BACKGROUND
The high prevalence of HIV among people who inject drugs (PWID) contributes substantially to global mortality rates (Degenhardt & Hall, 2012). Worldwide, 30% of HIV infections outside of sub-Saharan Africa are caused by injection drug use (International Harm Reduction Association [IHRA], 2012). It is estimated that between 11 and 21.2 million people inject drugs of which between .8 and 6.6 million are infected with HIV (Mathers, et al., 2008). Numerous scientific studies—including reports by The World Health Organization (WHO), the United Nations Office on Drugs and Crime (UNODC), and the Joint United Nations Programme on HIV/AIDS (UNAIDS)—conclude that opioid agonist therapy (OAT) and needle and syringe programs (NSP) are empirically-validated interventions to curb the spread of HIV/AIDS among PWID (Bluthenthal et al., 2004; Frischer & Elliot, 1993; Gibson, Flynn & Perales, 2001; Huang et al., 2014; Judd et al., 2004; Khoshnood, Blankenship, Pollack, Roan & Altice, 2000; Kral, Anderson, Flynn, & Bluthenthal, 2004; Miller, Tyndall, Spittal, Palepu, & Schechter, 2002; Millson et al., 2007; Obadia, Feroni, Perrin, Vlahov & Moatti, 1999; Pollack, Khoshnood, Blankenship & Altice, 2002; Rhodes et al., 2004; Riley, 2000; Strathdee et al., 2010; Van Den Berg, Smit, Van Brussel, Coutinho & Prins, 2007).
The provision of accessible HIV prevention interventions for PWID is a multisectoral, rights-based approach to reducing environmental and individual risk factors for HIV infection (Blankenship, Reinhard, Sherman, & El-Bassel, 2015; Beletsky et al., 2015; Cook et al., 2010; De Cock, El-Sadr, Ghebreyesus, 2011; Degenhardt, et al., 2010; De Jarlais, 1995; Ostlin et al., 2006; MacMaster, 2004; Rhodes, 2002; Rhodes et al., 2003; 2004; Strathdee et al., 2010; Strathdee et al., 2015). There is a large body of global literature calling for collaboration across sectors in the delivery of HIV prevention interventions (Degenhardt, 2010; Campbell & Williams, 1999; Ostlin, et al., 2006). This literature stresses the importance of including government actors, criminal justice agencies, public health organizations, social service offices, and international development agencies to in a socially inclusive agenda that is rooted in sound human-rights principles of equal access and medical treatment with dignity for PWID (Beletsky, Grau & White et al., 2011; Beletsky et al., 2015)
Harm reduction interventions have the potential to change environmental risk factors by providing places to obtain clean syringes rather than obtaining syringes in locations known to increase risk of HIV infection namely public locations or shooting galleries (Drucker, Lurie, & Wodak et al., 1998). Further, a safe and designated physical space to discard potentially infectious syringes is a promising alternative to discarding syringes in open spaces (Friedman, de Jong, & Rossi et al., 2007). Regarding individual-level factors, both NSP and OAT remove the mode of disease transmission by virtue of two different mechanisms of behavior change. Provision of an adequate dose of an opioid substitute for heroin consumption reduces injection behaviors thereby reducing the likelihood of sharing syringes (Mathers et al., 2010; Vlahov, 1997) The mechanism producing individual-level change for NSPs involves removing as many potentially infectious syringes from public access and syringe sharing, which reduces potential opportunities for transmission of HIV and other communicable diseases (World Health Organization, 2015).
Extant literature using patient-level data consistently identifies a reduction in the incidence of HIV as a result of access to treatment and retention in care (Springer, Chen, & Altice, 2010; Sorenson & Copeland, 2000; Rhoades, Creson, Elk, Schmitz, & Grabowski, 1998; Thiede, Hagan & Murill, 2000; Wells, Calsyn, Clark, Jackson & Saxon, 1996). A meta analysis by MacArthur et al., (2012) summarized findings from 12 studies and pooled 9 studies with 23608 person years of follow-up and found a 54% reduction in HIV infection among PWID. A later systematic review of reviews by MacArthur et al., (2014) found 13 reviews of interventions to treat HIV infection among PWID. Findings from the reviews suggest moderate support for NSPs and strong support for OST programs in reducing incidence rates of HIV among PWID.
In addition to individual-level behavior change and reducing environmental risk factors, the structural and macro level importance of adopting social policies in favor of the implementation of harm reduction programs are increasingly discussed in international literature (Beletsky et al., 2015; Degenhardt 2010; Harm Reduction Network, 2015; WHO, 2015). The transition from privately funded pilot programs to programs that are funded nationally constitutes a structural macro level shift in approaches to delivering harm reduction services with major implications for aggregate national-level rates of HIV (Harm Reduction Network, 2015). The adoption of social policies on the local, community and national level facilitate the implementation of NSP and OAT programs. Implementation of harm reduction programs increase access to the proper resources for building sustainable individual behavior change thereby reducing rates of HIV infection and other drug-related harms among PWID (Beletsky et al., 2015). By 2009, OAT existed in 70 nations and NSPs (formal and pilot) existed in 82 nations, with an estimated 61 per 100 PWID receiving OAT in Europe (Mathers et al., 2010).
On an aggregate level, no studies to date have examined the minimum coverage of OAT required to make an impact on the incidence of HIV cross-nationally. Nonetheless, ensuring open and rapid access to OAT is critical to maintain the effectiveness of OAT as primary HIV prevention (Bruce, 2009). Beyond the scope of harm reduction programs, total expenditures on the provision of healthcare programs may be associated with incidence rates of HIV among PWID as well as in the general population. Public and private expenditures on health could indicate a society’s commitment to promoting general population health (Or, 2000a; 2000b). Moreover, measurement of total expenditures on health indicates the level of financial resources allocated by local, regional, national governments and the private sector to improve health (Lu, Schneider, Gubbins, Leach-Kemon, Jamison, & Murray, 2010; Or, 2000a; 2000b). Studies have found that increasing health spending may attenuate rates of preventable deaths, particularly for heart disease, diabetes and infant mortality (Mays & Smith, 2011; Or, 2000a). However, research into the cross-national effects of healthcare expenditures on incidence rates of HIV is lacking. A significant relationship between greater spending on healthcare and lower incidence rates of HIV may provide some preliminary evidence for allocating more financial resources to healthcare.
