Abstract
Previous studies have shown that δ-octalactone is an important component of the tsetse-refractory waterbuck (Kobus defassa) repellent odour blend. In the present study, structure-activity comparison was undertaken to determine the effects of the length of the side chain and ring size of the lactone on adult Glossina pallidipes and Glossina morsitans morsitans. The responses of the flies to each compound were studied in a two-choice wind tunnel. Increasing the chain length from C3 (δ-octalactone) to C4 (δ-nonalactone) enhanced repellency to both species (G. pallidipes from 60.0 to 72.0 %, and G. m. morsitans from 61.3 to 72.6 %), while increasing the ring size from six (δ-octalactone) to seven members (ε-nonalactone) changed the activity from repellency to attraction that was comparable to that of the phenolic blend associated with fermented cow urine (p > 0.05). Blending δ-nonalactone with 4-methylguaiacol (known tsetse repellent) significantly (p < 0.05) raised repellency to 86.7 and 91.7 % against G. pallidipes and G. m. morsitans respectively. Follow-up Latin Square Designed field studies (Shimba hills in coastal areas in Kenya) with G. pallidipes populations confirmed the higher repellence of δ-nonalactone (with/without 4-methylguaiacol) compared to δ-octalactone (also, with/without 4-methylguaiacol). The results show that subtle structural changes of olfactory signals can significantly change their interactions with olfactory receptor neurons, and either shift their potency, or change their activity from repellence to attraction. Our results also lay down useful groundwork in the development of more effective control of tsetse by ‘push’, ‘pull’ and ‘push-pull’ tsetse control tactics.
Keywords: lactones, structure-activity, Glossina pallidipes, Glossina morsitans morsitans, repellency, attraction
1.0 INTRODUCTION
Previous surveys of different tsetse species (Glossina spp.) in varying habitats have shown differential attraction to and feeding on available vertebrates irrespective of their relative abundance (Weitz, 1963; Vale, 1974a; Grootenhuis, 1986; Turner, 1987; Moloo, 1993; Grootenhuis and Olubayo, 1993; Clausen et al 1998). In a follow up set of studies, comparison of the behaviour of teneral Glossina morsitans morsitans on waterbuck (Kobus defassa), a tsetse refractory bovid, and on two preferred hosts, buffalo (Syncerus caffer) and ox (Bos indicus), suggested the presence of volatile and short-range allomones on the waterbuck odour (Gikonyo et al., 2000). Examination of odour profiles of the three bovids by Gas chromatography-linked Mass spectrometry and electroantennography (GC-MS and GC-EAD, respectively), showed some constituents (phenols and aldehydes) that are common to the three bovids, but there were also a series of others (15 compounds) that were specific to waterbuck (Gikonyo et al., 2002). These included straight chain carboxylic acid (C5–C10), 2-alkanones (C8–C12 homologues and geranylacetone), phenols (guaiacol and carvacrol), and δ-octalactone (Fig 1). In a laboratory 2-choice wind tunnel, G. m. morsitans showed a pattern of responses to synthetic blends of these compounds that suggested avoidance behaviour, significantly different from their responses to attractive blends associated with odours of preferred hosts (Gikonyo et al., 2003).
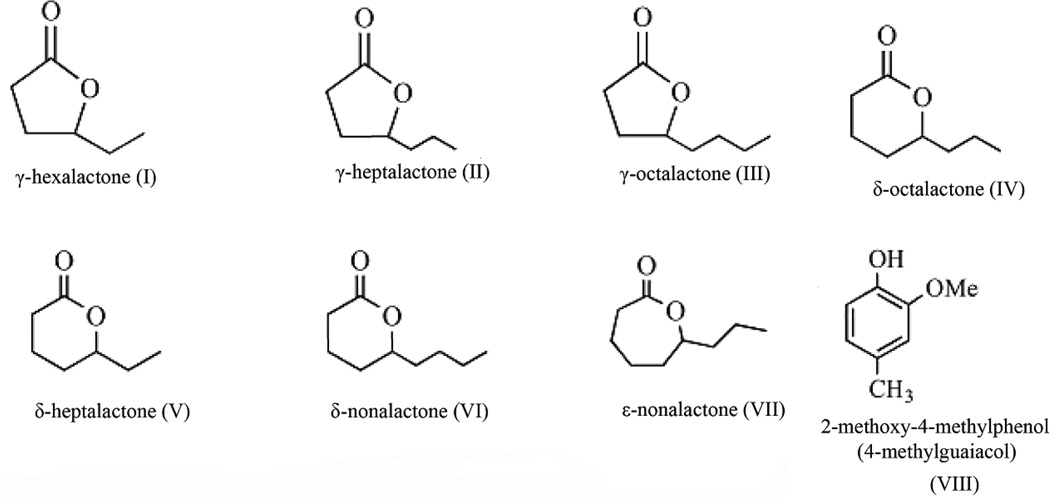
Fig. 1.
