Abstract
Understanding factors that contribute to the etiology of obesity is critical for minimizing the effects of obesity-related adverse physical health outcomes. Emotional eating or the inability to control intake of calorically dense diets (CDD) under conditions of psychosocial stress exposure is a potential risk factor for the development of obesity in people. Decreases in dopamine 2 receptors (D2R) availability have been documented in substance abuse and obesity in humans, as well as animal models of chronic stressor exposure. Social subordination in macaques is a well-established animal model of a chronic psychogenic stressor that results in stress axis dysregulation, attenuated striatal D2R levels, and stress-induced hyperphagia in complex dietary environment. However, it remains unclear how these phenotypes emerge as the stressor becomes chronic during the formation of new social groups. Thus, the goal of the current study was to assess how the imposition of social subordination over a four-month period would affect food intake, socioemotional behavior, and D2R binding potential (D2R-BP) in female rhesus monkeys maintained on a typical laboratory chow diet (LCD) compared with those having a choice between a LCD and a CDD. Results showed that access to a CDD leads to increased total caloric intake and preference for a CDD over a LCD. For the dietary choice condition, females directing less aggression towards group mates during the four-month period, a characteristic of lower social status, consumed progressively more calories over the four-month period than more aggressive females. This relation between agonistic behavior and appetite was not observed for females in LCD-only condition. Finally, decreased D2R-BP in the orbitofrontal cortex was predictive of increased overall caloric intake in all females regardless of dietary environment, suggesting that reduced availability of D2R within the prefrontal cortex is associated with unrestrained eating. Studies are continuing to determine how newly imposed dominance ranks continue to affect reward neurochemistry and appetite over time, and how this is influenced by the dietary environment.
Keywords: psychosocial stress, dopamine D2 receptors, diet choice, monkeys, emotional eating
Introduction
With the prevalence of obesity across the United States estimated to rise to 50% of the population by 2030 [1], understanding the its etiology is critical to minimizing the effects of obesity-related adverse physical health outcomes, such cardiovascular disease and type II diabetes [2, 3]. The inability to control intake of highly palatable foods, high in sugars and fats, is one behavioral phenotype that has been linked to increased risk for the development of obesity [4]. Importantly, environmental factors, including access to calorically dense diets (CDD; high in fat and sugar) during exposure to psychosocial stressors, increase individual risk for unrestrained emotional eating and accumulation of body weight and fat mass in humans [5, 6]. The importance of the dietary environment is highlighted by rodent data that show that exposure to diverse forms of physical and psychosocial stressors results in anorexia in the absence of CDD availability [7–9] and increased caloric intake in the presence of a CDD [10–12]. However, studies are most typically short term in nature, and do not completely address what initiates and sustains this phenotype in people [13–18].
Exposure to chronic psychosocial stressors may lead to stress-induced emotional eating phenotypes via stress-induced alterations in cortico-striatal-limbic reward pathways, similar to what has been described in individuals with substance abuse [19]. While multiple neurochemical systems are likely involved [20], the availability of dopamine D2 receptors (D2R) within the striatum, including the nucleus accumbens, is reduced in humans chronically abusing cocaine [21] or with an obese phenotype [22]. The importance diet for this change in D2R availability is supported by data in male rats fed an obesigenic diet [23]. While psychostimulants or CDDs may reduce D2R availability, results from animal models indicate that exposure to chronic stressors induces a hypodopaminergic state in part by decreases in D2R levels and increases individual risk for substance addiction [24–27]. However, it remains unclear how consumption of a CDD and exposure to a stressor alters D2R availability in cortico-striatal regions that are critical for modulating goal-directed behaviors such as feeding behavior [28].
One translational model of uncontrollable, unpredictable psychogenic stressor exposure typical of human populations that has been employed more recently to study a number of stress-related phenotypes, including hyperphagia in complex dietary environment, is social subordination in macaque monkeys. When group-housed, macaques (rhesus and cynomolgus monkeys) are organized socially by a matrilineal dominance hierarchies that function to maintain group stability [29] and are enforced via threat of aggression or harassment from higher-ranking monkeys towards lower-ranking animals within the social group [29, 30]. Lower ranking, or more subordinate animals, are thereby exposed continuously to an adverse social environment, similar to that experienced by people [31]. Critically, the experience of social subordination in female macaques results in diminished glucocorticoid negative feedback inhibition of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis [32, 33], a physiological phenotype similar to what has been describe in humans suffering from psychopathology such as depression [34, 35]. Furthermore, subordinate macaques show reduced D2R binding potential (D2R-BP) in striatal regions as assessed by positron emission tomography (PET) [27, 36].