Despite mounting scientific and theoretical support, the existing body of literature elucidating the effects of harm reduction programs on incidence rates of HIV suffers from several methodological shortcomings. A majority of studies have investigated the effects of harm reduction programs using cross-sectional, individual-level designs without a comparison group. Several studies have explored the link between incidence rates of HIV and harm reduction interventions through a cohort study methodology (Frischer & Elliot, 1993; Pollack et al., 2002; Judd et al., 2004; Van Den Berg et al., 2007; Huang et al. 2014) and pre-post study designs without control groups (e.g. Gibson et al., 2001; Millson et al., 2007). Most of these studies rely upon self-reported data, are based on cross-sectional designs, and focus on a single country or small number of nations. These studies neglect to consider the impact of policies cross-nationally and economic drivers as contributors to the epidemic.
Though some researchers have generated insights into cross-national drivers of incidence rates of HIV using epidemiologic techniques (e.g Grassly, et al., 2003; Vickerman et al., 2006; Strathdee et al., 2010; Alistar, Owens & Brandeau, 2011), none have used longitudinal incidence data across countries to explore the relationship between harm reduction and HIV rates. This study aims to address a substantive and methodological gap in the existing literature by examining the impact of years of methadone maintenance therapy (MMT) implementation, years of NSP implementation, and client utilization rates for MMT on HIV incidence rates among 28 European countries from 1995 to 2011 after adjusting for Gross Domestic Product (GDP) and total healthcare expenditures.
METHODS
Data and Measures
Data for this study were extracted from several publically available sources. The World Health Organization (WHO) Regional Office for Europe, and the European Center for Disease Control (ECDC) jointly compile HIV/AIDS surveillance data on the number of new cases of HIV infection in the general population for all 53 nations in the WHO European Region from 1985 to the present (World Health Organization [WHO], 2015). Annual numbers of new HIV cases for the 28 countries included in this study were divided by the total population and multiplied by 100,000 to produce a population-adjusted overall HIV incidence rate using yearly population data provided by the World Bank.
The European Monitoring Center for Drugs and Drug Addiction (EMCDDA) provided data reporting the year of introduction of harm reduction programs from 30 independent nations, annual number of clients enrolled in MMT from 1995–2011, and the number of cases of HIV infection among PWID. Incidence rates of HIV among PWID were converted from per-million to per 100,000. Retrospective reporting and implementation of new monitoring systems to track new cases of HIV among PWID warranted the exclusion of data points for Greece in 1999 and the Netherlands in 2002 to minimize the multiple counting of new infections. The years of NSP and MMT implementation were calculated by subtracting the year of first implementation from 2011 for each of the countries. For example, a country that initiated MMT in 1967 (Sweden) (the earliest date available) was subtracted from 2011. In this instance, the time range began at 17 years and reached 44 years in 2011.
Data were available for the greatest number of countries from the EMCDDA on year of MMT implementation compared to other OAT interventions. As a result, this study focuses exclusively on the effects of MMT implementation on incidence rates of HIV. Data on the annual numbers of clients receiving MMT for 26 countries (data were not available for Norway and Croatia) were retrieved and adjusted for population size using the same procedures that produced HIV incidence rates (per 100,000 of the population). Due to excessive missing data on key independent variables, Cyprus and Turkey were excluded leaving a final sample of 28 European nations for analysis of incidence rates of HIV in the general population and among PWID and 26 nations for the analysis of uptake in MMT utilization.
GDP and total expenditures on healthcare (public and private, as a percentage of GDP) were selected as control variables from the World Bank’s World Development Indicators Database (World Bank, 2015). Prior to entering GDP into the multivariable models, annual estimates of GDP were divided by 100,000. Incidence rates of HIV and service utilization rates were converted to the logarithm scale in order to reduce the potential bias introduced by heavily skewed data. The final dataset of 28 countries consisted of 477 observations over a time period from 1995 to 2011. For years of implementation of NSP and MMT, the analyses consisted of 426 observations for overall HIV rates and 376 for HIV rates among PWID. For MMT utilization rates, the analyses consisted of 418 observations for overall incidence rates of HIV and 365 observations for incidence rates among PWID.
Statistical Analyses
We used a year- and country-level fixed-effects model to estimate the potential association between years of implementation of harm reduction programs and incidence rates of HIV after adjusting for GDP and expenditures on health. A fixed-effects model introduces country and time dummy variables to remove the effects of time- and country-level invariant characteristics, whether observable or unobservable, on the outcome variable, thereby attenuating a significant source of potential omitted variable bias (Kreft, Kreft & Leeuw, 1998; Snijders, 2011; Rabe-Hasketh & Skrondel, 2008). Specifically, this analysis modeled only the net effects of time-variant predictor variables (across-country effects), thus minimizing the potential bias introduced by unmeasured within-country effects (Kreft, Kreft & Leeuw, 1998).
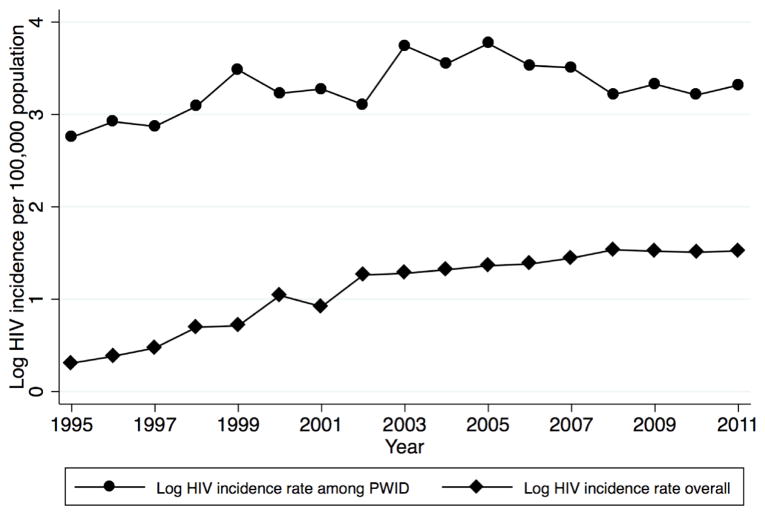
Our models tested for linear, quadratic and cubic country-specific trends that may have been correlated with years of implementation. The use of trends provides a relatively conservative test to evaluate models for potential bias from country-level trends (Rabe-Hasketh, 2008). The logged incidence rates of HIV appear to follow a linear and quadratic distribution (Figure 1) suggesting that linear and quadratic trends are most appropriately suited to control for the effects of country-specific trends. Given the quadratic distribution, results are presented for models that utilized quadratic trends. More information on subdivisions in the recording of data within countries is provided in the appendix.