Schematic diagrams of δ-octalactone and its selected analogues and 4-methylguaiacol evaluated.
In a recent study, the effects of different blends of these compounds on catches of Glossina pallidipes in attractant-baited NG2G traps were evaluated in the field (Bett et al., 2015). Each class of constituents (acids, ketones, phenols and δ-octalactone) was found to significantly reduce catches, indicating that each contributes incrementally to the repellency of the waterbuck odour. However, within each multi-component class of compounds (carboxylic acids, ketones, and phenols), large variations in intrinsic individual repellency to G. pallidipes were found. Among the carboxylic acids, the lower (C5–C7) were found to be repellent unlike the higher (C8–C10) homologues, which did not show any significant repellency. On the other hand, of the ketones, the higher molecular weight compounds (C11, C12 homologues and geranylacetone) were significantly more repellent than the lower (C8–C10) homologues. Of the two phenols, guaiacol was found to be more repellent than carvacrol. Guaiacol was previously shown to be a mild tsetse repellent (Torr et al., 1996). In a structure-activity study with different analogues of this phenol, replacement of H with a CH3 group (4-methylguiacol) was found to significantly increase repellency to tsetse (Saini and Hasanali, 2007). The G. m. morsitans and G. pallidipes, restricted to the savannah, are most widespread and common vectors of livestock and human trypanosomiasis in sub – Sahara Africa (Jordan, 1986; Onyango et al., 1966; Willett, 1965).
The objective of the present study was to extend the study on structural variants of δ-octalactone, and to see how these modifications affect the olfactory responses of G. pallidipes and G. m. morsitans flies to the different analogues. Specifically, the effects of two types of structural variants were studied: increased or decreased size of the lactone ring and that of the hydrocarbon side chain. In addition, the effect of blending more repellent lactones with 4-methylguiacol was also evaluated.
2.0 MATERIALS AND METHODS
2.1 Laboratory Test insects
Glossina pallidipes and G. m. morsitans were obtained from colonies maintained at Biotechnology Research Institute, Kenya Agricultural and Livestock Research Organization, Muguga, Kenya. Booth colonies were established from seed puparia material received from the large colonies maintained at the International Atomic Energy Agency (IAEA) labs, Seibersdorf, Austria. The IAEA G. pallidipes colony originated from wild pupae collected from Lugala, Uganda in 1975, and northern Zimbabwe in 1987 (Ciosi et al., 2014). The G. m. morsitans colony originated from wild pupae collected from Zimbabwe in 1983. The flies were reared in an insectary under controlled environmental conditions (25 ± 2 °C, 75 ± 2% RH and LD 12:12 h photoperiod), and received defibrinated bovine blood through an artificial feeding system three times per week (Moloo, 1971).
2.2 Test compounds
Racemic blends (98–99% pure) of γ-hexalactone, γ-heptalactone, γ-octalactone, δ-octalactone, δ-heptalactone, δ-nonalactone and 4-methylguaiacol (Fig 1) were sourced from Sigma-Aldrich, Taufkirchen, Germany. Racemic ε-nonalactone (Fig 1) was synthesized in the laboratory using the method of Gikonyo et al (2002) and its structure confirmed by HR-MS, 13C NMR, 1H-NMR and IR spectrophotometry. In addition, two blends (A, δ-octalactone + 4-methylguaiacol) and B (δ-nonalactone + 4-methylguaiacol) were prepared in 1:1 ratio.
