To the Editor:
Bronchiolitis is the leading cause of infant hospitalization in the US1 and cohort studies suggest that up to 50% of these hospitalized infants will develop childhood asthma.1 The two most common viral etiologies of severe bronchiolitis (i.e. bronchiolitis requiring hospitalization) are respiratory syncytial virus (RSV) and rhinovirus (RV).1 Although these two viruses have been the focus of most previous cohorts examining the association between bronchiolitis and asthma, RSV and RV infect infants through a respiratory tract colonized with bacteria.2, 3 Cross-sectional and prospective data suggest that the respiratory microbiota may also play a role in the development of childhood asthma.2 To date, no studies have examined the relationship between the respiratory viruses, RSV and RV, and the respiratory microbiota of infants hospitalized with bronchiolitis. This knowledge gap has hindered development of new conceptual models regarding the bronchiolitis to asthma pathway in this very high-risk population.
The 35th Multicenter Airway Research Collaboration (MARC-35) is a 17-center, prospective cohort study of 1,016 infants (age <1 year) hospitalized with bronchiolitis who we are following for the development of recurrent wheezing and eventual asthma. For 3 consecutive years (2011-2014) from November to April, researchers collected clinical data and used a standardized protocol to collect nasopharyngeal aspirates (NPA) within 24 hours of hospitalization.4 We tested these 1,016 NPAs for 17 viruses using real time PCR, as described previously,4 and also performed deep sequencing of 16S rRNA gene variable region 4 on the Illumina MiSeq platform (for a detailed description of the cohort and testing, see Methods in the online repository). We analyzed potential viral-microbial associations using 3 virus categories, RSV-only (i.e., no coinfecting viruses), RSV/RV coinfection, and RV-only (i.e., no coinfecting viruses). We tested the association between these 3 virus categories and the relative abundance of the dominant phyla and genera using linear regression and t-tests. We examined potential confounding variables using multiple regression; linear model assumptions were examined by evaluating residuals (see Methods in the online repository).
Of the 1,016 enrolled infants, there were 1,005 (99%) with NPAs that met the 16S rRNA gene sequence quality control requirements for analysis. Of these 1,005 NPAs, 978 (97%) had virus detected, of which 740 (74%) were in one of the 3 viral categories (580 RSV-only, 100 RSV/RV coinfection, or 60 RV-only) and comprised the analytic sample. At enrollment, the median age of the 740 infants was 3 months (IQR 2-5 months), 59% male, and 51% either non-Hispanic black or Hispanic. Table 1 shows the patient characteristics by virus category.
Table 1.
Demographic characteristics, medical history, and clinical presentation of infants hospitalized for bronchiolitis by virus, n=740
| Characteristica | RSV-only | RSV/RV coinfection |
RV-only |
P- value |
|---|---|---|---|---|
| (n=580) | (n=100) | (n=60) | ||
| Median age at enrollment in months (IQR) |
3 (1-5) | 3 (2-5) | 4 (1-7) | 0.03 |
| Age at enrollment | 0.02 | |||
| <2 months | 210 (36) | 27 (27) | 19 (32) | |
| 2-5.9 months | 268 (46) | 53 (53) | 21 (35) | |
| 6-11.9 months | 102 (18) | 20 (20) | 20 (33) | |
| Male sex | 331 (57) | 66 (66) | 41 (68) | 0.08 |
| Race/ethnicity | 0.41 | |||
| Non-Hispanic white | 267 (46) | 41 (41) | 19 (32) | |