Figure 1.
Logged HIV incidence in 28 European countries (1995–2011)
The first set of models tested the potential effects of years of NSP and MMT implementation on logged incidence rates of HIV in the general population and among PWID. In a second set of models, a fixed-effects regression model tested the potential effects of logged MMT utilization rates on logged incidence rates of HIV after adjusting for the same explanatory variables as the first set of models. Out of the total sample of 28 countries, NSPs, and MMT programs were nascent interventions at the beginning of the time period used in this study. To allow new harm reduction programs time to gain traction within the population, a three-year lag was applied to variables measuring years of NSP and MMT implementation.
RESULTS
Table 1 presents descriptive statistics and means for each country and the overall sample. The mean overall incidence rate of HIV was 5.70 (.40–30) (per 100,000 of the population) from 1995–2011. The numbers within parentheticals in Table 1 for both the overall population and PWID embody the minimum and maximum incidence rates of HIV for each country and the entire sample of countries over the time period from 1995–2011. Among PWID, the mean overall incidence rate of HIV was 123.04 (1.70–1608.99) (per 100,000 of the population). During the same time period, the mean GDP was 30891.23 (in hundreds of thousands) (PPP), and, on average, total healthcare expenditures (public and private) accounted for 7.99% of GDP (PPP).
Table 1.
Descriptive statistics for 28 European Nations for years of harm reduction implementation until 2011
| Overall HIV Rate1 Mean(SD9) | HIV Rate1 PWID2 Mean(SD9) | Years Public NSP3 | Years Public/Pilot NSP3 | Years MMT4 | MMT Utilization Rate1 Mean(SD9) | Health care spending5 (SD9) | GDP6(PPP7)8 Mean (SD9) | |
|---|---|---|---|---|---|---|---|---|
| Austria | 4.27(.69) | 53.07(18.17) | 22 | 22 | 25 | 43.28 | 10.33%(.58) | 39771.34(3390.28) |
| Belgium | 9.24(1.34) | 19.09(6.14) | 12 | 18 | 18 | 103.41(51.06) | 9.09%(1.10) | 38152.29(2915.56) |
| Bulgaria | 1.02(.79) | 32.96(36.37) | 13 | 17 | 16 | 23.13(14.78) | 6.62%(.98) | 11459.42(2687.31) |
| Croatia | 1.04(.40) | 5.57(3.42) | 16 | 16 | 21 | n.a | 7.05%(.58) | 17627.49(2943.52) |
| Czech Rep | .83(.44) | 4.33(2.86) | 21 | 25 | 14 | 5.07(1.43) | 6.80%(.46) | 23721.56(3696.40) |
| Denmark | 5.09(.53) | 37.46(16.21) | 26 | 26 | 41 | 72.96(44.64) | 9.48%(1.04) | 41370.57(2610.37) |
| Estonia | 30.15(23.33) | 1608.99(1426.96) | 11 | 15 | 11 | 56.23(31.34) | 5.60%(64) | 18268.51(4995.59) |
| Finland | 2.58(.80) | 41.11(44.97) | 16 | 16 | 37 | 6.42(4.19) | 8.08%(.59) | 35941.68(4646.69) |
| France | 8.56(1.00) | 24.25(6.53) | 17 | 23 | 17 | 21.87(16.65) | 10.76%(.55) | 35034.36(2237.34) |
| Germany | 2.75(.61) | 18.65(5.89) | 20 | 28 | 20 | 64.90(9.14) | 10.70%(.46) | 37984.91(2539) |
| Greece | 4.73(.96) | 25.83(51.01) | 14 | 14 | 19 | 15.26(7.68) | 9.04%(.65) | 27427.91(3669.00) |
| Hungary | .95(.39) | 1.70(.77) | 12 | 18 | 17 | 6.82(.90) | 7.65%(.52) | 198088.55(3024.47) |
| Ireland | 6.88(2.53) | 107.13(55.18) | 23 | 23 | 12 | 161.53(29.34) | 7.43%(1.22) | 40887.19(10113.05) |
| Italy | 3.98(1.39) | 53.88(26.20) | 22 | 22 | 36 | 130.75(16.95) | 8.26%(.76) | 35848.74(1620.32) |
| Latvia | 12.14(6.73) | 473.12(274.72) | 15 | 15 | 16 | 4.62(2.50) | 6.34%(.33) | 14715.18(4711.92) |
| Lithuania | 2.81(1.69) | 175.43(104.43) | 15 | 15 | 17 | 21.95(5.45) | 6.25%(.54) | 15833.31(4829.28) |
| Lux. | 9.51(1.67) | 101.04(42.53) | 19 | 19 | 23 | 193.32(76.30) | 7.00%(.99) | 81587.47(10220.62) |
| Malta | 3.87(2.09) | 49.28(24.54) | 15 | 15 | 15 | 244.62(9.24) | 7.60%(1.16) | 25117.28(2207.37) |
| Neth. | 5.97(1.31) | 12.69(8.87) | 26 | 28 | 43 | 60.82(7.18) | 9.69%(1.56) | 42145.99(3702.04) |
| Norway | 4.38(1.36) | 24.92(7.37) | 24 | 24 | 14 | n.a | 9.68%(.55) | 59440.40(4290.31) |
| Poland | 2.18(.28) | 50.91(33.35) | 23 | 23 | 20 | 3.75(1.31) | 6.2%(.52) | 16295.37(3417.11) |
| Portugal | 15.20(5.02) | 500.76(268.55) | 19 | 19 | 34 | 91.91(61.49) | 9.34(1.15) | 25585.81(1710.81) |
| Romania | 1.36(1.36) | 6.17(14.72) | 12 | 12 | 13 | 2.13(.94) | 4.77%(.85) | 13162(3042.83) |
| Slovakia | .40(.32) | 2.55(1.37) | 18 | 18 | 15 | 8.87(.41) | 6.80%(1.23) | 18967.66(4333.34) |
| Slovenia | 1.31(.72) | 9.05(6.55) | 16 | 20 | 22 | 85.15(34.01) | 8.27%(.49) | 24879.76(4009.66) |
| Spain | 5.17 (1.53) | 123.27(58.60) | 27 | 27 | 22 | 172.55(38.29) | 8.09%(.86) | 31207.8(2912.87) |
| Sweden | 3.72(.91) | 29.33(15.59) | 26 | 26 | 44 | 10.09(3.96) | 8.83%(.61) | 38596.54(4466.78) |
| UK | 9.20(3.16) | 26.99(4.88) | 25 | 25 | 43 | 180.21(16.54) | 7.91%(1.09) | 34115.32(3241.62) |
|
| ||||||||
| Overall | 5.70 (5.71) | 123.04(380.48) | 18.75 | 20.32 | 23.04 | 69.74(69.28) | 7.99%(1.73) | 30891.23(15474.45) |
per 100,000 population;
People who inject drugs; Needle and Syringe Programs;
Methadone Maintenance Therapy;
Annual spending on health as % of GDP in PPP;
Gross Domestic Product;
Purchasing Power Parity;
in hundreds of thousands;
Standard Deviation
Figure 1 shows temporal patterns in the overall incidence rate of HIV as well as the incidence rate of HIV among PWID during the time period from 1995 to 2011. The overall incidence rate of HIV increased steadily from 1995 to 2008, and then it began to level off for the remaining three years. A different pattern becomes apparent through visual inspection of the incidence rate of HIV among PWID, which increased until 2005, followed by a steady decline until 2011.
