Abstract
Hypertrophic cardiomyopathy (HCM) is a genetic disease of the sarcomere that can be found in both children and adults and is associated with many causative mutations. In children who are not the index case of HCM in their families, current recommendations call only for targeted genetic testing for familial mutations. However, clinical experience suggests that de novo mutations are possible, as are mutations inherited from apparently an unaffected parent. A chart review was conducted of all patients who received HCM genetic testing at Johns Hopkins from 2004 to 2013. In total, 239 patient charts were analyzed for personal and familial genetic findings. Eighty-one patients with sarcomere gene mutations were identified, of which 66 had a clinical diagnosis of HCM. Importantly, eight patients had >1 pathogenic or likely pathogenic mutation, including six patients who were diagnosed with HCM as children (18 or younger). In this analysis, when a sarcomere mutation is identified in a family, the likelihood of a child with HCM having >1 mutation is 25 % (6/24), compared to 4.8 % (2/42) for adults. The large number of children with multiple mutations suggests that broad panel rather than targeted genetic testing should be considered in HCM presenting during childhood even if the child is not the index case.
Keywords: Hypertrophic cardiomyopathy, Genetic counseling, Genetic testing
Introduction
Hypertrophic cardiomyopathy is clinically defined as unexplained left ventricular hypertrophy without an underlying disease condition that would explain the hypertrophy [11]. Recent guidelines suggest that left ventricular hypertrophy caused by infiltrative diseases, storage diseases, metabolic disorders or systemic/syndromic disorders such as Noonan syndrome should be excluded from this diagnosis [5]. Although there is a wide range of disease severity, hypertrophic cardiomyopathy can cause debilitating symptoms and is also the most common cause of sudden cardiac death in young athletes [13, 14]. The prevalence of hypertrophic cardiomyopathy is estimated to be at least 1/500 in the general population, with the majority of cases being asymptomatic [5, 17].
Hypertrophic cardiomyopathy is a genetic disease of the sarcomere typically inherited in an autosomal dominant manner. There are numerous genetic mutations of sarcomere genes that can lead to hypertrophic cardiomyopathy [19, 20]. While the knowledge of potential genetic causes of hypertrophic cardiomyopathy continues to increase, the current probability of detecting a probable disease-causing mutation in a person with hypertrophic cardiomyopathy ranges from about 30 % in a patient with sporadic disease to about 60 % in a patient with a positive family history of hypertrophic cardiomyopathy [1]. Thus, many patients with the disease do not have an identified mutation following genetic testing. Currently, commercial genetic testing panels for hypertrophic cardiomyopathy analyze about 20–50 genes, although the vast majority of mutations occur in just a few of these genes [16]. Increasingly these panels include several non-sarcomeric genes, such as GLA (the cause of Fabry disease) and LAMP2, which can produce phenotypes similar to that of hypertrophic cardiomyopathy [2].
Given the large number of mutations that can lead to hypertrophic cardiomyopathy, it is not uncommon for multiple potentially disease-causing mutations to be present in a single patient. These mutations may be in different genes or may be multiple (compound) mutations in a single gene. Mutations may be inherited from one or both parents or may arise as de novo mutations. Multiple studies have estimated these multiple mutations occur in about 5 % of patients [1, 6–8]. Triple mutations may also occur, with one study detecting their rate at <1 % in the particular study cohort [6]. Some studies suggest greater disease severity in patients with multiple mutations [4, 10, 15, 21]. Compounding the issue of interpreting multiple mutations in hypertrophic cardiomyopathy is the sheer number of possible mutations that may be present, recently estimated at 1400, and growing [12]. It is not uncommon for a particular mutation to be reported only in a single patient or family, leading to difficulty in establishing the true pathogenicity of some sequence variants. For this reason additional correlation of linkage within pedigrees, population-based studies and biological assays are necessary to understand pathogenicity [10].