| Non-Hispanic black | 126 (22) | 24 (24) | 18 (30) | |
| Hispanic | 162 (28) | 30 (30) | 21 (35) | |
| Other | 25 (4) | 5 (5) | 2 (3) | |
| Insurance | 0.13 | |||
| Public | 322 (56) | 66 (66) | 40 (68) | |
| Private | 247 (43) | 32 (32) | 19 (32) | |
| None | 10 (2) | 2 (2) | 0 (0) | |
| Parental history of asthma | 190 (33) | 44 (44) | 22 (37) | 0.08 |
| Maternal smoking during pregnancy | 84 (15) | 12 (12) | 11 (18) | 0.56 |
| Antibiotic use during pregnancy | 148 (26) | 24 (24) | 22 (39) | 0.09 |
| Mode of birth | 0.31 | |||
| Vaginal birth | 359 (63) | 70 (71) | 38 (63) | |
| C-section | 214 (37) | 29 (29) | 22 (37) | |
| Gestational age | 0.04 | |||
| ≤37 weeks | 104 (18) | 26 (26) | 17 (28) | |
| >37 weeks | 476 (82) | 74 (74) | 43 (72) | |
| Birth weight, kg | 0.08 | |||
| ≤1.3 kg | 1 (0.2) | 0 (0) | 0 (0) | |
| 1.4-2.2 kg | 32 (6) | 7 (7) | 8 (13) | |
| 2.3-3.1 kg | 183 (32) | 40 (40) | 23 (38) | |
| ≥3.2 kg | 359 (62) | 53 (53) | 29 (48) | |
| ICU or special care at birth | 88 (15) | 18 (18) | 15 (25) | 0.13 |
| Mostly breast milk for first 3 months | 0.66 | |||
| No | 259 (45) | 48 (48) | 31 (52) | |
| Yes | 248 (43) | 37 (37) | 21 (35) | |
| Unknown | 73 (13) | 15 (15) | 8 (13) | |
| Mostly breast milk from 3 to 6 months |
0.38 | |||
| No | 357 (62) | 63 (63) | 43 (72) | |
| Yes | 150 (26) | 22 (22) | 9 (15) | |
| Unknown | 73 (13) | 15 (15) | 8 (13) | |
| Previous breathing problemsb before index hospitalization |
76 (13) | 21 (21) | 21 (35) | <0.001 |
| Ever previously hospitalized overnight |
74 (13) | 12 (12) | 16 (27) | 0.01 |
| Child history of eczema | 77 (13) | 11 (11) | 11 (18) | 0.41 |
| Child history of antibiotic use before index hospitalization |
153 (26) | 27 (27) | 19 (32) | 0.68 |
| Ever had smoke exposure | 93 (16) | 9 (9) | 7 (12) | 0.15 |
| Ever attended daycare | 103 (18) | 34 (34) | 8 (13) | <0.001 |
| Number of other children at home | 0.44 | |||
| 0 | 123 (21) | 16 (16) | 12 (20) | |
| 1 | 232 (40) | 34 (34) | 26 (43) | |
| 2 | 127 (22) | 25 (25) | 12 (20) | |
| ≥3 | 98 (17) | 25 (25) | 10 (17) | |
| Month of enrollment | <0.001 | |||
| January | 189 (33) | 33 (33) | 10 (17) | |
| February | 155 (27) | 17 (17) | 9 (15) | |
| March | 62 (11) | 12 (12) | 14 (23) | |
| April | 23 (4) | 4 (4) | 12 (20) | |
| November | 43 (7) | 13 (13) | 5 (8) | |
| December | 108 (19) | 21 (21) | 10 (17) | |
| Weight at presentation (kg), median (IQR) |
5.7 (4.6-7.2) | 6.1 (4.7-7.6) | 6.8 (4.7- 8.5) |
0.11 |
| Respiratory rate per minute at presentation, median (IQR) |
48 (40-60) | 52 (40-60) | 45 (40- 58) |
0.21 |
| Initial oxygen saturation at presentation |
0.90 | |||
| <90% | 52 (9) | 8 (8) | 6 (10) | |
| 90%-93.9% | 93 (17) | 13 (13) | 8 (14) | |
| ≥94% | 417 (74) | 76 (78) | 44 (76) | |
| Received antibiotics during pre-hospitalization visit |
103 (18) | 14 (14) | 9 (15) | 0.59 |
| Received corticosteroids (systemic or inhaled) during pre- hospitalization visit |
36 (6) | 7 (7) | 9 (15) | 0.06 |
| Mechanical ventilation during hospitalization (CPAP and/or intubation) |
33 (6) | 2 (2) | 3 (5) | 0.30 |
Abbreviations: IQR, interquartile range; ICU, intensive care unit; CPAP, continuous positive airway pressure ventilation
Data were expressed as n (%) unless otherwise indicated.
A breathing problem is parent reported and defined as a child having cough that wakes him/her at night and/or causes emesis, or when the child has wheezing or shortness of breath without cough.