Countries varied widely in years of NSP and MMT implementation, in the number of clients enrolled in MMT, and in total spending on healthcare. In 1995, fifteen countries implemented publically funded NSPs and 18 implemented MMT. By the beginning of 2000, the number of countries that implemented publically funded NSPs and MMT was 24 and 27 respectively. Only Estonia had not yet implemented MMT, and Belgium, Estonia, Hungary, and Romania had not yet implemented NSPs.
By the end of the study period in 2011, all 28 countries had implemented publically funded NSPs and MMT programs. The country with the highest incidence rates of HIV (overall and among PWID), Estonia, implemented NSP and MMT for the shortest amount of time (11 years) and ranked 2nd from the bottom in expenditures on healthcare (5.89%).
Needle and syringe programs
The average number of years of implementing NSP was 18.80 for publically funded programs. This increased to 20.32 when accounting for additional years of pilot program implementation. Table 2 presents results from year- and country-level fixed-effects models examining the potential effects of years of NSP adoption on logged incidence rates of HIV.
TABLE 2.
Regression models of the effects of number of years of NSP2 and control variables on logged HIV incidence rates in 28 countries
| Unadjusted | Adjusted | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| logHIV rate9 overall no trend | logHIV rate9 overall with trend1 | logHIV rate9 PWID7 no trend | logHIV rate9 PWID7 with trend1 | logHIV rate9 overall no trend | logHIV rate9 overall with trend1 | logHIV Rate9 PWID7 no trend | logHIV rate9 PWID7 with trend1 | |||||||||
|
| ||||||||||||||||
| Variables | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 |
| Year and country level fixed effects | ||||||||||||||||
| NSP2 (lagged3) | −.07*** | .01 | −.04** | .01 | −.05* | .02 | −.02 | .03 | −.06*** | .01 | −.04** | .01 | −.05* | .02 | −.07* | .029 |
| GDP4(PPP5)6,9 | 1.57 | .89 | −.91 | .98 | 2.01 | 1.53 | −.54 | 1.76 | ||||||||
| Healthcare spending8 | −.18*** | .04 | −.10* | .04 | −.37*** | .08 | −.16 | .087 | ||||||||
| Constant | 1.44*** | .20 | 2.43*** | .44 | 3.73*** | .39 | 3.81 | 2.95 | 2.24*** | .42 | 3.50*** | .63 | 5.83*** | .81 | 9.67 | 5.69 |
|
| ||||||||||||||||
| Model F-Test | 20.05*** | 26.14*** | 1.74* | 13.12*** | 20.09*** | 25.81*** | 2.83*** | 9.78*** | ||||||||
p<.05;
p<.01;
p<.001;
Model contains linear and quadratic country-specific trend variables;
Needle Exchange Programs;
Lagged by 3 years;
Gross Domestic Product;
Purchasing Power Parity;
In hundreds of thousands;
People who inject drugs;
Annual Expenditure on health as a percent of GDP in PPP from public and private sources;
Per 100,000 population;
Coefficient;
Standard Error
In the unadjusted models, each year of NSP implementation with a 3-year lag operator was associated with a decline in the overall incidence rate of HIV of .07 (p<.05). Each year of NSP implementation was associated with a decline of 0.04 (p<.01) in the logged overall incidence rate of HIV after adding country-specific trends into the model. In the unadjusted model with no trend, each additional year of NSP was associated with a significant decrease in the logged incidence rate of HIV among PWID of 0.05 (p<.05). The effects of years of NSP implementation were robust to country-specific trends in both the unadjusted and the adjusted models. Each additional year of NSP implementation was associated with a decline of .06 (p<.001) in the incidence rate of HIV among PWID after controlling for expenditures on health, GDP and country-specific trends.
In experimental models, the potential effects of NSP implementation on reducing the overall logged incidence rate of HIV were robust to cubic trends suggesting that the potential effects of years of NSP implementation were able to withstand the most conservative tests of confounding by trends at the country level (results not shown but available upon request).
Methadone maintenance therapy
The average number of years of implementation for MMT was 23.04 years. Table 3 presents year- and country-level fixed-effects models with a 3-year lag, examining the effects of years of MMT on the logarithms of the overall incidence rate of HIV and the incidence rate of HIV among PWID.
TABLE 3.