The relatively large number of patients with multiple mutations, and the fact that multiple mutations may signify an increased risk of severe disease, presents particular challenges to clinicians who coordinate genetic testing for patients with hypertrophic cardiomyopathy, particularly in younger patients. Few studies have quantified the prevalence of multiple mutations in children with hypertrophic cardiomyopathy, although one study suggests it is similar to that of adults [9]. It has been hypothesized, however, that the true rate of multiple mutations may be higher in children with hypertrophic cardiomyopathy relative to adults with the disease, because earlier presentation may be correlated with increased disease severity, and thus increased risk of having more than one causative mutation. The question arises whether full-panel genetic testing is appropriate in a child with clinically apparent hypertrophic cardiomyopathy, even if a familial mutation has been previously identified. In this study, we retrospectively studied a sequential group of patients who had or were at risk of hypertrophic cardiomyopathy, both children and adults, who were genetically tested. The frequency of multiple mutations was compared in children (<18 years at diagnosis) and adults with a goal of quantifying whether children with the disease are at increased risk of multiple mutations, thus informing our approach to genetic counseling and testing in these children.
Methods
Patient Selection
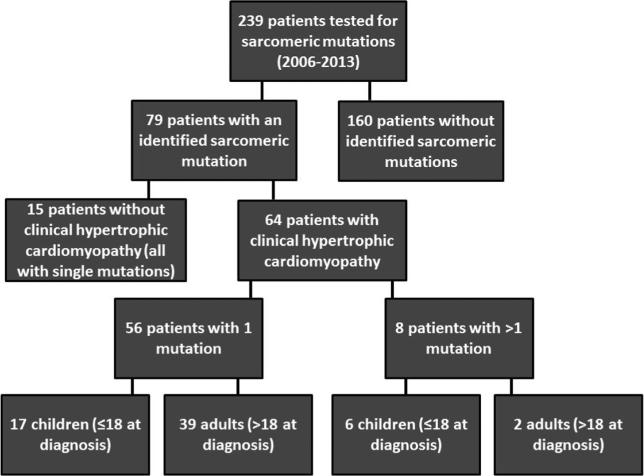
There were 239 patients at Johns Hopkins Hospital who received genetic testing for HCM between 11/2004 and 5/2013. The testing was all performed in CLIA-certified commercial laboratories. Some of these patients had a clinical diagnosis of HCM, while others did not have HCM but were screened for familial mutations (Fig. 1).
Fig. 1.
Flow chart describing separation of patients in the clinical study. The majority of tested patients did not have a sarcomeric mutation (160/239). Of the 79 patients with an identified mutation, 64 were also phenotype positive for HCM. Of these genotype and phenotype positive patients, eight had multiple mutations. Children were overrepresented in the multiple mutation group (6/8) relative to adults
Genetic Testing
In total, 82 patients received only targeted testing (i.e., testing for a known familial mutation or mutations), with the remainder receiving comprehensive panel testing for multiple sarcomeric genes as well as for mutations in nonsarcomeric genes associated with cardiac hypertrophy. Over the time period of the study, advances in clinical genetic testing allowed for more comprehensive genetic panels, and thus patients tested later in the study period were more likely to have been tested with larger gene panels. More specifically, 18 patients were tested with a five-gene panel (MYH7, MYPBC3, TNNT2, TNNI3 and TPM1), six patients were tested with an eight-gene panel (including ACTC, MYL2 and MYL3), and 32 patients were tested with an 11-gene panel. Four patients were tested with a more comprehensive panel for unexplained cardiac hypertrophy (UHC). The remaining 97 patients were tested using a 17- or 18-gene HCM panel. Mutations were defined as pathogenic or likely pathogenic or similar terminology that distinguished this from variants of uncertain significance. Mutations defined as variants of uncertain significance (VUS) were not included in the analysis, but may be reclassified in the future.
Statistical Methods
The prevalence of multiple mutations in the HCM group diagnosed as children (≤18 years) was compared to the adult HCM group using a two-tailed Fisher's exact test.
Results
In total, 239 patients had genetic testing between 2004 and 2013 (Table 1). This group included probands and those tested because of familial mutations. The majority of these patients did not have a mutation identified (57 %). A total of 79 (33 %) patients had a pathogenic or likely pathogenic mutation identified. However, 15 of these patients did not yet meet clinical criteria for a clinical diagnosis of HCM. A total of 64 patients (27 %) had at least one mutation identified as well as a clinical diagnosis of HCM. One patient was found to have a LAMP2 mutation, and another was found to have Fabry disease. Finally, 21 patients (9 %) had failed or uncertain test results or results were not available at time of data collection.
Table 1.