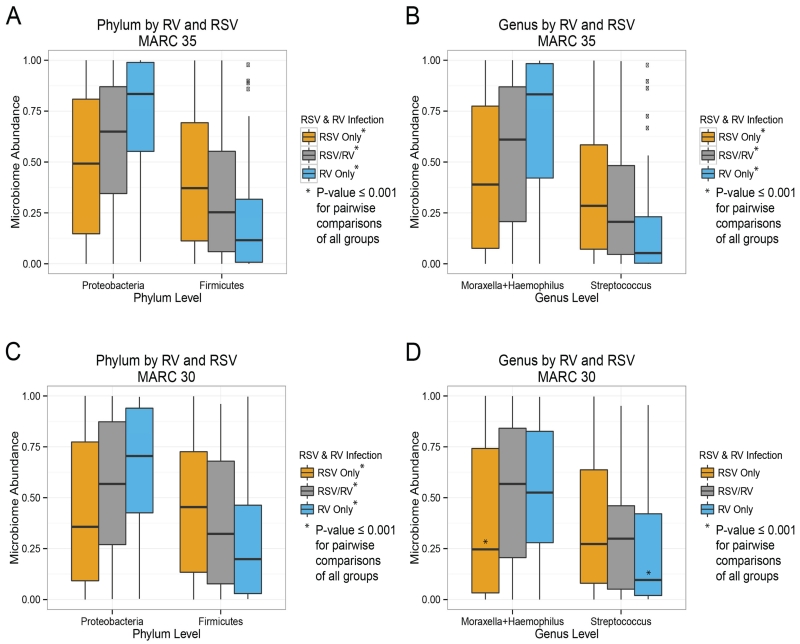
Examining the nasopharyngeal microbiota within these 3 virus categories demonstrated significant differences at the phylum and genus taxonomic levels as shown in Figure 1, A and B. Infants with RSV-only had a high abundance of Firmicutes and the genus Streptococcus and a low abundance of Proteobacteria and the genera Haemophilus and Moraxella (P<0.001). By contrast, infants with RV-only had the opposite pattern at both the phylum (i.e., low Firmicutes and high Proteobacteria) and genus levels (i.e., low Streptococcus and high Haemophilus and Moraxella; P<0.001). Interestingly, infants with RSV/RV coinfections had intermediate abundances of these phyla and genera. These viral-microbial findings remained highly significant after controlling for clinical covariates: age, mode of birth, prematurity, being fed mostly breast milk for the first 3 months of life, previous breathing problems, antibiotic use, smoke exposure (ever), daycare, month of hospitalization, and recent and lifetime corticosteroid use (see Figure E1 in the online repository).
Figure 1.
Association of respiratory syncytial virus (RSV) and rhinovirus (RV) with nasopharyngeal microbiota at phylum and genus levels in two severe bronchiolitis cohorts
Panels A and B depict phylum and genus level microbiota composition, respectively, for the MARC-35 cohort. Viral infection states of RSV-only, RSV/RV co-infection and RV-only are shown. Compared with RV-only, RSV-only infected infants had significantly higher levels of Firmicutes and lower levels of Proteobacteria; for RV, this trend is reversed; the RSV/RV co-infection state is intermediate. In panels C and D this analysis is replicated in the MARC-30 cohort and shows concordant viral-microbial patterns of association. P-values in these analyses are based on one-way ANOVA and corresponding t-tests of microbiota relative composition. Wilcoxon tests produced consistent results.
After observing these significantly different viral-microbial associations, we performed a replication analysis in 307 children (94% age <1 year) from our well characterized MARC-30 severe bronchiolitis cohort from 2007-2010.4 Since these 307 MARC-30 children are part of an ongoing study on microbial predictors of mechanical ventilation, the population differs slightly from the MARC-35 population (for additional details comparing the 2 cohorts, see Table E1 in the online repository). The NPAs from both studies, however, were collected, processed, and tested using the same protocol.4 Despite population differences between these 2 severe bronchiolitis cohorts, the viral-microbial associations for RSV and RV found in MARC-35 were reproducible in MARC-30 (Figure 1, C and D).