Regression models of the effects of number of years of MMT2 and control variables on logged HIV incidence rates in 28 countries
| Unadjusted | Adjusted | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| logHIV rate overall no trend | logHIV rate overall with trend1 | logHIV rate PWID7 no trend | logHIV rate PWID with trend | logHIV rate overall no trend | logHIV rate overall with trend1 | logHIV rate PWID no trend | logHIV rate PWID with trend | |||||||||
|
| ||||||||||||||||
| Variables | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 | Coef10 | SE11 |
| Year and country level fixed effects | ||||||||||||||||
| MMT2(lagged3) | −.03*** | .004 | −.01 | .15 | −.03*** | .008 | −.002 | .01 | −.03*** | .004 | −.008 | .01 | −.02** | .01 | −.004 | .01 |
| GDP4(PPP5)6 | 1.24 | 1.42 | −.69 | 1.00 | 1.59 | 1.51 | −.22 | 1.78 | ||||||||
| Healthcare spending8 | −.17*** | .04 | −.10* | .05 | −.35*** | .08 | −.15 | .09 | ||||||||
| Constant | .91*** | 0.13 | 2.01*** | 0.40 | 3.44*** | 0.24 | 6.41 | 5.59 | 1.24*** | 0.4 | 3.02*** | .60 | 5.41*** | 0.76 | 8.42 | 5.71 |
|
| ||||||||||||||||
| Model F-Test | 21.07*** | 25.69*** | 2.10** | 9.72*** | 20.71*** | 25.34*** | 2.97*** | 12.31*** | ||||||||
p<.05;
p<.01;
p<.001;
Model contains linear and quadradic country-specific trend variables
Methadone Maintenance Therapy;
Lagged by 3 years;
Gross Domestic Product;
Purchasing Power Parity;
in hundreds of thousands;
People who inject drugs;
Annual Expenditure on health as a percent of GDP in PPP from public and private sources;
Per 100,000 population;
Coefficient;
Standard Error
The unadjusted models identified a significant negative effect of years of MMT on logged overall incidence rates of HIV in which each year’s increase in MMT resulted in a decline in the log overall incidence rate of HIV of .03 (p<.001). In the unadjusted model for incidence rates of HIV among PWID, each year of MMT predicted a decline in the incidence rate of HIV among PWID of .03 (p<.01). After adjusting for GDP and public expenditures on healthcare, each year of MMT implementation resulted in a decline in the logged overall incidence rate of HIV of .03 (p<.001) and of .02 (p<.001) in the logged incidence rate of HIV among PWID. The addition of country-specific trend terms resulted in insignificant findings for potential effects of years of MMT on both logged overall incidence rates of HIV and incidence rates of HIV among PWID in the unadjusted and adjusted models.
Uptake of methadone maintenance therapy
The mean annual rate of MMT utilization from 1995 to 2011 was 69.74 clients (per 100,000 people in the population). Table 4 presents year- and country-specific fixed-effects models investigating the effect of the logarithm of the rate of MMT clients (per 100,000) on the logarithms of the overall incidence rate of HIV and the incidence rate of HIV among PWID (per 100,000).
TABLE 4.
Regression models of the effects of logged rates of methadone service utilization on logged HIV incidence rates in 26 countries
| Unadjusted | Adjusted | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| logHIV Rate2 overall no trends1 | logHIV Rate2 overall with trend1 | logHIV Rate2 PWID6 no trends | logHIV Rate2 PWID7 with trends1 | logHIV Rate2 overall no trends | logHIV Rate2 overall with trend1 | logHIV Rate8 PWID7 no trend | logHIV Rate2 PWID7 with trends1 | |||||||||
|
| ||||||||||||||||
| Variables | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 | Coef9 | SE10 |
| Logged rates of MMT8 | −0.24*** | 0.05 | −0.19 | 0.09 | −0.36*** | 0.11 | −.29 | .21 | −0.23*** | 0.05 | −.09 | .09 | −0.34** | 0.01 | −0.35 | 0.21 |
| Clients2 | ||||||||||||||||
| GDP3 (PPP4)5 | 1.23 | 0.69 | −.71 | 0.79 | 2.71* | 1.24 | 0.51 | 1.72 | ||||||||
| Expenditure on Health7 | −0.10* | 0.05 | −.05 | .06 | −0.54*** | 0.09 | −0.22 | 0.15 | ||||||||
| Constant | 1.62*** | 0.33 | 1.24* | 0.35 | 5.24*** | 0.44 | 5.14*** | 0.85 | 2.05*** | 0.50 | 1.90** | .67 | 8.96 | 1.10 | 7.06*** | 1.70 |
|
| ||||||||||||||||
| Model F-Test | 7.58*** | 10.26*** | 2.38** | 4.95*** | 7.62*** | 9.96*** | 5.33*** | 4.70*** | ||||||||
p<.05;
p<.01;
p<.001;
With linear, squared and cubic trend variables and variables for each trend interacted with country-level dummy variables
Per 100,000 population;
Gross Domestic Product;
Purchasing Power Parity;
in hundreds of thousands;
People who inject drugs;
Annual Expenditure on health as a percent of GDP in PPP from public and private sources;
Methadone Maintenance Therapy;
Coefficient;
Standard Error
In the unadjusted model, each increase in the logged rates of MMT clients was associated with a decrease in the logged overall incidence rate of HIV of 0.24 (p<.001) and a decrease of 0.36 (p<.001) in the logged incidence rate of HIV among PWID. In the adjusted model, each unit increase in rates of MMT clients was associated with a significant decline in the logged overall HIV rate of .23 (p<.001) and 0.34 (p<.01) in the incidence rates of HIV among PWID. These results became insignificant after introducing country-specific trend terms. In all of the models without country-specific trends, expenditures on health were associated with lower incidence rates of HIV in the general population and among PWID. In the models for years of MMT and NSP implementation, expenditures on healthcare remained significant after controlling for country-specific trends. Across models, greater total expenditures on healthcare were associated with lower incidence rates of HIV. Gross domestic product was not a significant predictor of incidence rates of HIV in any of the models.
DISCUSSION
The central aim of this exploratory study was to examine the association between harm reduction programs and incidence rates of HIV in both the general population and PWID in Europe.
Needle and syringe programs
After controlling for year- and country-level fixed-effects and country-level trend terms, this study found an association between years of implementation of NSPs and lower incidence rates of HIV among the general population and PWID.
Methadone maintenance therapy
This study found a significant association between years of implementation of MMT and lower incidence rates of HIV among the general population and PWID. However, the parameter estimates of the effects of MMT became insignificant after adding country-level trend terms thus indicating the possibility of confounding in our results due to country-specific trends.
Uptake of methadone maintenance therapy
In the model without country-specific trends, greater rates of clients entering treatment were associated with lower incidence rates of HIV in the general population and among PWID. After controlling for country-specific trend terms, the association between rates of service utilization of MMT and incidence rates of HIV became insignificant.
Implications
Our findings converge with extant literature suggesting early implementation of MMT and NSP in the HIV epidemic and countries who enrolled greater numbers of PWID may have significantly lower incidence rates of HIV than countries who were delayed in their responses to injection drug use. This is an important lesson for countries with rapidly growing populations of PWID. Heroin addiction and intravenous drug use are on the rise, due in large part to increased availability of heroin worldwide and the growth of prescription opioid abuse (United Nations, 2015). Moreover, empirical studies point to the relationship between concomitant patterns of heroin abuse and HIV infection (Strathdee, 2010, 2011).
