Abstract
Intra- and extra-muscular hematomas result from repetitive injury as well as sharp and blunt limb trauma. The clinical consequences can be serious, including debilitating pain and functional deficit. There are currently no short-term treatment options for large hematomas, only lengthy conservative treatment. The goal of this work was to evaluate the feasibility of a high intensity focused ultrasound (HIFU)-based technique, termed histotripsy, for rapid (within a clinically relevant timeframe of 15–20 min) liquefaction of large volume (up to 20 mL) extra-vascular hematomas for subsequent fine-needle aspiration. Experiments were performed using in vitro extravascular hematoma phantoms—fresh bovine blood poured into 50 mL molds and allowed to clot. The resulting phantoms were treated by boiling histotripsy (BH), cavitation histotripsy (CH) or a combination in a degassed water tank under ultrasound guidance. Two different transducers operating at 1 MHz and 1.5 MHz with f-number = 1 were used. The liquefied lysate was aspirated and analyzed by histology and sized in a Coulter Counter. The peak instantaneous power to achieve BH was lower than (at 1.5 MHz) or equal to (at 1 MHz) that which was required to initiate CH. Under the same exposure duration, BH-induced cavities were one and a half to two times larger than the CH-induced cavities, but the CH-induced cavities were more regularly shaped, facilitating easier aspiration. The lysates contained a small amount of debris larger than 70 μm, and 99% of particulates were smaller than 10 μm. A combination treatment of BH (for initial debulking) and CH (for liquefaction of small residual fragments) yielded 20 mL of lysate within 17.5 minutes of treatment and was found to be most optimal for liquefaction of large extravascular hematomas.
Keywords: High intensity focused ultrasound (HIFU), Histotripsy, Boiling histotripsy, Intramuscular hematoma, Compartment syndrome, Trauma, Thrombolysis, Fine needle aspiration
INTRODUCTION
Intramuscular hematomas are characterized by blood extravasation into the body of the muscle affected by trauma, with preserved integrity of the epimysium. Significant limb hematomas occur in diverse populations, from professional athletes to amateur runners and exercise enthusiasts who sustain muscle injuries from repetitive overuse, as well as sharp and blunt limb trauma (Conforti 2013). The main symptom related to the onset of an intramuscular hematoma is pain, which may be debilitating. There are currently no short-term treatment options for large hematomas; drainage is generally not possible because even large percutaneously placed drains are inefficient due to the firm gelatinous consistency of the hematomas. Conservative treatment includes rest, ice and compression, and the return to full activity is generally not possible before a period of 10–20 wk (Smith et al. 2006). In addition, post-traumatic myositis ossificans—calcification of muscle—occurs as a complication in approximately 20% of large hematomas. It is responsible for considerable morbidity, with symptoms of prolonged pain, diminished flexibility, local tenderness and stiffness lasting an average of 1.1 y. One of the most devastating sequelae of large intramuscular hematomas is extremity compartment syndrome (ECS), which occurs when muscle tissues take on excess fluid (moderate to large hematoma or muscle swelling due to inflammation) creating pressure that reduces blood flow and ischemic injury. Increased pressures can cause irreversible damage over time, resulting from loss of vascular perfusion leading to loss of limb function/viability, in some cases requiring amputation (Garner et al. 2014).
Clearly, a rapid definitive intervention aiming at evacuation of the space-occupying hematoma would reduce pain, improve function and avoid long term sequelae. Pulsed high intensity focused ultrasound (HIFU)-induced bubble activity could provide a means for such an intervention—non-invasive liquefaction of the hematoma followed by fine-needle aspiration. In fact, ultrasound is known to promote intravascular clot breakdown, as both a stand-alone procedure and used in conjunction with thrombolytic drugs and/or microbubbles (Birnbaum et al. 1998; Datta et al. 2008; Porter and Xie, 2001). Innumerable in vitro and in vivo studies have been conducted over the y, and acoustic cavitation is widely accepted as the dominant mechanism for mechanical disruption of the clot integrity and partial or complete recanalization of the vessel (Bader et al. 2015). Recently, a technique termed histotripsy has been demonstrated to be an efficient, non-invasive tool in dissolving large in vitro and in vivo vascular clots without thrombolytic drugs within 1.5–5 min into debris 98% of which were smaller than 5 microns (Maxwell et al. 2009, Maxwell et al. 2011). The main challenge in the application of this same approach to the large extravascular hematomas is their large volume (20–50 mL) compared to the intravascular clots, which necessitates higher thrombolysis rates to complete the treatment within clinically relevant times (~15–20 min). On the other hand, the concerns regarding the size of the debris that can embolize into downstream vessels and the degree of hemolysis are not important compared with intravascular thrombus.