Although viral-bacterial interaction in respiratory illness has been noted for ~100 years, a clear understanding of the directionality of viral-bacterial interactions is lacking.5 In other words, do viral infections increase certain bacterial populations within a community and/or do microbial community populations create environments suitable for specific viruses? In a prospective study of 308 school-age children, Kloepfer and colleagues demonstrated that RV infection preceded the increased odds of detection of Streptococcus and Moraxella by one week.6 By contrast, Gulraiz and colleagues demonstrated in human bronchial epithelial cells that nontypeable Haemophilus influenza increased the generation of RV infectious particles and also enhanced the inflammatory response to RSV.7
In addition to reproducing the present viral-microbial associations in two different severe bronchiolitis cohorts, the composition of our nasopharyngeal samples at hospitalization matched the composition of the nasopharyngeal microbiota in 234 infants with outpatient respiratory infections.8 Taken together these data suggest that both viruses and bacteria may contribute to the acute symptoms of bronchiolitis and its long-term respiratory consequences (e.g., asthma).1-3, 5, 8 Although much remains to be learned about viral-bacterial interactions (e.g., interaction types and dynamics), we plan to examine in MARC-35 the relation of respiratory virus infections, the nasopharyngeal microbiota, and other factors to the subsequent development of recurrent wheezing and asthma.
Our analysis has potential limitations. First, these are viral-bacterial profiles at the time of a severe illness and therefore, may not be reproducible in healthy infants. Second, RV has many subtypes and may be either a bystander virus or a pathogen,9 but the observed RV-Proteobacteria profile suggests that in infants hospitalized with bronchiolitis, RV is not simply a bystander.
We observed in two prospective multicenter cohorts of US infants hospitalized with bronchiolitis that RSV and RV are associated with distinct nasopharyngeal taxa. Further understanding the relation of these viral-microbial profiles to acute bronchiolitis symptoms and future asthma risk may help generate new conceptual models for both respiratory infections and for the bronchiolitis to asthma pathway.
Online Repository
Methods
Study Design
We performed a 17-center, prospective cohort study of hospitalized infants (age <1 year) with an attending physician’s diagnosis of bronchiolitis. Research teams at each center used a standardized protocol to enroll these hospitalized infants for 3 consecutive bronchiolitis seasons from November 1 to April 30 (2011-2014). This study was the 35th Multicenter Airway Research Collaboration (MARC-35). MARC is a program of the Emergency Medicine Network (EMNet) (www.emnet-usa.org). The overall objective of MARC-35 is to examine the relationship between severe bronchiolitis (i.e., bronchiolitis requiring hospitalization) and recurrent wheezing of childhood and eventual asthma.
Inclusion criteria were: 1) age <1 year; 2) admitted to hospital with attending physician diagnosis of bronchiolitis defined by the Academy of Pediatrics: acute respiratory illness with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and retractions;1 3) expect to have stable address, phone number, email, and primary care provider for next year; and 4) ability of the parent/guardian to give informed consent.
Exclusion criteria were: 1) enrolled into the current study during an earlier bronchiolitis admission; 2) parents who do not agree to the collection of all biologic specimens (i.e., nasopharyngeal aspirate, nasal swab, blood) or who do not agree to the possible future use of specimens; 3) consented >24 hours after admission to ward or ICU; 4) patient transferred to participating hospital >24 hours after the original time of admission; 5) already meets the primary endpoint of the overall study (i.e., current treatment is 2nd oral steroid course in 6 months, or 4th episode of wheezing in past year); or 6) known heart-lung disease, immunodeficiency, immunosuppression, or gestational age <32 weeks.
All patients were treated at the discretion of the treating physician in the hospital and after discharge. The intensive participant retention procedures and follow-up are not discussed herein as they are not relevant to the present analysis. All consent and data forms were translated into Spanish. The institutional review board at each of the 17 participating hospitals approved the study.
Data Collection
Investigators conducted a structured interview that assessed patients’ demographic characteristics; medical, environmental, nutritional, and family history; and details of the acute illness. Mothers, fathers or, in special circumstances (e.g., adoption), a guardian completed a pregnancy and birth questionnaire. Emergency department (ED) and hospital chart reviews provided further details about the acute illness. All data were reviewed at the EMNet Coordinating Center and site investigators were queried about missing data and discrepancies identified by manual data checks.