Few studies have explored the relationship between public spending on general health and incidence rates of HIV cross-nationally. Our results suggest that allocation of greater financial resources to general health may mitigate the growth of incidence rates of HIV in European nations. Though abundant research highlights the effectiveness of public and private healthcare spending on incidence rates of HIV by virtue of scale-up in low-to-middle-income countries (Bor et al., 2013; De Cock et al., 2011; El-Sadr & Abrams, 2007; Parker, 2009), no studies have explored this issue longitudinally and cross-nationally using fixed-effect models as of this writing. Our findings provide new insight surrounding the benefits accrued from expenditures on healthcare particularly in relation to attenuating the spread of HIV.
Avenues of future research
The findings presented in this analysis allow for a new line of inquiry and are a compelling call to action for additional cross-national research into harm reduction policies. Additional socioeconomic, economic, demographic, and global health variables are now being collected in Europe and other regions as well, with timespans that are becoming increasingly feasible to analyze in empirical inquiry (Cook, Bridge, & Stimson, 2010). Therefore, future comparative studies must explore the effects of harm reduction cross-nationally, using additional potential confounders and focusing on other geographic regions. Additional research must consider expanding dependent variables to include other communicable diseases such as the Hepatitis C and B viruses and sexually transmitted infections.
In addition to other communicable diseases, this study did not investigate the impact of harsh sentencing laws, compulsory treatment protocols, mandatory drug user registration laws, and other indicators of the degree to which a country criminalizes drug use on incidence rates of HIV. Moreover, limited data on dates of implementation for other types of OAT—buprenorphine maintenance therapy, morphine assisted therapies, suboxone maintenance therapies, and heroin assisted therapies, etc.—precluded inclusion of other types of OAT into the research design of this study. Additional cross-national research into the effects of adoption of multiple types of OAT is necessary to expand knowledge surrounding benefits accrued to countries from implementing a combination of approaches to treatment for heroin addiction.
Limitations
This study has some limitations that are worth noting. Despite the HIV/AIDS epidemic officially beginning in 1981, gaps in publicly available data restricted the time window to 1995–2011. Also, this study’s methodological approach assumes that each country engages in the same level of service provision. The literature indicates otherwise, reporting that some European countries are considered to be at the forefront of innovation, while others are deemed to have a weaker framework for harm-reduction program development (Cook et al., 2010). We partially addressed this limitation by including client utilization rates of MMT. However, we were unable to use service utilization data for syringe distribution. Cross-national data were available on number of syringes distributed from 2003 to 2011 from a limited number of countries, which would have hindered our capacity to produce results for the widest time period possible. In addition to utilization rates, this study did not account for differences across countries in rates of coverage of MMT and NSP. Future empirical inquiry into the effects of harm reduction on incidence rates of HIV must account for heterogeneity in rates of coverage of MMT and NSP across countries. Similarly, this study did not control for the quality of care provided in harm reduction programs, which may influence aggregate country-level rates of HIV. Future research should investigate the impact of aggregate quality indicators on incidence rates of HIV including retention in care and dosing levels.
Another limitation involves not accounting for HIV incidence among PWID as a potential driver of overall HIV rates in the general population. We did not include HIV among PWID in the general population due to differences in reporting systems of new cases between the overall rates and the rates of PWID. Since the data originates from two different repositories, the ECDC and the ECDDMA, it is not possible to compare data in the same analytical model. It is possible that cases of HIV were reported or cited by the ECDDMA and not reported to the ECDC. A more likely scenario involves cases being reported to the ECDC that are not detected as HIV acquired through injection drug use. In either scenario, it would not be empirically prudent to control for aggregate rates of HIV among PWID in the model predicting overall HIV rates. This is a limitation in that it is possible that HIV among PWID is a major driver of overall HIV rates in Europe due to high rates of injection drug use. One avenue of future research involves using a standardized reporting system to calculate the ratio of the incidence rate of HIV among PWID to the incidence rate of HIV among the overall population. A statistical model in which the outcome is a rate ratio between the incidence of HIV among PWID and incidence in the overall population would estimate the degree to which harm reduction programs reduce the overall share of PWID relative to the overall HIV incidence rate cross-nationally.
Although countries are differentiated based on GDP, and spending on healthcare, this analysis did not adjust for the effects of other cross-national demographic variables on incidence rates of HIV. Complete data for all of the countries included in this study were not available for cross-national demographic characteristics. In order to retain the greatest number of countries for the longest time period possible, this study only controlled for GDP and expenditures on healthcare as potential confounders. The empirical justification for the utility of MMT and NSP would be strengthened substantially if the effects of implementation on HIV rates remain significant after controlling for cross-national demographic heterogeneity. These preliminary findings warrant additional research that includes more cross-national demographic data.
Another limitation pertains to the ecological study design used to analyze a panel dataset of aggregate rates of HIV infection. Although temporal analysis provides a more rigorous design than cross-sectional or retrospective designs, this exploratory study is associational rather than causal. Specifically, it is critical to avoid inferring causality or associations between variables from findings on an aggregate country-level to an individual-level. Generalization of findings from this study must occur only between countries rather than to a population. This would be a classic example of an ecological fallacy. Other unmeasured potential contributors to national-level declines in incident rates of HIV include health promotion campaigns, changes in testing rates and uptake of anti-retroviral therapies (ART). Finally, the effect of the proliferation of drugs on incidence rates of HIV, either through rates of heroin seizures or the number of drug users seeking services, was not explored in this study.
CONCLUSION
Given the absence of cross-national analyses of correlates of HIV incidence rates, this study opens the door to a new approach to studying the effects of harm reduction policies globally. Our findings reinforce extant literature suggesting that MMT and NSP implementation not only protects the basic human rights and health of PWID, but also promotes the health and wellbeing of societies at large by virtue of reducing overall rates of HIV.
HIGHLIGHTS.
Greater years of needle and syringe program implementation were associated with lower incidence rates of HIV generally and among PWID.
Greater years of methadone maintenance program implementation were associated with lower incidence rates of HIV generally and among PWID.
Higher rates of clients enrolled in methadone maintenance therapy were associated with lower incidence rates of HIV generally and among PWID.
Greater total expenditures on healthcare were associated with lower incidence rates of HIV generally and among PWID.