The advent of an automated feeding system that allows for the continuous quantification of food intake in socially housed, free-feeding monkeys has been leveraged to assess how exposure to chronic psychogenic stress influences food intake in a complex dietary environment wherein animals can choose freely between a CDD and a low calorie standard laboratory monkey diet (LCD) [37]. Several studies using long established social groups of consume significantly more calories compared to more dominant group mates [38, 39] during a brief (two-three week) exposure to this choice dietary condition, a phenotype that is reversed during administration of the corticotropin releasing factor (CRF) type 1 receptor antagonist, Antalarmin [40]. While both dominant and subordinate females prefer the CDD to the LCD in the choice dietary condition, only subordinate females become hyperphagic [39]. This stress-induced hyperphagia in complex dietary environment was observed specifically in subordinate females and occurred in tandem with body weight gain [38]. While these findings parallel reports of stress-induced comfort food ingestion in people [41, 42], it is unclear how appetite and changes in D2R-BP in a rich dietary environment are affected as a social stressor is imposed and becomes chronic, a situation that can be modeled in rhesus monkeys as social subordination is imposed during the formation of new social groups. Thus, we sought to determine how the formation of new social groups and the imposition of social subordination over a four-month period would affect food intake in adult female rhesus monkeys maintained on a typical laboratory chow diet compared with those having a choice between the chow diet and CDD. Based on our previous findings, we hypothesized that more subordinate female monkeys who receive more aggression from other group-mates would show significantly greater caloric intake, particularly of a CDD. We also sought to determine how D2R-BP in striatal and prefrontal regions was associated with total and CDD-specific caloric intake after four-months living in new social groups in the two dietary conditions. We hypothesized that increased caloric intake in the presence of the choice dietary environment would exacerbate the reduction of D2R BP in striatal and prefrontal D2R-BP associated with subordinate status.
Methods
Subjects and group formation
Adult female rhesus monkeys (n = 31) living in one of the six breeding groups located at the Yerkes National Primate Research Center (YNPRC) Field Station in Lawrenceville, Georgia were selected as subjects of the current study based on age and familiarity with other females. Upon study inclusion, females were removed from their natal groups to form new social groups of four to six females each as previously described [43]. Briefly, a staggered group formation process was employed to sequentially introduce females in indoor-outdoor pens measuring approximately 144 ft2 (12×12 ft.). The first step of the group formation process was to place two females together in two adjacent pens. After 24 hours, the two females were reduced to a single indoor-outdoor pen. A third female was then placed in the adjacent pen separated by a Plexiglas door that allowed for only visual and olfactory access to other females for 24 hours, after which she was introduced to the pair of females by reducing the available space down to one pen. This protocol was repeated until all five females were living together in one pen where they remained housed for the duration of the current study. Importantly, the order of introduction was randomized and behavioral data were collected throughout the introduction process to monitor agonistic behavior and to intervene, if necessary [43]. More specifically, the outcome of dyadic interactions between females obtained from weekly 30-minute observations using an established ethogram [43] were used to capture the frequency of aggression received and submission emitted – the behavioral phenotypes used previously to determine dominance ranks of individuals within each group [44]. Based on the outcome of these dyadic agonistic interactions, ordinal ranks within each social group were assigned. Because groups differed in the number of members (4 to 6), relative ranks were calculated by dividing a female’s ordinal rank by the number of animals in the group (i.e., rank 1 out of 6 monkeys would equal 0.17). This same ethogram was used during behavioral observations to quantify rates of anxiety-like behavior (i.e. yawning, body shakes, self groom/explore, and body scratching) and social behavior, including proximity initiated towards others and grooming duration [43]. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
Experimental Design
After all females were introduced to form new social groups as described above, the dietary intervention was imposed so that three social groups (n = 15) only had access to the standard monkey chow (LCD; 3.45 kcal/g, Purina 5038) and the other three groups (n = 16) had access to a choice dietary environment wherein both the LCD and a CDD (3.73 kcal/g Purina Typical American Diet #5038) were available. The caloric composition of the LCD was 12% fat, 18% protein, and 4.14% sugar carbohydrate and 65.9% fiber carbohydrate. The calories of the CDD were distributed as 36% fat, 18% protein, 16.4% sugar carbohydrate and 29.6% fiber-starch carbohydrate. The rationale for providing access to both the LCD and CDD is based on well-established data showing that that dietary choice sustains intake of high caloric diets [45] and people have dietary choices.