Characteristics of patients genetically tested for HCM
| Patients genetically tested | 239 |
| HCM (+), mutation (+) | 64 (27 %) |
| HCM (–), mutation (+) | 15 (6 %) |
| Mutation (+) | 137 (57 %) |
| LAMP2 mutation (+) | 1 (<1%) |
| Fabry mutation (+) | 1 (<1%) |
| Failed, pending and uncertain results | 21 (9 %) |
The majority of patients (57 %) tested negative for sarcomeric mutations. A smaller number (27 %) tested positive for at least one sarcomeric mutation, while 6 % of patients tested positive for a pathogenic or likely pathogenic mutation but did not yet meet criteria for a clinical diagnosis of HCM
In the 79 patients in whom a sarcomeric mutation was identified, a total of 88 mutations were found. In patients diagnosed with HCM after the age of 18 years, 54 total mutations were found (Table 2). The greatest number of mutations were in the MYBPC3 gene (54 % of mutations), followed by MYH7 (22 %), TNNT2 (11 %), TNNI3 (5 %), TPM1 (5 %) and MYL2 (5 %). Among children 18 years and younger with at least one mutations identified, 34 total mutations were found. The distribution of mutations was similar to that of adults except for an increase in MYH7 mutations (32 %) relative to MYBPC3 mutations (38 %).
Table 2.
Sarcomeric mutation frequency in adults and children
| Gene | Number of mutations identified (adults) (%) | Number of mutations identified (children) (%) |
|---|---|---|
| MYBPC3 | 29 (54) | 13 (38) |
| MYH7 | 12 (22) | 11 (32) |
| TNNT2 | 6 (11) | 4 (12) |
| TNNI3 | 3 (5) | 2 (6) |
| TPM1 | 2 (5) | 2 (6) |
| MYL2 | 2 (5) | 2 (6) |
| Total | 54 (100) | 34 (100) |
Includes pathogenic and likely pathogenic mutations in patients with and without clinical HCM at the time of testing
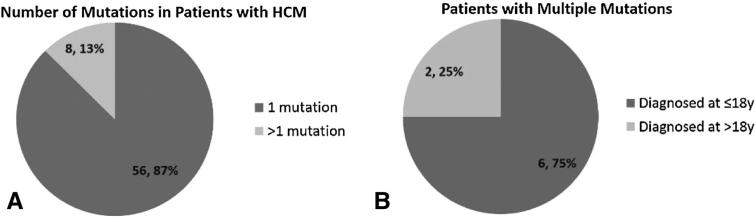
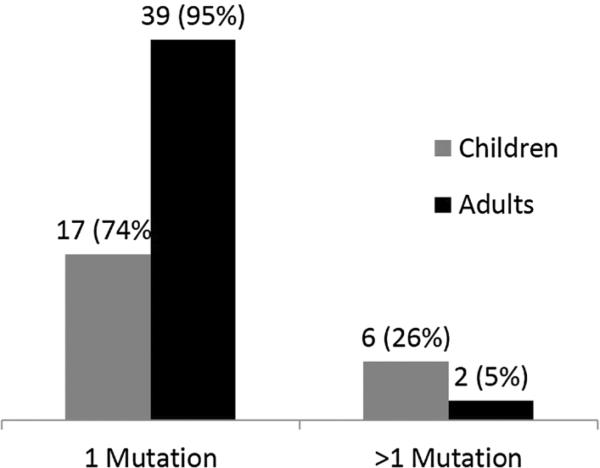
In patients who were both genotype and phenotype positive, 56 patients (87 %) had a single pathogenic or likely pathogenic mutation, while eight patients (13 %) had more than one mutation (Fig. 2a). Among these eight patients with multiple mutations, seven had two mutations, while one had three mutations. In patients with multiple mutations identified, 6/8 were diagnosed with HCM as children (≤18 years), while only 2/8 were diagnosed with HCM as adults (Fig. 2b). Put another way, in patients diagnosed with HCM at 18 years of age or younger, 6/23 (26 %) harbored more than one mutation, while only 2/41 (5 %) of genotype positive adults had more than one mutation (Fig. 3).
Fig. 2.
Frequency of single versus multiple mutations in genotype positive patients with HCM and age at clinical diagnosis for patients with multiple mutations. While the majority of patients had a single mutation, 13 % harbored more than one mutation (a). Patients with multiple mutations were also more likely to be diagnosed with HCM at a younger age (b)
Fig. 3.