Acknowledgments
Funding: Phillip L. Marotta was supported by a T-32 Training Grant from the National Institute on Drug Abuse (NIDA) Grant #: 1T32DA037801-01 during the writing and analysis of this research report. The authors gratefully acknowledge the support provided by Irwin Garfinkel, Neeraj Kaushal, Elwin Wu and Nabila El-Bassel
Appendix
Since Belgium was reported in two groups according to the Flemish or French communities’ data was reported for the first region that officially adopted NSP. This represents the first year in which public adoption occurred for a specific region in the country. Subsequent adoption in the Flemish Community signifies greater adoption and implementation of an already existing social policy. Similarly, the EMCDDA subdivides dates of implementation for the United Kingdom according to England, Wales, Northern Ireland or Scotland. For the purposes of this study, dates of implementation chosen for the United Kingdom consist of the earliest year of implementation which corresponds to years of adoption for England. Data from the United Kingdom does not include Wales and Northern Ireland.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors of this manuscript have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Medicine. 2011;8 doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L, Grau LE, White E, Bowman S, Heimer R. Prevalence, characteristics, and predictors of police training initiatives by US SEPs: Building an evidence base for structural interventions. Drug and Alcohol Dependence. 2011;119(1):145–149. doi: 10.1016/j.drugalcdep.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L, Cochrane J, Sawyer AL, Serio-Chapman C, Smelyanskaya M, Han J, … Sherman SG. Police encounters among needle exchange clients in Baltimore: drug law enforcement as a structural determinant of health. American Journal of Public Health. 2015;105(9):1872–1879. doi: 10.2105/AJPH.2015.302681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship KM, Reinhard E, Sherman SG, El-Bassel N. Structural Interventions for HIV Prevention Among Women Who Use Drugs: A Global Perspective. Journal of Acquired Immune Deficiency Syndromes. 2015;69:S140–S145. doi: 10.1097/QAI.0000000000000638. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Malik MR, Grau LE, Singer M, Marshall P, Heimer R, et al. Sterile syringe access conditions and variations in HIV risk among drug injectors in three cities. Addiction. 2004;99(9):1136–1146. doi: 10.1111/j.1360-0443.2004.00694.x. [DOI] [PubMed] [Google Scholar]
- Bruce RD. Methadone as HIV prevention: high volume methadone sites to decrease HIV incidence rates in resource limited settings. International Journal of Drug Policy. 2010;21(2):122–124. doi: 10.1016/j.drugpo.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris S, Blankenship KM, Donoghoe M, Sherman S, Vernick JS, Case P, … Koester S. Addressing the “risk environment” for injection drug users: the mysterious case of the missing cop. Milbank Quarterly. 2004;82(1):125–156. doi: 10.1111/j.0887-378X.2004.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, Williams B. Beyond the biomedical and behavioural: towards an integrated approach to HIV prevention in the southern African mining industry. Social Science & Medicine. 1999;48(11):1625–1639. doi: 10.1016/s0277-9536(98)00449-3. [DOI] [PubMed] [Google Scholar]
- Cook C, Bridge J, Stimson GV. The diffusion of harm reduction in Europe and beyond. In: Rhodes T, Hedrich D, editors. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Harm reduction: evidence, impacts and challenges. 10 2010. Scientific Monograph Series. [Google Scholar]
- De Cock KM, El-Sadr WM, Ghebreyesus TA. Game changers: why did the scale-up of HIV treatment work despite weak health systems? Journal of Acquired Immune Deficiency Syndromes. 2011;57:S61–S63. doi: 10.1097/QAI.0b013e3182217f00. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, et al. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC. Harm reduction--a framework for incorporating science into drug policy. American Journal of Public Health. 1995;85(1):10–12. doi: 10.2105/ajph.85.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker E, Lurie P, Wodak A, Alcabes P. Measuring harm reduction: the effects of needle and syringe exchange programs and methadone maintenance on the ecology of HIV. AIDS-LONDON-CURRENT SCIENCE THEN RAPID SCIENCE PUBLISHERS THEN LIPPINCOTT RAVEN- 1998;12:S217–S230. [PubMed] [Google Scholar]
- Elliott R, Csete J, Wood E, Kerr T. Harm reduction, HIV/AIDS, and the human rights challenge to global drug control policy. Health and Human Rights. 2005:104–138. [PubMed] [Google Scholar]
- El-Sadr WM, Abrams EJ. Scale-up of HIV care and treatment: can it transform healthcare services in resource-limited settings? AIDS. 2007;21:S65–S70. doi: 10.1097/01.aids.0000298105.79484.62. [DOI] [PubMed] [Google Scholar]
- European Monitoring Center for Drugs and Drug Addiction. Dataset. 2015 http://www.emcdda.europa.eu/index.cfm.
- Friedman SR, de Jong W, Rossi D, Touzé G, Rockwell R, Des Jarlais DC, Elovich R. Harm reduction theory: Users’ culture, micro-social indigenous harm reduction, and the self-organization and outside-organizing of users’ groups. International Journal of Drug Policy. 2007;18(2):107–117. doi: 10.1016/j.drugpo.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer M, Elliott L. Discriminating needle exchange attenders from non attenders. Addiction. 1993;88(5):681–687. doi: 10.1111/j.1360-0443.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Gibson DR, Flynn NM, Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 2001;15(11):1329–1341. doi: 10.1097/00002030-200107270-00002. [DOI] [PubMed] [Google Scholar]
- Grassly NC, Lowndes CM, Rhodes T, Judd A, Renton A, Garnett GP. Modelling emerging HIV epidemics: the role of injecting drug use and sexual transmission in the Russian Federation, China and India. International Journal of Drug Policy. 2003;14(1):25–43. [Google Scholar]
- Hartel DM, Schoenbaum EE. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public Health Reports. 1998;113(Suppl 1):107. [PMC free article] [PubMed] [Google Scholar]
- Huang Y-F, Yang J-Y, Nelson KE, Kuo H-S, Lew-Ting C-Y, et al. Changes in HIV Incidence among People Who Inject Drugs in Taiwan following Introduction of a Harm Reduction Program: A Study of Two Cohorts. PLoS Medicine. 2014;11(4):e1001625. doi: 10.1371/journal.pmed.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHRA. The Global State of Harm Reduction 2012: Towards an Integrated Response 2012 [Google Scholar]
- Judd A, Hickman M, Jones S, McDonald T, Parry JV, Stimson GV, Hall AJ. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. British Medical Journal. 2004;330(7481):24–25. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnood K, Blankenship KM, Pollack HA, Roan CT, Altice FL. Syringe source, use, and discard among injection-drug users in New Haven, Connecticut. AIDS & Public Policy Journal. 2000;15(3–4):88–94. [PubMed] [Google Scholar]