Thus, the main goal of this work was to evaluate the feasibility of using histotripsy to rapidly (within ~20 min) liquefy large extravascular hematomas for subsequent fine-needle aspiration. The treatment time frame limitation was chosen based on the review of the existing methods of soft tissue hematoma evacuation: drain placement, which averages 45–60 min (including preparation and post placement cleaning), and fasciotomy, which averages 30–45 min. It is preferred that the procedure can be performed in an outpatient setting, under regional anesthesia/sedation, which reinforces these time limits. Further extending the treatment time substantially increases the procedure costs and makes it less likely to become clinically adopted.
Two different histotripsy methods have been developed over the past decade: cavitation histotripsy (CH) and boiling histotripsy (BH) (Khokhlova et al. 2015). In BH, non-linear propagation effects on the way from the transducer to the focus lead to the formation of a shock front at the focus. Absorption and heating at the shock front is very large and leads to a highly localized temperature rise of over 100ºC, resulting in a boiling vapor bubble in only a few milliseconds (Canney et al. 2010). The explosion of the millimeter-sized boiling bubble and its further interaction with the shocks causes localized mechanical erosion of tissue at the focus (Simon et al. 2012). If the ultrasound pulse does not significantly exceed the time-to-boil and the duty factor is low enough to avoid heat buildup (below 2%), thermal injury to tissue is negligible (Khokhlova et al. 2011; Wang et al. 2013). In CH, shorter (microsecond instead of millisecond) ultrasound pulses with higher pressure amplitude, repeated with low duty factor (less than 1%) periodically produce dense energetic bubble clouds in tissue. The activity of the bubble clouds mechanically disintegrates tissue in the focal area to a sub-cellular level (Maxwell et al. 2011; Parsons et al. 2006).
Thus, both histotripsy techniques induce transient bubble activity in the focal area of the transducer and lead to the same outcome of tissue liquefaction. In both techniques, reliable treatment monitoring is achieved by B-mode ultrasound imaging of the highly reflective bubbles that appear as bright hyperechoic regions. Furthermore, both techniques have previously demonstrated advantageous tissue selectivity, both in vivo and ex vivo; for example, connective tissue structures e.g., blood vessels, biliary structures or fascia surrounding muscle groups and organs, proved to be very resistant to mechanical damage and were largely unaffected by BH treatment while the more fragile cellular components were completely lysed (Khokhlova et al. 2014).
One notable difference that is relevant here is that the individual lesions produced by BH are larger than those produced by CH for the same treatment time, using the same ultrasound frequency. On the other hand, a unique feature of the CH technique is the formation of fluid vortices adjacent to the bubble cloud that were shown to attract, trap and erode millimeter-sized thrombus fragments if induced in a large blood vessel (Maxwell et al. 2014; Park et al. 2013). Thus, we hypothesized that a combination of BH (for large-scale debulking of a large hematoma) and CH (for eroding residual fragments) would optimize liquefaction treatment times for large extravascular hematomas. In this work CH, BH and a combination thereof were investigated in an in vitro model of a large hematoma in an effort to maximize the rate of thrombolysis for subsequent fine-needle aspiration.