Frequency of single versus multiple sarcomeric mutations in children and adults with genotype positive HCM. Children were more likely than adults to have multiple mutations identified (26 vs 5 %)
In total, eight patients were found to have multiple sarcomeric mutations (Table 3). Patients A–F were diagnosed with HCM at ≤18 years of age, while patients G–H were diagnosed at >18 years. The age range of diagnosis in these patients was between 0 (in utero diagnosis) and 44 years of age. All children with multiple mutations were symptomatic. Patients C, D and F had ICDs placed, while patient E underwent a myectomy due to exertional symptoms. Patient C has a history of heart failure from severe HCM as well as decreased ejection fraction. Patient A underwent a heart transplant early in life, while patient B experiences exertional symptoms. In contrast to the younger patients, only one of two adults was symptomatic (patient G).
Table 3.
Characteristics of patients with multiple sarcomeric mutations
| Patient | Sex | Age at Dx | Mutations | Clinical notes |
|---|---|---|---|---|
| A | F | Utero | MYBPC3: c. 1227-13 G>A (intron), E542Q | Heart transplant; sister with DCM |
| B | F | 1 | MYBPC3: G490R, IVS30+5G>C | SOB with moderate exertion; brother with HCM (patient C) |
| C | M | 4 | MYBPC3: G490R, IVS30+5G>C, TNNI3: T143N, TPM1: S215L | ICD; heart failure class II; sister with HCM (patient B) |
| D | M | 7 | MYBPC3: R943X, TNNI3: T143N, TPM1: S215L | ICD; father, paternal aunt, two paternal cousins with HCM |
| E | F | 11 | MYH7: R663H, MYH7: A893sp | Fatigue and SOB; myectomy |
| F | M | 17 | TNNT2: 287X, MYL2: R120G | ICD; chest pain on exertion; father, paternal grandfather with HCM |
| G | M | 22 | TNNI3: T143N, TPM1: S215L | Sister and son (patient D) with HCM. Deceased from colon cancer. |
| H | M | 44 | TNNT2: W287stop, MYL2: R120G | Asymptomatic; HCM in son (patient F) |
Patients A–F were diagnosed with HCM at ≤18 years of age, while patients G–H were diagnosed at >18 years. Patient A had characteristics of both HCM and DCM and received a heart transplantation before a definitive diagnosis could be established. Decisions to place ICDs were based on traditional clinical factors for primary or secondary prevention, or in one case patient/family preference due to lifestyle considerations, and not based solely on multiple mutations
Both pathogenic and likely pathogenic mutations were included in the analysis of mutation frequency, but variants of uncertain significance were excluded. Both mutations of patient A (Table 3) are classified as likely pathogenic. The mutations of patients B and C (siblings) are known to be pathogenic. For patient D, MYBPC3 p.Gly490Arg is known pathogenic, while TNNI3 p.Thr143Asn and TPM1 p.Ser 215Leu are likely pathogenic. In patient E, MYH7 p.Arg 943Ter is known pathogenic, while MYH7 p.Ala893sp is likely pathogenic. For patients F and H (father and son), TNNT2 p.Trp287Ter is pathogenic, while MYL2 p.Arg120-Gly is likely pathogenic. For patient G, both mutations are classified as likely pathogenic.
Discussion
Genetic counseling is recommended for individuals diagnosed with hypertrophic cardiomyopathy and their families and some expert groups recommend routine genetic testing [1, 5, 7]. As a relatively common (at least 1/500 on a population basis), autosomal dominant condition that can have significant risk as well as recommended changes in lifestyle, counseling families is imperative. For families who decline genetic testing, clinical surveillance with echocardiography and ECG is recommended with relatively frequent assessment during adolescence. When an individual with a clinical diagnosis of hypertrophic cardiomyopathy is identified and clear pathogenic mutation is identified, predictive testing to offspring, siblings or parents can be offered and extended to other family members who are at risk by cascade testing. Those who carry the pathogenic mutation but without ventricular hypertrophy have ongoing surveillance; however, at risk family members who test negative for the familial mutation typically do not need further clinical surveillance. On the surface this would appear to be straightforward,; however, in our cumulative experience a number of clinical scenarios have made decision making more complex. Examples include children affected prior to adolescence, with severe hypertrophy or early onset symptoms. In addition, there may be both maternal and paternal relatives who had apparent sudden cardiac death in adulthood attributed to “heart attack” without prior history of coronary disease and without autopsy information to confirm the cause of death [3, 18]. In practice, it is essential to obtain detailed and extensive family histories. It is also crucial to probe for the many ways that hypertrophic cardiomyopathy can present including manifest cardiomyopathy, unexplained sudden death including deaths from drowning or single occupant motor vehicle accidents, early onset presentation of atrial fibrillation or late systolic heart failure that may be end stage hypertrophic cardiomyopathy. The value of obtaining primary data such as autopsy reports cannot be underestimated.