Nasopharyngeal aspirate collection
Nasopharyngeal aspirates (NPAs) were collected by site teams using the same standardized protocol we utilized in a previous cohort study, MARC-30.2 All of the sites used the same collection equipment (e.g., sample traps and suction catheters from Medline Industries, Mundelein, IL) and collected the samples within 24 hours of hospitalization. The NPA sample was added to transport medium, immediately placed on ice and then stored at −80°C. Frozen samples were shipped overnight on dry ice to Dr Piedra’s laboratory at Baylor College of Medicine, where they were stored again at −80°C.
Polymerase Chain Reaction assay
All PCR assays were conducted as singleplex or duplex two-step real time PCR (rtPCR). Real time reverse transcriptase-PCR (rtRT-PCR) was used for the detection of respiratory viruses which included RSV types A and B, human rhinovirus (HRV), parainfluenza virus (PIV) types 1, 2 and 3, influenza virus types A and B, 2009 novel H1N1, human metapneumovirus (hMPV), coronaviruses NL-65, HKU1, OC43 and 229E, adenovirus, human bocavirus type 1 and enterovirus. These tests are routinely conducted in Dr Piedra’s laboratory and details of the primers and probes have been described. 3-5
16S rRNA gene sequencing and compositional analysis
16S rRNA gene sequencing methods were adapted from the methods developed for the NIH-Human Microbiome Project.6, 7 Briefly, bacterial genomic DNA was extracted using MO BIO PowerSoil DNA Isolation Kit (Mo Bio Laboratories; Carlsbad, CA).
The 16S rDNA V4 region was amplified by polymerase chain reaction (PCR) and sequenced in the MiSeq platform (Illumina; SanDiego, CA) using the 2×250 bp paired-end protocol yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes allowing pooling and direct sequencing of PCR products.8, 9
The 16S rRNA gene pipeline data incorporates phylogenetic and alignment-based approaches to maximize data resolution. The read pairs were demultiplexed based on the unique molecular barcodes, and reads were merged using USEARCH v7.0.1090,10 allowing zero mismatches and a minimum overlap of 50 bases. Merged reads were trimmed at first base with Q5. In addition, a quality filter was applied to the resulting merged reads and reads containing above 0.05 expected errors were discarded.
The Alkek Center for Metagenomics and Microbiome Research (CMMR) pipeline for 16S analysis leverages custom analytic packages and pipelines developed at the CMMR to provide summary statistics and quality control measurements for each sequencing run, as well as multi-run reports and data-merging capabilities for validating built-in controls and characterizing microbial communities across large numbers of samples or sample groups. Rarefaction curves of bacterial Operational Taxonomic Units (OTUs) were constructed using sequence data for each sample to ensure coverage of the bacterial diversity present. Samples with suboptimal amounts of sequencings reads (<80% of the taxa are represented) were re-sequenced to ensure that the majority of bacterial taxa were encompassed in our analyses.
16S rRNA gene sequences were clustered into OTUs at a similarity cutoff value of 97% using the UPARSE algorithm.11 OTUs were mapped to an optimized version of the SILVA Database11, 12 containing only the 16S v4 region to determine taxonomies. Abundances were recovered by mapping the demultiplexed reads to the UPARSE OTUs. A custom script constructed a rarefied OTU table from the output files generated in the previous two steps for downstream analyses of alpha-diversity, beta-diversity,13, 14 and phylogenetic trends.
Statistical methods
Initial analysis and visualization of nasopharyngeal microbiota were conducted in R,15 utilizing the phyloseq package16 to import sample data and calculate alpha- and beta-diversity metrics. Further statistical analysis was continued in R, version 3.01 and Stata 14 (StataCorp). Analyses were performed using base packages and custom scripts. The analyses utilized the rarefaction proportion of reads for each OTU as the input data.
We used one-way analysis of variance (ANOVA) to analyze the association between the virus (independent variable) and the relative abundance of taxa within the nasopharyngeal microbiota (dependent variable) at all levels of the taxonomic tree (i.e., phylum to genus). T-tests were utilized to analyze the difference of the means between RSV and RV for each taxonomic level (i.e., mean relative abundance of taxa for RSV minus the mean relative abundance of taxa for RV). The ANOVA estimate of within group error variance, together with sample size, was used to scale the mean differences to determine the T-statistics.