MATERIALS AND METHODS
Experimental arrangement
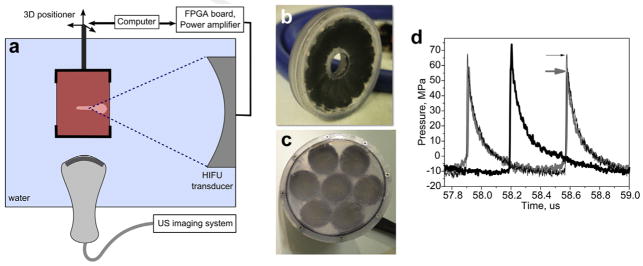
All experiments were performed using an in vitro model of a large hematoma. Fresh bovine blood obtained from a local butcher was poured into plastic molds (50 mL per mold) and allowed to clot at room temperature for 20 min. The resulting phantoms were then transferred into a custom-built holder attached to a 3-D positioning system and treated with BH, CH or a combination treatment in a degassed water tank (Fig. 1a). Treatments were monitored and targeted by B-mode ultrasound (Epiq, Phillips, Bothell, WA, USA), with the imaging probe (C5-2, Phillips) positioned in the tank perpendicular to the transducer axis. The position of the HIFU focus was determined with an initial pulse to generate a bubble and marked on the ultrasound image before the start of the treatments. The focus was placed at a 2-cm depth below the surface of the clot phantom. Two different spherically focused custom built transducers with an f-number of 1 were used in this study: a 12-element sector 1.5 MHz transducer with an aperture of 8 cm (Fig. 1b) and a 7-element 1 MHz transducer with an aperture of 12 cm (Fig. 1c). The transducers were manufactured from flat PZT-8 modules distributed on a flat surface and bonded by tungsten-epoxy matching layer to an acoustic lens manufactured with a stereolithography system following Kim et al. (2014). The transducers were powered by a 2.5 kW custom built amplifier and a field-programmable gate array board controlled by a personal computer.
Fig. 1.
(a) Schematic of the experimental setup; (b, c) multi-element custom built HIFU transducers operated at 1.5 MHz and 1 MHz, respectively; (d) focal waveforms for BH (thick gray line) and CH (thin black line) produced by the 1.5 MHz transducer and 1 MHz transducer (thick black line). The waveforms were measured in water by fiber-optic probe hydrophone and derated to a 2-cm depth in the hematoma phantom. The arrows indicate the respective peak positive pressures. FPGA = field-programmable gate array; HIFU = high intensity focused ultrasound; US = ultrasound.
The focal pressure waveforms produced by the transducers at different output power levels were measured in water by a fiber optic probe hydrophone (FOPH 2000, RP Acoustics e.K., Leutenbach, Germany). The waveforms were then derated into the clot material at a 2-cm depth, using the derating algorithm previously developed for non-linearly distorted waveforms (Khokhlova et al. 2011). The properties of clot material needed for derating were taken from the literature as follows: non-linear parameter β = 4.15, attenuation α = 0.19 Np/cm at 1 MHz and α = 0.21 Np/cm at 1.5 MHz (Duck 1990, Nahirnyak et al. 2006). The derated waveforms corresponding to the output power used for BH and CH treatments are shown in Figure 1d.
Exposure parameters
In both CH and BH treatments, the pulse durations and the duty factors were kept constant throughout all exposures, and corresponded to the optimal parameters identified previously for soft tissue disintegration (for BH) or for intravascular clot lysis (for CH) as follows: BH treatments used 10-ms pulse durations at a pulse repetition frequency (PRF) of 1 Hz, resulting in 1% duty factor (Khokhlova et al. 2011) and CH treatments used 5-cycle pulses at a PRF of 1 kHz, resulting in 0.33–0.5% duty factor (for 1.5 MHz and 1 MHz frequencies, respectively). The instantaneous output power of the transducer was set at the threshold level for initiating CH or BH treatment, which was determined in the first series of experiments. That is, for BH, single 10-ms pulses with increasing output power were fired consecutively until a bright hyperechoic region was observed on the B-mode ultrasound image (Fig. 2b). This derated focal waveform is shown in Figure 1d. The threshold peak focal pressures being p− = 10 MPa, p+ = 58 MPa and shock amplitude (As) = 64 MPa at 1.5 MHz; and p− = 12 MPa, p+ = 82 MPa and As = 64 MPa at 1 MHz. These thresholds are in line with the theoretical estimations of the time to reach boiling, according to weak shock theory—6 ms and 4.3 ms for 1.5 MHz and 1 MHz, respectively. The corresponding peak acoustic intensities were ISPPA = 19.2 kW/cm2, ISPTA = 192 W/cm2 for 1 MHz and ISPPA = 18.5 kW/cm2, ISPTA = 185 W/cm2 for 1.5 MHz. For CH, the 5-cycle pulses were fired at 1 kHz PRF while increasing the output power until the hyperechoic cavitation bubble cloud seen on B-mode ultrasound became stable. At 1 MHz the threshold output power was the same as for BH (ISPPA = 19.2 kW/cm2, ISPTA = 96 W/cm2), and at 1.5 MHz it was higher and corresponded to the peak focal pressures of p−=12 MPa, p+=68 MPa and As = 79 MPa (ISPPA = 32 kW/cm2, ISPTA = 106 W/cm2).