These nuances in clinical presentations and family history have led us to hypothesize that in some circumstances targeted genetic testing for a single known familial pathogenic mutation may not be sufficient to rule out risk in relatives at risk of hypertrophic cardiomyopathy by pedigree analysis. In this retrospective study, we asked whether individuals diagnosed with hypertrophic cardiomyopathy at 18 years or younger in an unselected group of patients who were referred and tested in our center were more likely to have more than one pathogenic or likely pathogenic mutation. In the group of patients, we studied those who were genotype positive as well as having hypertrophic cardiomyopathy, the frequency of having multiple pathogenic or likely pathogenic mutations was approximately five times higher in patients who were diagnosed with hypertrophic cardiomyopathy at or less than 18 years of age, relative to patients diagnosed at greater than 18 years (26 % vs. 5 %). Identifying these additional mutations with comprehensive panel genetic testing may be critically important for management of family members at risk of the disease, who may otherwise have been dismissed from surveillance if they had received only targeted testing.
It is possible that diagnosis at a young age may be correlated with greater disease severity. Other studies have supported the concept that disease severity has an association with a higher likelihood of having multiple mutations [4, 10, 15, 21, 22]. Whether this factor alone should be a determining factor for performing targeted versus full-panel testing cannot be determined definitively by this study however, since individuals were referred for testing as both index cases and due to the family pedigree and risk status. Furthermore, in some cases a clinical decision was made to do broad panel testing rather than targeted testing in individuals at risk based on complex family histories that raised the possibility of a second mutation. Importantly, it is also possible that second mutations were missed in some individuals included in this report when a targeted testing strategy was undertaken with family members of the pro-band. Given these complexities, an effective testing strategy must focus on comprehensive (broad panel) genetic testing of an unequivocally affected proband. If more than one family member is clearly affected, the individual with the youngest age of onset and/or the individual affected with disease which is significantly more severe than the remainder of the family should be the initial focus of testing [3, 18].
Finally, the cost of comprehensive genetic testing compared to targeted testing may be a consideration. While the cost effectiveness of different testing approaches was not analyzed in this research, single gene testing is less expensive than full-panel testing. In our experience, the out of pocket cost of testing for single mutation is in the range of a few hundred dollars whereas panel testing is often tenfold greater. For many families, this is a barrier for comprehensive testing if insurance reimbursement is denied.
Limitations
There are several limitations to this study that must be considered. First, due to the long period of time in which genetic date was collected (2004–2013), not all participants in the study were tested for the same number of disease-causing genes. Near the beginning of the study period, most patients were tested using an 11-gene panel, which has now increased to a 20-gene panel and in current practice expanded further to assess both sarcomere genes and many conditions that are considered mimics of hypertrophic cardiomyopathy such as Fabry disease. Theoretically, this may mean that possible disease-causing mutations may have been missed in earlier participants. However, it is important to remember that the vast majority of disease-causing mutations would be identified using the 11-gene panel, as all of the common disease-causing genes are tested for in this panel. In fact, none of the participants tested with the >11-gene panels had a mutation identified that would not have been found using the 11-gene panel. Secondly, referral bias may limit the application of these findings in community settings. Because the study patients were treated at a tertiary care center, it is possible that they presented with more severe disease or are more likely to have multiple mutations. In fact, it is well known that the majority of patients with hypertrophic cardiomyopathy are asymptomatic or become symptomatic only later in life, and may never be aware of or seek care for their disease [5]. Thus, it is possible that the rate of multiple mutations in non-tertiary care settings is lower than seen in our study. However, given the correct set of predisposing factors, namely an affected child with a family history of hyper-trophic cardiomyopathy and a known mutation in a family member, we believe these conclusions may applicable to any clinical setting.