All females had access to experimental diets ad libitum via previously validated automated feeders that allow for constitutive quantification of caloric intake in socially housed, free feeding monkeys [37, 38]. Briefly, activation of a radio-frequency antenna by an identification chip within each animal’s wrist signals a computer to dispense a single pellet of food via a pellet dispenser. Each social group had access to two different automated feeders and a computer recorded each feeding event in a log. A previous validation of the feeding system showed that high-ranking females rarely (<1%) take the pellet from subordinate animals or guard feeders, and pellets are never discarded [37]. Food intake was monitored for four months following group formation to assess the effects of social status and dietary environment on daily caloric intake. Morning blood samples (1 hr after sunrise) were collected four-months following group formation to assess cortisol levels. All animals were habituated to being removed from their group for conscious venipuncture using previously described procedures [46]. Blood samples were obtained within 10 minutes from entering the animal area to minimize arousal [47]. Plasma levels of cortisol were measured by LC-ESI-tandem mass spectrometry using a Discovery 5cm × 2.1mm C18 column (Supelco, PA) eluted at flow rate of 0.5 ml/min at the YNPRC Biomakers Core. The intra- and inter-assay coefficient of variation (CV%) was 1.21% and 5.78%, respectively.
Acquisition and analyses of D2R-BP
A subset of females comprised of the two highest and two lowest ranking females in each of the six social groups from each dietary condition (n=12 for both groups; 24 total) received a PET scan at the end of the four-month period to assess D2R-BP. PET scan using [18F]-fallypride was undertaken to assess D2R-BP at, on average, 13.4 ± 0.4 weeks from group formation. Subjects excluded from PET scans were those middle ranking females in each group. [18F]-fallypride was used as the D2R radioligand because it provides robust in vivo measures of both striatal and extra-striatal D2R binding due to its high D2R affinity [48]. [18F]-fallypride-PET has been validated previously in monkeys and in humans [49]. Quantitative PET images were acquired using a MicroPET Focus 220 scanner system (CTI Concorde Microsystems LLC, Knoxville, TN) and cyclotron located in the YNPRC Imaging Core. PET imaging occurred at the same time of day to control for any diurnal effects [50]. Animal anesthesia (isoflurane 1 to 2% to effect) and monitoring followed standard veterinary practices [51]. A transmission scan was obtained with a germanium-68 source for attenuation correction of the emission data. [18F]-fallypride was infused over 1 min. Emission data were collected continuously over 120 min after injection and then binned into appropriate time frames. Structural MR images were obtained within three weeks of the PET scan using a 3T magnet (Siemens Trio) for co-registration of PET and calculation of D2R-BP in the region(s) of interest (ROIs).