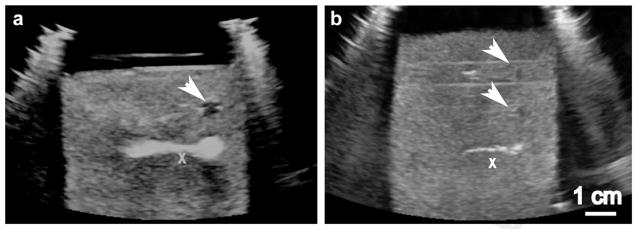
Fig. 2.
Ultrasound B-mode images of the hematoma phantom during treatment by (a) BH and (b) CH. The treatment pulses are incident from the right of the images. An “X” is positioned at the geometric focus of the transducer but off-axis, so as not to obscure the bubble activity. The images were taken at the end of a 20-s exposure; echogenic bubble clouds can be readily observed in both images. The white arrows indicate the hypoechogenic liquefied regions treated in previous exposures.
Outcome measures
In the first series of experiments, BH or CH exposures of variable duration (5–60 seconds per spot) were applied to separate focal spots in the phantom to evaluate the dose dependence of the liquefied void size. All the voids were contained within the gelatinous clot. The samples were then bisected along the axial dimension of the voids, and photographed for later analysis. In the second series of experiments, the focus was translated within the sample in a 2-D rectangular grid, using 2–5 mm spacing between spots to cover the cross-sectional area of either 2 × 2 cm or 3 × 3 cm. A higher frequency imaging transducer was then used to image the void and to guide the fine-needle (21-gauge) aspiration of the lysate. The contents of the lysate were analyzed by histology and sized in a Coulter Counter after filtering the entire collected volume through a 70-micron nylon filter.
RESULTS
Ultrasound imaging of BH and CH exposures
Examples of the B-mode images recorded during BH and CH exposures at 1 MHz are presented in Figures 2a and 2b, respectively. The hematoma phantom is mildly hyperechoic, which is consistent with the appearance of a fresh clot as well as organized hematoma, and represents the irregular fibrin matrix (Wicks et al. 1978). The bright hyperechoic region represents the areas of either bubble activity originating from boiling (Fig. 2a) or the cavitation cloud (Fig. 2b). A few seconds after the exposure is finished, all the residual bubbles dissolve leaving behind a hypoechoic area corresponding to the resulting void, indicating that the fibrin matrix in that area is mechanically destroyed and the contents liquefied.
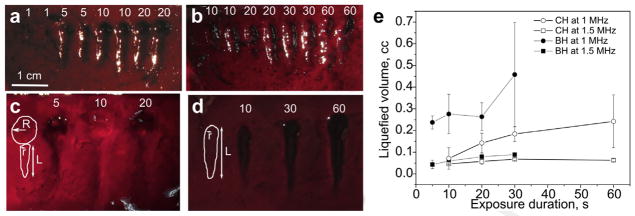
Dose dependence of the single BH and CH lesions
Figure 3 shows representative examples of the gross appearance of individual BH and CH lesions induced in the clot material using variable exposure durations. These results were replicated at least three times in three different clot samples. As seen in Figures 3a and 3c, BH lesions have a characteristic tadpole shape, and the linear dimensions of the lesion approach saturation relative to the exposure duration after only 10 seconds. The corresponding maximum linear dimensions at 1.5 MHz were 4.6 mm × 13.4 mm, and at 1 MHz were 22.6 mm × 7.2 mm. To evaluate the liquefied volume achieved with each exposure, the lesions were represented as a combination of a sphere (the proximal part of the void) and a half-ellipsoid (the distal part of the void), as shown in an insert to Figure 3c. The corresponding dependence of the volume on the exposure duration is shown in Figure 3e (filled symbols). Each point on the curves in Figure 3e can be used to estimate the upper limit of the thrombolysis rate per min, given the liquefied volume achieved within the corresponding exposure duration and assuming that the same liquefaction volume will be achieved in consecutive focal points. Under these conditions, the highest thrombolysis rate is achieved at 5 s exposure duration and is 2.8 mL/min for 1 MHz and 0.5 mL/ min for 1.5 MHz. As seen in Figures 3b and 3d, CH lesions are substantially smaller than BH lesions produced in the same amount of time, but have a much more regular shape. The volume of CH voids was approximated by a half ellipsoid, as shown in an insert to Figure 3d.