2.3 Responses of G. pallidipes and G. m. morsitans to δ-octalactone, analogues and selected blends in a wind tunnel
The study was undertaken in a two-choice cuboidal plexi-glass wind tunnel (195 cm × 20 cm × 20 cm) following the procedure outlined by Gikonyo et al. (2003). Briefly, the effect of each test compound and selected blends at 3 doses (0.05, 0.25 and 0.5 g/ml) in 1000 µl dichloromethane (CH2Cl2) was compared with that of the pure solvent (CH2Cl2) in three sets of replicates, each with ten individual flies of each species. The experiment was conducted in the mornings (0800 – 1200 hrs) and afternoons (1500 – 1700 hrs) coincident with the natural tsetse feeding hours. Purified air from an air cylinder flowed from both sides of the tunnel at 12.63 l/min. The following observations were recorded three minutes post-exposure: (i) number of flies departing from the midsection, (ii) initial direction of flights upwind, and (iii) final landing and resting position (control or treated arms of the tunnel). After each replicate cycle, the tunnel, metallic racks and release cages were cleaned with water and then with 70% ethanol. Blank tests were conducted to confirm no residual effects of previous test materials.
2.4 Responses of G. pallidipes to δ-octalactone and δ-nonalactone with/without 4-methylguaiacol in the field
Studies were conducted at Shimba Hill National Reserve (004° 15' 26''S, 039° 23'16''E) altitude 403 m) in Kwale County, Kenya where wild populations of G. pallidipes occur. Large mammal populations in the reserve include sable antelopes, elephants, buffalos, bushbucks, warthogs, bush pigs, giraffes, leopards, duikers, hartebeest and monkeys. The vegetation consists mostly of coastal rainforest and semi-evergreen woodland and grassland. The area experiences long and short rainy seasons from April to June, and October to November, respectively. The mean annual rainfall level is between 855 and 1682 mm. Maximum daily temperatures are highest in March and November, often reaching 31°C. June to July are the coolest months, with daily maximum temperatures of about 27°C.
The effects of δ-octalactone and δ-nonalactone with/without 4-methylguaiacol on G. pallidipes catches in NG2G traps baited with acetone (~500mg/h) and fermented cow urine (1000mg/h) (Brightwell et al., 1991) were evaluated in the field in randomized Latin squares design (5 targets × 5 sites × 5 days) arrangement (Mireji et al., 2003; Bett et al., 2015). The traps were deployed in clearings about 300m apart. δ-Octalactone, δ-nonalactone, and 50:50 2-component blends of each of these with 4-methylguaiacol were dispensed from sealed thin-walled polythene sachets constructed from polyethylene layflat tubing with 0.15mm thick walls and a surface area of 50 cm2 folded into tetrahedrons. The sachets, replaced daily, were placed, on the ground close to the attractive baits ~30 cm downwind side of the traps. Daily (24 hrs.) catches of G. pallidipes in the traps were recorded. The G. pallidipes was identified using morphological characteristics. The experiments were replicated in three independent blocks two kilometers apart. Release rates of the lactones and the 2-component blends in the field was computed from their lost masses of 48 hrs post-deployment and was found to be ≈17 mg/hr.
2.5 Statistical analyses
Average distances of upwind flight in the treated (with odors) and the control (without odor) arms in the laboratory wind-tunnel experiments were compared using Chi Square non-parametric test. The proportional responses of tsetse to various odors in the laboratory wind-tunnel experiments were rank-transformed before being subjected to analysis of variance (ANOVA) and SNK post hoc analyses. Catches of G. pallidipes in the field experiment were log (n+1) transformed to normalize the distributions and homogenize the variances. Effects of day, site and bait were separated using analysis of variance and SNK post hoc analyses. Data from wind tunnel experiments and the field were analyzed using SPSS Version 22 (SPSS, Inc., Chicago, IL, U.S.A.) with significance level set at 5%. Only de-transformed mean catches are reported and expressed as a proportion of the mean control catch (baited NG2G trap).
3.0 RESULTS
3.1 Responses of G. pallidipes and G. m. morsitans to δ-octalactone, analogues and selected blends in a wind tunnel
Gross flight behaviors of the flies in the wind tunnel depended on the presence of attractants or repellents, similar to those reported by Gikonyo et al. (2003). Whereas in the absence of any odours or presence of attractants the flies flew straight upwind, in the presence of repellents flight behaviour was characterized by zigzag movements in and out of plume, short hops, sudden turning at 180°, and gliding or fanning wings against the wall while stationary. Two parameters were specifically quantified at the three doses of all the tested odours: flight distance upwind on treated and control sides of the wind tunnel, and proportion of the flies that rested on the control and treated sides of the tunnel. Similar pattern of responses of the flies were obtained at the three doses of each test material, so only those of 1000 µl of 0.5 g/ml in dichloromethane are provided in Tables 1 and 2 for G. pallidipes and G. m. morsitans, respectively.