The underlying statistical assumptions used in ANOVA were checked. First, we found that statistical independence exists between samples, but not between microbiota groups within an individual sample. Second, we examined normality using QQplots to visualize the residuals. Third, using the arcsin square root transformation of the frequency data, we found that the P-values and directionality of our findings were not affected by transformation. For reasons of transparency and simplicity we have presented the untransformed data in the manuscript. To assure ourselves that normality assumptions did not play a major role in our conclusions, we also performed non-parametric analyses using Wilcoxon rank sum tests; these non-parametric analyses had results consistent to those of the ANOVA.
Analyses presented are at the phylum and genus level. For the genus level analyses, we analyzed only the dominant genera within the phyla. For the Proteobacteria phylum, the microbiota was dominated by Haemophilus (55%) and Moraxella (37%). For the Firmicutes phylum, the microbiota was dominated by Streptococcus (84%) alone with the next highest taxa being Staphylococcus (6%).
In addition to hypothesis testing using ANOVA and non-parametric analyses, we performed exploratory analyses including bivariate scatter plots as well as multi-factorial boxplots using the R method ggplot2 version 1.01. These approaches were employed to examine the relationships between microbiota and clinical variables and to visualize relationships to guard against potential confounding. To further confirm our virus-microbiota findings, we used linear regression to examine the possibility that the findings were driven by confounding associations with other clinical variables (see Figure 1 in the online repository).
Supplementary Material
Acknowledgements
The authors thank Ginger A. Metcalf, Donna M. Muzny, and Richard A. Gibbs in the Human Genome Sequencing Center at Baylor College of Medicine for their assistance in performing the sequencing for this project. We also thank the participating medical centers for the collection of patient samples and ongoing dedication to bronchiolitis research.
Funding: This work was supported by the grant U01 AI-087881 from the National Institutes of Health (Bethesda, MD) and a grant from the William F. Milton Fund (Boston, MA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations
- (RSV)
respiratory syncytial virus
- (RV)
rhinovirus
- (NPA)
nasopharyngeal aspirates
- (IQR)
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasegawa K, Mansbach JM, Camargo CA., Jr Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12(7):817–828. doi: 10.1586/14787210.2014.906901. [DOI] [PubMed] [Google Scholar]
- 2.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135(1):25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vissers M, de Groot R, Ferwerda G. Severe viral respiratory infections: are bugs bugging? Mucosal Immunol. 2014;7(2):227–238. doi: 10.1038/mi.2013.93. [DOI] [PubMed] [Google Scholar]
- 4.Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of Pediatrics and Adolescent Medicine. 2012;166(8):700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9(1):e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–1307. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulraiz F, Bellinghausen C, Bruggeman CA, Stassen FR. Haemophilus influenzae increases the susceptibility and inflammatory response of airway epithelial cells to viral infections. FASEB J. 2015;29(3):849–858. doi: 10.1096/fj.14-254359. [DOI] [PubMed] [Google Scholar]
- 8.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerna G, Piralla A, Rovida F, Rognoni V, Marchi A, Locatelli F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. Journal of Medical Virology. 2009;81(8):1498–1507. doi: 10.1002/jmv.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- E1.Lieberthal AS, Bauchner H, Hall CB, Johnson DW, Kotagal U, Light MJ, et al. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- E2.Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of Pediatrics and Adolescent Medicine. 2012;166(8):700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, et al. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50(4):322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Knorr L, Fox JD, Tilley PA, Ahmed-Bentley J. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect Dis. 2006;6:62. doi: 10.1186/1471-2334-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Winchell JM, Thurman KA, Mitchell SL, Thacker WL, Fields BS. Evaluation of three real-time PCR assays for detection of Mycoplasma pneumoniae in an outbreak investigation. Journal of Clinical Microbiology. 2008;46(9):3116–3118. doi: 10.1128/JCM.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.A framework for human microbiome research. Nature. 2012;486(7402):215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- E11.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- E12.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5(2):169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Team R Core . R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A language and environment for statistical computing″. [Google Scholar]
- E16.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.