Fig. 3.
(a, c) BH and (b, d) CH liquefied lesions produced in the hematoma phantom at 1.5 MHz (a, b) and 1 MHz (c, d). The numbers at the top of each lesion indicate the duration of exposure (in seconds). Given the same exposure duration, the BH lesions are substantially larger in linear dimensions compared to CH lesions. Similarly, all lesions produced at 1 MHz were almost twice as large as those produced at 1.5 MHz (e). Dependence of the estimated volume of the BH and CH lesions, produced at 1 MHz and 1.5 MHz on the exposure duration. The inserts in (c) and (d) illustrate the way the lesion volume was estimated, i.e., a sphere with radius R and a half-ellipsoid with half-axes r and L for BH: V = 4/3π (Lr2/2 + R3); a half ellipsoid with half-axes r and L for CH: V = 2πLr2/3. The largest resulting thrombolysis rate corresponded to BH at 1 MHz, with 5 s exposure per spot, and was estimated as 2.8 mL/min. BH = boiling histotripsy; CH = cavitation histotripsy.
Large-volume lesions and fine-needle aspiration
As mentioned in the previous section, the highest rate of thrombolysis was observed using BH for 5 s per spot. However, the BH lesions have an irregular tadpole shape, and in order to achieve unobstructed fine-needle aspiration of a large-volume void, the individual lesions need to merge somewhat. In producing a large-volume void, two different lesion-placement strategies were used. In the initial experiments, the 1.5 MHz transducer was used to produce lesions placed at 2-mm distances from each other. An exposure duration of 30 s per spot was used to achieve merging of the distal parts of the lesion. Thus, liquefaction of a 2 × 2 cm cross-sectional area took 50 min to complete. A higher frequency imaging transducer was then used to guide the fine-needle (21-gauge) aspiration of the lysate (Fig. 4a). After 8 mL of lysate was aspirated from the void, the sample was removed from the holder and bisected as shown in Figure 4b. The aspiration was somewhat hindered by the shape of the cavity—the presence of small, millimeter-sized filaments of intact clot protruding from the side of the void that was distal to the transducer during treatment. These filaments were due to the tadpole shape of the individual lesions and their incomplete merging.
Fig. 4.
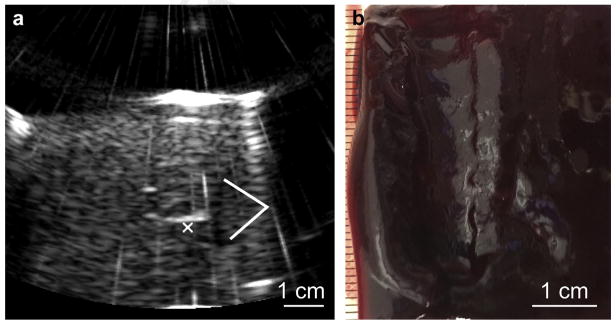
A large boiling histotripsy liquefied lesion was produced in a hematoma sample by translating the focus of the 1.5 MHz transducer in a 2 × 2 cm grid in 2-mm steps. (a) B-mode image of the fine-needle (18-gauge) aspiration of lysate from the cavity. The cavity appears as a hypoechoic region; note the irregular filaments protruding from the side of the void (the white “x” denotes one of them). These filaments are due to the incomplete merging of the distal parts of the individual liquid lesions. (b) Photograph of the bisected treated hematoma sample.