Table 1.
Responses of G. pallidipes to cow urine attractant blend, δ-octalactone and analogues, 4-methylguaiacol, and two selected blends in a choice wind tunnel
| Dose (g/ml) |
Test material | Average distance of upwind flight (cm ± SE) | Final resting choice | |
|---|---|---|---|---|
| C | T | (C−T)/(C+T)100 ± SE | ||
| 0.00 | Air | 51.07 ± 3.79 | 52.64 ± 4.95 | 0.00±0.00e |
| Dichloromethane | 48.20 ± 5.25 | 48.67 ± 6.91 | 0.00±0.00e | |
| Cow urine# | 41.00 ± 6.87 | 62.65 ± 5.14* | −58.52±1.48f | |
| 0.50 | δ-Octalactone | 43.25 ± 4.56 | 30.17 ± 5.22 | 60.00±3.2c |
| γ-Hexalactone | 59.33 ± 4.92 | 47.82 ± 4.94 | 23.70±5.37d | |
| γ-Heptalactone | 40.19 ± 5.12 | 42.63 ± 4.05 | 28.89±4.44d | |
| γ-Octalactone | 53.89 ± 5.43 | 38.50 ± 4.72 | 45.59±5.19c | |
| δ-Heptalactone | 50.91 ± 4.80 | 43.29 ± 4.26 | 51.11±4.89c | |
| δ-Nonalactone | 47.75 ± 5.55 | 29.20 ± 3.12* | 71.93±3.93b | |
| ε-Nonalactone | 37.43 ± 3.01 | 58.30 ± 5.65* | −53.33±4.65f | |
| 4-Methylguaiacol | 54.74 ± 4.94 | 35.57 ± 4.22 | 53.33±3.67c | |
| Blend A | 56.12 ± 5.21 | 14.50 ± 2.87*** | 73.33±5.62b | |
| Blend B | 59.54 ± 4.24 | 15.00 ± 1.03*** | 86.67±4.32a | |
Number of tsetse flies used in each test (N = 10 × 3); C = control arm; T = treated arm;
fermented for 3 days; Blend A= δ-octalactone and 4-methylguaiacol (1:1); Blend B= δ-nonalactone and 4-methylguaiacol (1:1). Each pair of average distance of upwind flight in C and T were compared by χ2;
= p < 0.05,
= p < 0.01,
= p < 0.001;
Means followed by the same letter in final resting choice are not significantly different (P > 0.05, SNK test).
Table 2.
Responses of G. m. morsitans to cow urine attractant blend, δ-octalactone and analogues, 4-methylguaiacol, and two selected blends in a choice wind tunnel
| Dose (g/ml) |
Test material | Average distance of upwind flight (cm ± SE) | Final resting choice | |
|---|---|---|---|---|
| C | T | (C−T)/(C+T)100 ± SE | ||
| 0.00 | Air | 47.00 ± 6.86 | 52.60 ± 5.40 | 0.00 ± 0.00f |
| Residual solvent | 57.75 ± 3.17 | 54.50 ± 4.47 | 0.00 ± 0.00f | |
| Cow urine# | 32.50 ± 7.50 | 60.38 ± 4.02*** | −60.00 ± 1.58g | |
| 0.50 | δ-Octalactone | 58.00 ± 8.22 | 32.00 ± 1.90** | 61.33 ± 3.02c |
| γ-Hexalactone | 46.71 ± 9.31 | 30.67 ± 7.06 | 26.67 ± 6.67e | |
| 0.50 | γ-Heptalactone | 40.00 ± 8.04 | 31.00 ± 6.00 | 45.19 ± 5.19d |
| γ-Octalactone | 54.00 ± 7.40 | 43.67 ± 8.29 | 46.67 ± 4.58d | |
| δ-Heptalactone | 58.14 ± 9.32 | 46.00 ± 3.51 | 46.67 ± 3.04d | |
| δ-Nonalactone | 56.33 ± 8.36 | 22.00 ± 3.25*** | 72.59 ± 2.33b | |
| ε-Nonalactone | 24.50 ± 4.50 | 73.43 ± 8.74*** | −58.52 ± 1.48g | |
| 4-Methylguaiacol | 62.29 ± 8.95 | 36.67 ± 4.48* | 51.85 ± 3.06cd | |
| Blend A | 52.33 ± 8.81 | 9.00 ± 1.04*** | 79.33 ± 4.23b | |
| Blend B | 59.00 ± 7.84 | 7.00 ± 2.03*** | 91.67 ± 4.29a | |
Number of tsetse flies used in each test (N = 10 × 3); C = control arm; T = treated arm;
fermented for 3 days; Blend A= δ-octalactone and 4-methylguaiacol (1:1); Blend B= δ-nonalactone and 4-methylguaiacol (1:1). Each pair of average distance of upwind flight in C and T were compared by χ2;
= p < 0.05,
= p < 0.01,
= p < 0.001;
Means followed by the same letter in final resting choice are not significantly different (P > 0.05, SNK test).