- Kral AH, Anderson R, Flynn NM, Bluthenthal RN. Injection risk behaviors among clients of syringe exchange programs with different syringe dispensation policies. Journal of Acquired Immune De ciency Syndromes. 2004;37(2):1307–1312. doi: 10.1097/01.qai.0000127054.60503.9a. [DOI] [PubMed] [Google Scholar]
- Kreft IG, Kreft I, de Leeuw J. Introducing multilevel modeling. Sage; 1998. [Google Scholar]
- Lu C, Schneider MT, Gubbins P, Leach-Kemon K, Jamison D, Murray CJ. Public financing of health in developing countries: a cross-national systematic analysis. The Lancet. 2010;375(9723):1375–1387. doi: 10.1016/S0140-6736(10)60233-4. [DOI] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ (Clinical research ed.) 2012;345 doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, Taylor A, Roy K, Aspinall E, Goldberg D, Rhodes T, Hedrich D, Salminen M, Hickman M, Hutchinson SJ. Interventions to prevent HIV and Hepatitis C in people who inject drugs: A review of reviews to assess evidence of effectiveness. The International Journal on Drug Policy. 2014;25:34–52. doi: 10.1016/j.drugpo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- MacMaster SA. Harm reduction: A new perspective on substance abuse services. Social Work. 2004;49(3):356–363. doi: 10.1093/sw/49.3.353. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, … Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. The Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Mays GP, Smith SA. Evidence links increases in public health spending to declines in preventable deaths. Health Affairs. 2011:10–1377. doi: 10.1377/hlthaff.2011.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Tyndall M, Spittal P, Li K, Palepu A, Schechter MT. Risk-taking behaviors among injecting drug users who obtain syringes from pharmacies, fixed sites, and mobile van needle exchanges. Journal of Urban Health. 2002;79(2):257–265. doi: 10.1093/jurban/79.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, et al. Reduction in injection-related HIV risk after 6 months in a low-threshold methadone treatment program. AIDS Education & Prevention. 2007;19(2):124– 136. doi: 10.1521/aeap.2007.19.2.124. [DOI] [PubMed] [Google Scholar]
- Obadia Y, Feroni I, Perrin V, Vlahov D, Moatti JP. Syringe vending machines for injection drug users: An experiment in Marseille, France. American Journal of Public Health. 1999;89(12):1852–1854. doi: 10.2105/ajph.89.12.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or Z. Labour Market and Social Policy Occasional-Papers No. 46. Paris: Organization for Economic Cooperation and Development; 2000a. Exploring the Effects of Health Care on Mortality across OECD Countries. [Google Scholar]
- Or Z. OECD Economic Studies: No. 30, 2000/1. Paris: Organisation for Economic Cooperation and Development; 2000b. Determinants of Health Outcomes in Industrialized Countries: A Pooled, Cross-Country, Time-Series Analysis. [Google Scholar]
- Östlin P, Eckermann E, Mishra US, Nkowane M, Wallstam E. Gender and health promotion: A multisectoral policy approach. Health Promotion International. 2006;21(suppl 1):25–35. doi: 10.1093/heapro/dal048. [DOI] [PubMed] [Google Scholar]
- Parker RG. Civil society, political mobilization, and the impact of HIV scale-up on health systems in Brazil. Journal of Acquired Immune Deficiency Syndromes (1999) 2009;52(Suppl 1):S49. doi: 10.1097/QAI.0b013e3181bbcb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack HA, Khoshnood K, Blankenship KM, Altice FL. The impact of needle exchange-based health services on emergency department use. Journal of General Internal Medicine. 2002;17(5):341–348. doi: 10.1046/j.1525-1497.2002.10663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. STATA press; 2008. [Google Scholar]
- Riley EDS. Comparing new participants of a mobile versus a pharmacy- based needle exchange program. Journal of Acquired Immune De ciency Syndromes. 2000;24(1):57–61. doi: 10.1097/00126334-200005010-00010. [DOI] [PubMed] [Google Scholar]
- Rhoades HM, Creson D, Elk R, Schmitz J, Grabowski J. Retention, HIV risk, and illicit drug use during treatment: methadone dose and visit frequency. American Journal of Public Health. 1998;88(1):34–39. doi: 10.2105/ajph.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T, Judd A, Mikhailova L, Sarang A, Khutorskoy M, Platt L, et al. Injecting equipment sharing among injecting drug users in Togliatti City, Russian Federation: Maximizing the protective effects of syringe distribution. Journal of Acquired Immune Deficiency Syndromes. 2004;35(3):293–300. doi: 10.1097/00126334-200403010-00011. [DOI] [PubMed] [Google Scholar]
- Rhodes T. The ‘risk environment’: a framework for understanding and reducing drug-related harm. International Journal of Drug Policy. 2002;13(2):85–94. [Google Scholar]
- Snijders TA. Multilevel analysis. Springer; Berlin Heidelberg: 2011. pp. 879–882. [Google Scholar]
- Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. Journal of Urban Health. 2010;87(4):592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, West BS, Reed E, Moazan B, Azim T, Dolan K. Substance use and HIV among female sex workers and female prisoners: risk environments and implications for prevention, treatment, and policies. Journal of Acquired Immune Deficiency Syndromes. 2015;69:S110–S117. doi: 10.1097/QAI.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis b and C risk reduction among injectors in the Seattle area. Journal of Urban Health. 2000;77(3):331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam cohort studies among drug users. Addiction. 2007;102:1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman P, Kumaranayake L, Balakireva O, Guinness L, Artyukh O, Semikop T, … Watts C. The cost-effectiveness of expanding harm reduction activities for injecting drug users in Odessa, Ukraine. Sexually Transmitted Diseases. 2006;33(10):S89–S102. doi: 10.1097/01.olq.0000221335.80508.fa. [DOI] [PubMed] [Google Scholar]
- Vlahov D, Junge B, Brookmeyer R, Cohn S, Riley E, Armenian H, Beilenson P. Reductions in high-risk drug use behaviors among participants in the Baltimore needle exchange program. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1997;16(5):400–406. doi: 10.1097/00042560-199712150-00014. [DOI] [PubMed] [Google Scholar]
- Wells EA, Calsyn DA, Clark LL, Jackson TR, Saxon AJ. Retention in methadone maintenance is associated with reductions in different HIV risk behaviors for women and men. The American Journal of Drug and Alcohol Abuse. 1996;22(4):509–521. doi: 10.3109/00952999609001677. [DOI] [PubMed] [Google Scholar]
- World Bank. Data. 2015 http://data.worldbank.org/indicator/SI.POV.GINI.
- World Health Orgainization. Needle and Syringe Programs. 2015. [Google Scholar]
- World Health Organization, Regional Office For Europe, European Center for Disease Prevention and Control. Dataset. 2015 http://www.euro.who.int/en/home.