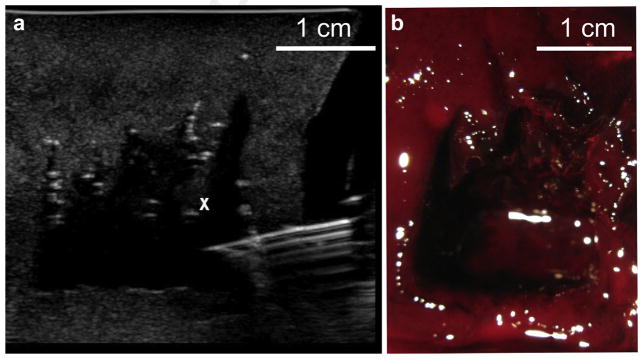
In a second strategy, designed to improve thrombolysis rates, the 1 MHz transducer was used to produce BH cavities with only 5 pulses, and the cavities were separated by 5 mm, so that only the proximal parts of the cavities merged. A larger cross-sectional area of 4 × 2 cm was treated in only 10.5 min. Treatment was then switched to the CH regime and applied to the distal parts of the lesions (Fig. 5a) to liquefy the filaments, so that fine-needle aspiration would not be hindered by the filaments. The same rectangular scanning pattern was used as for the BH, with 3-s exposures per spot, which overall took 7 min. Total treatment time was 17.5 min. Small needle (21-gauge) aspiration was then performed and 20 mL of lysate were removed. The bisected sample is shown in Figure 5b, and shows that the proximal parts of the lesions were clearly merged and did not interfere with aspiration. This strategy amounted to an overall thrombolysis rate of 1.3 mL/min, and is within a clinically relevant time frame (15–20 min) for an outpatient procedure.
Fig. 5.
Combined treatment with BH and CH using a 1 MHz transducer to accelerate liquefaction rate. (a) B-mode image of the cavitation bubble cloud (white cross) distal to the merged voids created by BH (white lines). (b) Bisected large void after the combined treatment and drainage with 18-gauge needle. Total drained volume was 20 mL, and the total treatment duration was 17 min.
Analysis of the lysate
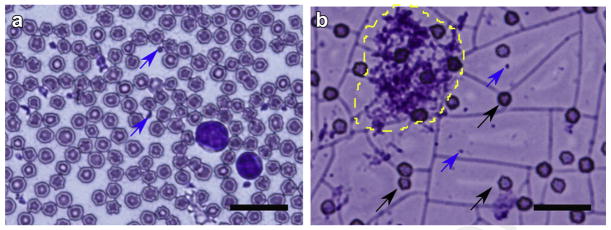
Smears of the lysates aspirated from the large-volume voids were analyzed histologically using Wright-Giemsa stain and compared to a smear of untreated, non-coagulated bovine blood (Fig. 6). Regardless of the method employed for clot lysis, the lysate contents were very similar. Some intact red blood cells were detected, although intense background indicated a higher degree of hemolysis compared with the untreated blood. Few larger particulates containing red blood cells embedded into what appeared as fibrin matrix residuals ranging within 10–25 microns in size were seen on some histologic slides (see example in Fig. 6b). However, these residual clot fragments did not hinder fine-needle aspiration. According to the sizing of the samples of the aspirated volume by Coulter Counter, no debris were larger than 25 microns, and 99% of the particles were smaller than 10 microns, likely representing intact RBCs.
Fig. 6.
(a) Histologic sample of fresh bovine blood stained with Wright-Giemsa stain. A multitude of red blood cells, platelets (blue arrows) and two neutrophils are seen. (b) Histologic sample of the hematoma lysate. The blue arrows indicate platelets, the black arrows indicate intact red blood cells. Note the pink background likely stemming from lysed red blood cells. An ~25-micron residual clot fragment is outlined in yellow. The scale bar is 20 microns.