In all the tests, 93–100% of the flies of both species were activated to leave the midsection. In the presence of the phenolic blend (4-cresol and 3-n-propylphenol) of cow urine (Hassanali et al. 1986, 1988), both tsetse species showed significant preference for upwind flight on the treated arm of the wind tunnel (Tables 1 and 2). Interestingly, a similar behaviour was demonstrated in the presence of ε-nonalactone. In both cases, 58.30 – 73.43 % of the flies also rested on the treated arm of the tunnel. On the other hand, δ-nonalactone and δ-octalactone elicited a reverse behaviour, with significantly more flies flying upwind in the control arm of the tunnel. More flies also preferred to rest on the control arm of the tunnel (Tables 1 and 2).
Blending δ-octalactone or δ-nonalactone with 4-methylguaiacol (blends A and B, respectively) appeared to significantly enhance the avoidance responses of both species of tsetse flies (P < 0.05), with δ-nonalactone plus 4-methylguaiacol combination eliciting a higher repellency against both tsetse species as reflected in their final resting choices (Tables 1 and 2).
3.2 Responses of G. pallidipes to δ-octalactone and δ-nonalactone with/without 4-methylguaiacol in the field
The higher repellence of δ-nonalactone compared with δ-octalactone was confirmed in the Latin Square Design field experiment with G. pallidipes (Table 3). The results showed that δ-nonalactone reduced tsetse catches by 76% compared with 68% by δ-octalactone. In addition, the blend of δ-nonalactone and 4-methylguaiacol reduced catches of the flies by 85% compared with 79% by the blend of δ-octalactone and 4-methylguaiacol.
Table 3.
Mean catches of G. pallidipes to attractant-baited NG2G traps in the presence of δ-octalactone, δ-nonalactone, and 1:1 blends of each of these with 4-methylguaiacol
| Treatment | Mean catch ± SE | Index of catch# | (% Reduction) |
|---|---|---|---|
| Control¥ | 103.73±11.82d | 1.00 | |
| δ-Octalactone | 43.53±8.59c | 0.32 | (68) |
| δ-Nonalactone | 27.93±6.99b | 0.27 | (76) |
| Blend A | 22.27±5.79b | 0.21 | (79) |
| Blend B | 15.80±3.90a | 0.15 | (85) |
=Fermented cow urine, acetone and 3-n-propylphenol.
= Total mean catch expressed as proportion of that from baited trap; Blend A= δ-octalactone and 4-methylguaiacol (1:1); Blend B= δ-nonalactone and 4-methylguaiacol (1:1); Means followed by the same letter are not significantly different (P > 0.05, SNK test).
4.0 DISCUSSION
δ-Octalactone with a six-membered ring and a propyl (CH3CH2CH2) side chain at δ position was previously shown to be an important repellent component of waterbuck, a tsetse-refractory member of the related wildlife (Bett et al., 2015). In the present study, the effects of two structural features of the compound, ring size and the length of the side chain, on the responses of G. pallidipes and G. m. morsitans were first studied in a 2-choice wind tunnel. Reducing the ring size to five membered ring (γ-heptalactone) was found to significantly (P < 0.05) reduce repellency to both tsetse species. However, increasing the ring to seven members (ε-nonalactone) transformed the molecule to an attractant of comparable potency with the phenolic blend of fermented cow urine (Okech and Hassanali, 1990). Reduction of the alkyl side chain of δ-octalactone from a 3-carbon to a 2-carbon (CH3CH2) ethyl unit gave a structure (δ-heptalactone) with reduced repellency (P < 0.05). On the other hand, increasing the alkyl chain to 4C (CH3CH2CH2CH2) butyl unit (δ-nonalactone) significantly (P < 0.05) increased its repellency. This was confirmed with a field population of G. pallidipes where the effect of the presence of δ-nonalactone (with/without 4-methylguaiacol) on tsetse catches with baited NG2G was compared with that of δ-heptalactone (also with/without 4-methylguaiacol).
