007/s10194-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen JM, Goadsby PJ, Charles A. Reduced efficacy of sumatriptan in migraine with aura vs without aura. Neurology. 2015 doi: 10.1212/WNL.0000000000001535. [DOI] [PubMed] [Google Scholar]
- 9.(IHS) HCCotIHS. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 10.Ueberall MA, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology. 1999;52(7):1507–1510. doi: 10.1212/wnl.52.7.1507. [DOI] [PubMed] [Google Scholar]
- 11.Winner P, Rothner AD, Saper J, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics. 2000;106(5):989–997. doi: 10.1542/peds.106.5.989. [DOI] [PubMed] [Google Scholar]
- 12.Rothner AD, Winner P, Nett R, et al. One-year tolerability and efficacy of sumatriptan nasal spray in adolescents with migraine: results of a multicenter, open-label study. Clinical therapeutics. 2000;22(12):1533–1546. doi: 10.1016/s0149-2918(00)83051-9. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Sato K, Nishioka H, Sakai F. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia. 2014;34(5):365–375. doi: 10.1177/0333102413510213. [DOI] [PubMed] [Google Scholar]
- 14.Derosier FJ, Lewis D, Hershey AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics. 2012;129(6):e1411–e1420. doi: 10.1542/peds.2011-2455. [DOI] [PubMed] [Google Scholar]
- 15.Linder SL. Subcutaneous sumatriptan in the clinical setting: the first 50 consecutive patients with acute migraine in a pediatric neurology office practice. Headache. 1996;36(7):419–422. doi: 10.1046/j.1526-4610.1996.3607419.x. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald JT. Treatment of juvenile migraine with subcutaneous sumatriptan. Headache. 1994;34(10):581–582. doi: 10.1111/j.1526-4610.1994.hed3410581.x. [DOI] [PubMed] [Google Scholar]
- 17.Rothner AD, Wasiewski W, Winner P, Lewis D, Stankowski J. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache. 2006;46(1):101–109. doi: 10.1111/j.1526-4610.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis DW, Winner P, Hershey AD, Wasiewski WW Adolescent Migraine Steering C. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics. 2007;120(2):390–396. doi: 10.1542/peds.2007-0085. [DOI] [PubMed] [Google Scholar]
- 19.Christensen ML, Eades SK, Fuseau E, Kempsford RD, Phelps SJ, Hak LJ. Pharmacokinetics of naratriptan in adolescent subjects with a history of migraine. Journal of clinical pharmacology. 2001;41(2):170–175. doi: 10.1177/00912700122009980. [DOI] [PubMed] [Google Scholar]
- 20.Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Cephalalgia : an international journal of headache. 2012;32(10):750–765. doi: 10.1177/0333102412451358. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt DJ, Pearlman E, Hamalainen M, et al. Long-term open-label safety study of rizatriptan acute treatment in pediatric migraineurs. Headache. 2013;53(1):104–117. doi: 10.1111/j.1526-4610.2012.02285.x. [DOI] [PubMed] [Google Scholar]
- 22.Linder SL, Mathew NT, Cady RK, Finlayson G, Ishkanian G, Lewis DW. Efficacy and tolerability of almotriptan in adolescents: a randomized, double-blind, placebo-controlled trial. Headache. 2008;48(9):1326–1336. doi: 10.1111/j.1526-4610.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 23.Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA : the journal of the American Medical Association. 2007;297(13):1443–1454. doi: 10.1001/jama.297.13.1443. [DOI] [PubMed] [Google Scholar]
- 24.McDonald SA, Hershey AD, Pearlman E, et al. Long-term evaluation of sumatriptan and naproxen sodium for the acute treatment of migraine in adolescents. Headache. 2011;51(9):1374–1387. doi: 10.1111/j.1526-4610.2011.01965.x. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoph-Diener H, Ferrari M, Mansbach H, Group SDS. Predicting the response to sumatriptan: the Sumatriptan Naratriptan Aggregate Patient Database. Neurology. 2004;63(3):520–524. doi: 10.1212/01.wnl.0000133207.70312.30. [DOI] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Zanchin G, Geraud G, et al. Early vs. non-early intervention in acute migraine-'Act when Mild (AwM)'. A double-blind, placebo-controlled trial of almotriptan. Cephalalgia : an international journal of headache. 2008;28(4):383–391. doi: 10.1111/j.1468-2982.2008.01546.x. [DOI] [PubMed] [Google Scholar]
- 28.Hansen JM, Goadsby PJ, Charles A. Reduced efficacy of sumatriptan in migraine with aura vs without aura. Neurology. 2015;84(18):1880–1885. doi: 10.1212/WNL.0000000000001535. [DOI] [PubMed] [Google Scholar]
- 29.Hauge AW, Asghar MS, Schytz HW, Christensen K, Olesen J. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009;8(8):718–723. doi: 10.1016/S1474-4422(09)70135-8. [DOI] [PubMed] [Google Scholar]
- 30.Charles A, Hansen JM. Migraine aura: new ideas about cause, classification, and clinical significance. Curr Opin Neurol. 2015;28(3):255–260. doi: 10.1097/WCO.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 31.Gelfand AA, Reider AC, Goadsby PJ. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology. 2013;81(5):431–436. doi: 10.1212/WNL.0b013e31829d872a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19(2):115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]