DISCUSSION
Large limb hematomas are relatively common in sports injuries and lead to long layoffs for athletes and exercise enthusiasts. Standard conservative treatment of rest, ice, compression and elevation (i.e., RICE protocol) delays a return to full activity for a period of 10–20 wk, and calcification complications in approximately 20% of large hematomas leads to prolonged reduction of use lasting an average of 1.1 y. An interventional technique that can rapidly and safely liquefy hematomas for fine-needle aspiration could significantly reduce layoffs and allow return to full activities quickly. In this study, we demonstrated the ability of histotripsy to liquefy large (up to 20 mL) in vitro hematoma models within clinically relevant timeframe (under 20 min), and subsequently aspirate the lysate using small needle.
In an effort to optimize the thrombolysis rate, the efficiency of two different histotripsy techniques were compared—CH and BH, as well as two different center frequencies for each technique, 1 MHz and 1.5 MHz. The choice of this frequency range was dictated because the size of the histotripsy-induced cavities in soft tissue is known to decrease with frequency (Khokhlova et al. 2015). However, both techniques require the formation of a shock front at the focus, and the fabrication of a transducer capable of achieving and sustaining output powers sufficient for that becomes increasingly difficult in the sub-MHz range. The pulsing protocols selected for each technique were those typically used for fractionation of soft tissues in the previous studies: 10 ms and a 5-cycle pulse duration, 1 Hz and 1000 Hz for BH and CH, correspondingly. The peak focal pressures required to initiate bubble activity and thrombolysis in the two techniques were determined at each frequency, and were similar for CH and BH at 1 MHz, but were substantially higher for CH at 1.5 MHz. This was to be expected, because the threshold for inducing a cavitation cloud increases with frequency, whereas shock formation is not frequency dependent.
According to the results presented in Figure 3e, the fresh hematomas proved to be much more susceptible to histotripsy-induced damage than soft tissues in terms of the number of pulses sufficient to produce a sizeable cavity. In particular, delivering five BH pulses per spot produced the fastest overall thrombolysis rate, and allowed the formation of a large cavity, whereas in healthy soft tissues, e.g., liver and myocardium, 30–60 pulses per spot are usually needed (Khokhlova et al. 2011). This is a distinct advantage in liquefaction of hematomas contained within healthy tissue (e.g., muscle)—the threshold for inducing gross damage in soft tissues is much higher. In translation of this technology from bed to bedside, safety evaluation, i.e., ensuring the absence of mechanical or thermal damage to the adjacent tissue, will be of major importance. In the case when the region of bubble activity is fully contained within the remaining clot, this intervention is not expected to induce any collateral damage. If the bubble cloud appears immediately adjacent or overlaps with the fascial plane enclosing the muscle, some mechanical damage in the form of bruising may potentially occur in the muscle. This risk will have to be evaluated in vivo in a large animal model and can be avoided (if proven substantial) by careful targeting. This may imply the ability to adjust the length of the liquefaction zone according to the shape and size of the hematoma by adjusting the HIFU frequency and/or the exposure duration per treatment spot.
Although BH generates larger treatment zones compared to CH in the same amount of time (Fig. 3), the cavities are tadpole-shaped and have to be placed very close together to fully merge, which slows the treatment down. If the cavities are positioned further apart, filamental structures remain in the cavity after treatment and can obstruct the needle during fine-needle aspiration. Conversely, the cavities induced by CH in the volume of coagulated blood are smaller, but if the cavitation cloud is induced in the area already liquefied by BH, it is very efficient in eroding the small filaments next to it. Thus, we found that a combination of BH for initial debulking and CH for erosion of residual filaments was most beneficial in terms of overall treatment speed and efficiency.
CONCLUSIONS
Two different histotripsy regimes (CH and BH) were used to optimally lyse large hematomas in vitro within a clinically relevant time period. The highest rate of thrombolysis was achieved by delivering five BH pulses per focus location at 1 MHz and then removing the remaining clot filaments using CH, which allowed fine-needle (18-guage) aspiration of the liquefied hematoma. These results suggest that a rapid definitive intervention with histotripsy may be clinically useful for treatment of intramuscular hematomas. Ultrasound imaging of histotripsy treatment was facilitated by the highly reflective bubbles—cavitation or boiling—making treatment monitoring relatively simple. Thus, ultrasound-guided histotripsy of large intra-muscular hematomas may be clinically achievable without significant tool development. Although our focus was in limb hematomas, future work will include histotripsy applications to intra-abdominal and sub-dural hematomas, provided a sufficient acoustic window.