These findings show that subtle changes in the structure of δ-octalactone can significantly change the olfactory responses of the tsetse flies, and raises interesting questions about the mode(s) of interactions of this group of signals with olfactory receptor neurons. For example, the increased size of the hydrophobic side chain of the 6-membered ring lactone (to give δ-nonalactone) may facilitate improved interaction of this moiety with specific site on the δ-octalactone receptor and/or associated odorant binding protein, and it will be interesting to study the effects of other hydrophobic groups on the repellency of the resulting molecules. On the other hand, the shift in the olfactory responses of the tsetse flies resulting from increased size of the ring (ε-nonalactone) may suggest that it may be interacting with a different receptor. Follow up functional genomics and electrophysiological expression studies may provide insights on this.
From a practical perspective, the results of the present study lay down some useful groundwork for improving the efficiency of the bait (‘pull’ tactic) technologies (with ε-nonalactone), the ‘push’ tactic for protecting cattle (with δ-nonalactone or analogues with 4-methylguaiacol, and perhaps, other waterbuck repellent compounds), as well as the ‘push-pull’ strategy for area-wide control of savannah tsetse flies, and potentially other riverine species with poorly understood olfactory responses.
Highlights.
Increasing the chain length of δ-octalactone structure from C3 to C4 significantly enhanced repellency of G. pallidipes and G. m. morsitans.
Increasing the ring size of δ-octalactone structure from six to seven members changed the activity against G. pallidipes and G. m. morsitans from repellency to attraction.
There was synergistic effect on blending δ-octalactone or δ-nonalactone with 4-methylguaiacol on repellency of G. pallidipes and G. m. morsitans.
Acknowledgments
We Thank Prof. Serap Aksoy for reviewing the manuscript, and for her useful comments. We thank Mr. G. Ndiangui of Science workshop (Kenyatta University) for facilitating construction of the wind tunnel; Department of Chemistry (Kenyatta University) for allocating laboratory space for synthesis. We also thank Dr. Florence Wamwiri (HOD Entomology) and Mr. P. Obore (technician Entomology) of Biotechnology Research Institute for providing facilities and technical support in tsetse flies behavioral studies. This study was funded by National Commission for Science, Technology and Innovation (NACOSTI/RCD/ST&I 5th CALL M.Sc/254) fellowship to BM. Research reported in this publication received support from Fogarty International Center of the National Institutes of Health award R03TW009444 and NIH/NIAID supported award U01AI115648. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ciosi M, Masiga DK, Turner CM. Laboratory colonization and genetic bottlenecks in the tsetse fly Glossina pallidipes. PLoS Neglected Tropical Diseases. 2014;8(2):e2697. doi: 10.1371/journal.pntd.0002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett MK, Saini RK, Hassanali A. Repellency of tsetse-refractory waterbuck (Kobus defassa) body odour to Glossina pallidipes (Diptera: Glossinidae): assessment of relative contribution of different classes and individual constituents. Acta tropica. 2015;146:17–24. doi: 10.1016/j.actatropica.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Brightwell R, Dransfield RD, Kyorku C. Development of a low-cost tsetse trap and odour baits for Glossina pallidipes and G. longipennis in Kenya. Medical and Veterinary Entomology. 1991;5:153–164. doi: 10.1111/j.1365-2915.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Clausen PH, Adeyemi I, Bauer B, Breloeer M, Salchow F, Staak C. Host preferences of tsetse flies (Diptera: Glossinidae) based on blood meal identifications. Medical and Veterinary Entomology. 1998;12:169–180. doi: 10.1046/j.1365-2915.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- Gikonyo NK, Hassanali A, Njagi PG, Saini RK. Behaviour of Glossina morsitans morsitans Westwood (Diptera: Glossinidae) on waterbuck, Kobus defassa Ruppel and feeding membranes smeared with waterbuck sebum indicates the presence of allomones. Acta Tropica. 2000;77:295–303. doi: 10.1016/s0001-706x(00)00153-4. [DOI] [PubMed] [Google Scholar]