Finally, this is a single institution and relatively small study. Larger registry or population-based studies may be necessary to provide greater specificity as to which clinical characteristics might indicate need for comprehensive genetic testing in the setting of a known familial mutation.
Conclusions
This study reinforces the need for a comprehensive clinical assessment and genetic counseling strategy in assessing children presenting with hypertrophic cardiomyopathy. A comprehensive rather than targeted genetic testing approach for a family member should be considered in patients who present with hypertrophy at young ages, such as less than the 12 years of age time point when it is recommended that clinical screening should commence [5]. Broad panel testing should also be considered when the family history suggests there may be cardiomyopathy on both sides of an individual's family even if a pathogenic mutation has been identified in a proband on one side. Finally, very severe disease, such those 18 and younger with severe LVH and obstruction or significant exercise intolerance or arrhythmia with a known familial mutation, might prompt consideration of a more comprehensive genetic testing strategy.
Acknowledgments
Mr. Bales was funded by the Johns Hopkins School of Medicine Deans Research fund for medical student.
Footnotes
Compliance with Ethical Standards
Conflict of interest Nicole M. Johnson began employment at Invitae Corporation, a diagnostic genetic testing laboratory, in January of 2014. The data collection and analysis preceded that date.
Ethical Approval All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was found to be exempt from need for formal consent.
References
- 1.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Arad M, Maron BJ, Gorham JM, Johnson WH, Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 3.Arscott P, Caleshu C, Kotzer K, Kreykes S, Kruisselbrink T, Orland K, Rigelsky C, Smith E, Spoonamore K, Haidle JL, Marvin M, Ackerman MJ, Hadi A, Mani A, Ommen S, Cherny S. A case for inclusion of genetic counselors in cardiac care: a case for genetic counselors. Cardiol Rev. 2016;24:49–55. doi: 10.1097/CRD.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201–211. doi: 10.1016/j.jacc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 5.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hyper-trophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 6.Girolami F, Ho CY, Semsarian C, Baldi M, Will ML, Baldini K, Torricelli F, Yeates L, Cecchi F, Ackerman MJ, Olivotto I. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J Am Coll Cardiol. 2010;55:1444–1453. doi: 10.1016/j.jacc.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 7.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA. Genetic evaluation of cardiomyopathy— a Heart Failure Society of America practice guideline. J Cardiac Fail. 2009;15:83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hyper-trophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet. 2005;42:e59. doi: 10.1136/jmg.2005.033886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaski JP, Syrris P, Esteban MT, Jenkins S, Pantazis A, Deanfield JE, McKenna WJ, Elliott PM. Prevalence of sarcomere protein gene mutations in preadolescent children with hyper-trophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:436–441. doi: 10.1161/CIRCGENETICS.108.821314. [DOI] [PubMed] [Google Scholar]
- 10.Kelly M, Semsarian C. Multiple mutations in genetic cardiovascular disease: a marker of disease severity? Circ Cardiovasc Genet. 2009;2:182–190. doi: 10.1161/CIRCGENETICS.108.836478. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 13.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 15.Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hyper-trophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 16.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 17.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Tan BY, Judge DP. A clinical approach to a family history of sudden death. Circ Cardiovasc Genet. 2012;5:697–705. doi: 10.1161/CIRCGENETICS.110.959437. [DOI] [PubMed] [Google Scholar]
- 19.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 20.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hyper-trophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 21.Tsoutsman T, Kelly M, Ng DC, Tan JE, Tu E, Lam L, Bogoyevitch MA, Seidman CE, Seidman JG, Semsarian C. Severe heart failure and early mortality in a double-mutation mouse model of familial hypertrophic cardiomyopathy. Circulation. 2008;117:1820–1831. doi: 10.1161/CIRCULATIONAHA.107.755777. [DOI] [PubMed] [Google Scholar]
- 22.Van Driest SL, Vasile VC, Ommen SR, Will ML, Tajik AJ, Gersh BJ, Ackerman MJ. Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:1903–1910. doi: 10.1016/j.jacc.2004.07.045. [DOI] [PubMed] [Google Scholar]