Acknowledgments
We would like to thank Mr. Michael Breshock for assistance in performing experiments. This work was sponsored in part by the National Institute of Health and the Washington State Life Science Discovery Fund.
References
- Bader KB, Gruber MJ, Holland CK. Shaken and stirred: Mechanisms of ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2015;41:187–196. doi: 10.1016/j.ultrasmedbio.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li S, Kricsfeld D, Porter TR, Siegel RJ. Noninvasive in vivo clot dissolution without a thrombolytic drug: Recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol. 2010;36:250–267. doi: 10.1016/j.ultrasmedbio.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti M. The treatment of muscle hematomas. In: Bisciotti GN, Eirale C, editors. Muscle injuries in sport medicine. 1. Croatia: InTech; 2013. Available at: http://www.intechopen.com/books/muscle-injuries-in-sport-medicine/the-treatment-of-muscle-hematomas. [Google Scholar]
- Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–1433. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck F. Physical properties of tissue: A comprehensive reference book. London: Academic Press; 1990. [Google Scholar]
- Garner MR, Taylor SA, Gausden E, Lyden JP. Compartment syndrome: Diagnosis, management, and unique concerns in the twenty-first century. HSS J. 2014;10:143–152. doi: 10.1007/s11420-014-9386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130:3498–3510. doi: 10.1121/1.3626152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova TD, Wang YN, Simon JC, Cunitz BW, Starr F, Paun M, Crum LA, Bailey MR, Khokhlova VA. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci USA. 2014;111:8161–8166. doi: 10.1073/pnas.1318355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, Hall TL, Maxwell AD, Wang YN, Cain CA. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. Int J Hyperthermia. 2015;31:145–162. doi: 10.3109/02656736.2015.1007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Maxwell AD, Hall TL, Xu Z, Lin KW, Cain CA. Rapid prototyping fabrication of focused ultrasound transducers. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:1559–1574. doi: 10.1109/TUFFC.2014.3070. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy - histotripsy. Ultrasound Med Biol. 2009;35:1982–1994. doi: 10.1016/j.ultrasmedbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011a;22:369–377. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Park S, Vaughan BL, Cain CA, Grotberg JB, Xu Z. Trapping of embolic particles in a vessel phantom by cavitation-enhanced acoustic streaming. Phys Med Biol. 2014;59:4927–4943. doi: 10.1088/0031-9155/59/17/4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Wang TY, Cain CA, Fowlkes JB, Sapozhnikov OA, Bailey MR, Xu Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am. 2011b;130:1888–1898. doi: 10.1121/1.3625239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahirnyak VM, Yoon SW, Holland CK. Acousto-mechanical and thermal properties of clotted blood. J Acoust Soc Am. 2006;119:3766–3772. doi: 10.1121/1.2201251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Maxwell AD, Owens GE, Gurm HS, Cain CA, Xu Z. Non-invasive embolus trap using histotripsy—An acoustic parameter study. Ultrasound Med Biol. 2013;39:611–619. doi: 10.1016/j.ultrasmedbio.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115–129. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Porter TR, Xie F. Ultrasound, microbubbles, and thrombolysis. Prog Cardiovasc Dis. 2001;44:101–110. doi: 10.1053/pcad.2001.26441. [DOI] [PubMed] [Google Scholar]
- Simon JC, Sapozhnikov OA, Khokhlova VA, Wang YN, Crum LA, Bailey MR. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57:8061–8078. doi: 10.1088/0031-9155/57/23/8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TO, Hunt NJ, Wood SJ. The physiotherapy management of muscle haematomas. Phys Ther Sport. 2006;7:201–209. doi: 10.1016/j.ptsp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Wang YN, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39:424–438. doi: 10.1016/j.ultrasmedbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks JD, Silver TM, Bree RL. Gray scale features of hematomas: An ultrasonic spectrum. Am J Roentgenol. 1978;131:977–980. doi: 10.2214/ajr.131.6.977. [DOI] [PubMed] [Google Scholar]