- Gikonyo NK, Hassanali A, Njagi PGN, Gitu PM, Midowo JO. Odor composition of preferred (buffalo and ox) and non-preferred (waterbuck) hosts of some savanna tsetse flies. Journal of Chemical Ecology. 2002;28:961–973. doi: 10.1023/a:1015205716921. [DOI] [PubMed] [Google Scholar]
- Gikonyo NK, Hassanali A, Njagi PG, Saini RK. Responses of Glossina morsitans morsitans to blends of electroantennographically active compounds in the odors of its preferred (buffalo and ox) and non-preferred (waterbuck) hosts. Journal of Chemical Ecology. 2003;29:2331–2345. doi: 10.1023/a:1026230615877. [DOI] [PubMed] [Google Scholar]
- Grootenhuis JG. Trypanotolerance in wildlife. Kenya Veterinarian. 1986;10:45–46. [Google Scholar]
- Grootenhuis JG, Olubayo RO. Disease research in the wildlife-livestock interface in Kenya. (Review papers) Veterinary Quarterly. 1993;15:55–59. doi: 10.1080/01652176.1993.9694372. [DOI] [PubMed] [Google Scholar]
- Hassanali A, McDowell PG, Owaga ML, Saini RK. Identification of tsetse attractants from excretory products of a wild host animal, Syncerus caffer. Insect Science and Its Application. 1986;7:5–9. [Google Scholar]
- Hassanali A, Owaga ML, McDowell PG. The role of 4-methylphenol and 3-n-propylphenol in the attraction of tsetse flies to buffalo urine. Insect Science and Its Application. 1988;9:95–100. [Google Scholar]
- Jordan AM. Trypanosomiasis control and African rural development. New York: Longman Inc.; 1986. p. 357. [Google Scholar]
- Mireji PO, Mabveni AM, Dube BN, Ogembo JG, Matoka CM, Magwiro TNC. Field Responses of Tsetse Flies (Glossinidae) and Other Diptera to Oils in formulations of deltamethrin. International Journal of Tropical Insect Science. 2003;23:317–323. [Google Scholar]
- Moloo SK. An artificial feeding technique for Glossina. Parasitology. 1971;63:507–512. doi: 10.1017/s0031182000080021. [DOI] [PubMed] [Google Scholar]
- Moloo SK. The distribution of Glossina species in Africa and their natural hosts. Insect Science and its Application. 1993;14:511–527. [Google Scholar]
- Okech M, Hassanali A. The origin of phenolic tsetse attractants from hosturine: studies on the pro-attractants and microbes involved. Insect Science and its Application. 1990;11:363–368. [Google Scholar]
- Onyango RJ, Van Hoeve K, De Raadt P. The epidemiology of Trypanosoma rhodesiense sleeping sickness in Alego location, Central Nyanza, Kenya. I. Evidence that cattle may act as reservoir hosts of trypanosomes infective to man. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1966;60:175–182. doi: 10.1016/0035-9203(66)90024-1. [DOI] [PubMed] [Google Scholar]
- Saini RK, Hassanali A. A 4-Alkyl-substituted analogue of guaiacol shows greater repellency to savannah tsetse (Glossina spp.) Journal of Chemical Ecology. 2007;10:1–11. doi: 10.1007/s10886-007-9272-7. [DOI] [PubMed] [Google Scholar]
- Torr SJ, Mangwiro TN, Hall DR. Responses of Glossina pallidipes (Diptera: Glossinidae) to synthetic repellent on the field. Bulletin of Entomological Research. 1996;86:609–616. [Google Scholar]
- Turner DA. The population ecology of Glossina pallidipes Austen (Diptera: Glossinidae) in the Lambwe Valley, Kenya. I. Feeding behaviour and activity patterns. Bulletin of Entomological Research. 1987;77:317–333. [Google Scholar]
- Vale GA. The responses of tsetse flies (Diptera: Glossinidae) to mobile and stationary baits. Bulletin of Entomological Research. 1974a;64:545–588. [Google Scholar]
- Weitz B. The feeding habits of Glossina. Bulletin of the World Health Organization. 1963;29:711–729. [PMC free article] [PubMed] [Google Scholar]
- Willett KC. Some Observations on the Recent Epidemiology of Sleeping Sickness in Nyanza Region, Kenya, and Its Relation to the General Epidemiology of Gambian and Rhodesian Sleeping Sickness in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1965;59:374–394. doi: 10.1016/0035-9203(65)90055-6. [DOI] [PubMed] [Google Scholar]