ROI drawing
ROIs were based on neuroanatomical definitions previously published in rhesus by our group [52, 53]. ROIs were manually traced on both hemispheres [left(l) and right(r)] for each monkey after realignment of sagittal, coronal and axial orthogonal planes into stereological space. Rhesus macaque brain atlases [54, 55] were used to guide ROI tracing within structural MRI images in coronal and sagittal views [52]. Sub-regions of the PFC were drawn, including the dorsolateral PFC (dlPFC) and the orbitofrontal cortex (OPFC). Ventral striatum included the nucleus accumbens (NACC), ventromedial caudate and anteroventral putamen and has established functional connections with PFC [56]. Ventral striatum was traced using an adaptation of the technique applied to macaques [57] and humans [58, 59], wherein the line that segregates ventral from dorsal striatum is made by connecting (a) the intersection between the outer edge of the putamen with a vertical line through the most superior and lateral point of the internal capsule and (b) the center of the portion of the anterior commissure transaxial plane overlying the striatum [58]. Finally, the non-vermis cerebellar gray matter served as reference region due to the relative absence of D2R binding sites. Regional measures D2R-BP were determined using the simplified reference tissue method (SRTM) as BP = (DVROI/DVCER)-1= (Bmax/Kd)f2 = k3/k4, where BP is the binding potential, DV is the distribution volume, ROI is the region of interest, CER as the cerebellum (reference tissue), Bmax is the available D2R density, KD is the equilibrium dissociation constant, f2 is the free fraction of [18F]-fallypride in tissue, k3 is the association rate [18F]fallypride to D2R, and k4 is the dissociation rate of [18F]-fallypride from D2R [60, 61].
Statistical analyses
Data were summarized as mean ± standard error of the mean (SEM) and a p≤0.05 was considered significant. Post-hoc analyses were conducted when necessary. Repeated-measures ANOVA (RM-ANOVA) was used to determine the effects of dietary environment over the course of the four-month study on agonistic and affiliative behavior and caloric intake using SPSS v23. Linear mixed modeling for repeated measures was used to assess the fixed effects of cumulative rates of agonistic behavior (aggression received and directed at group mates and submissive behaviors), dietary environment (choice vs. no-choice), time (months from group formation), and their interaction on total caloric intake over the course of the four months. Cumulative rates of behavior better reflect the social experience over time in the group as opposed to the average rates of behavior for a specific month. We chose to use rates of behavior, rather than categorical ranks (e.g., high vs. low) to better assess the “dosing” effect of social experience provided by including these continuous variables in our statistical models. The random effect of individual subject was also controlled for in this linear mixed model. Linear mixed modeling was undertaken using R “lme4” package [62]. Linear regression in SPSS was used to assess the effects of dietary environment (choice vs. no-choice) and cumulative rates of agonistic behavior (aggression received and emitted, and submission emitted) on morning cortisol levels four-months following group formation. Finally, bivariate correlations were used to assess relationships between ROI of D2R-BP, agonistic behavior, and caloric intake. ROIs significantly associated with total caloric and CDD intake were then added to a stepwise linear regression models to assess whether they were predictive of feeding behavior.
Results
Relation between relative rank, social behaviors and cortisol levels
Table 1 summarizes bivariate correlations between relative dominance rank, agonistic behaviors, affiliative behaviors, anxiety-like behaviors, and morning cortisol levels at month four of the study for all females independent of diet condition. More subordinate females (reflecting a larger relative rank number) tended to be less aggressive towards group mates, receive more aggression, emit more submissive behaviors, and initiate proximity to group mates less frequently. Not surprisingly, animals that received more aggression also showed significantly higher rates of submissive behavior. More submissive females initiated proximity less often to group mates. In contrast, females that were more aggressive towards other animals in their group, showed lower rates of submissive behavior but higher rates of initiating proximity. Finally, plasma cortisol and the frequency of anxiety-like behaviors were not associated with any agonistic and affiliative behaviors (all p>0.05).
Table 1.
Correlations between relative rank, total frequencies of agonistic behaviors (aggression emitted and received, and submission emitted), affiliative behaviors (proximity initiated and total time spent grooming), anxiety-like behaviors, and morning cortisol levels four-months after group formation.
| Relative Rank |
Aggression Emitted |
Aggression Received |
Submission Emitted |
Proximity Initiated |
Grooming Duration |
Anxiety-like | Cortisol | |
|---|---|---|---|---|---|---|---|---|
| Relative Rank | - | - | - | - | - | - | - | - |
| Aggression Emitted | r=−0.35 p=0.05* |
- | - | - | - | - | - | - |
| Aggression Received | r=0.59 p<0.001* |
r=−0.24 p=0.19 |
- | - | - | - | - | - |
| Submission Emitted | r=0.77 p<0.001* |
r=−0.36 p=0.05* |
r=0.84 p<0.001* |
- | - | - | - | - |
| Proximity Initiated | r=−0.43 p=0.017* |
r=0.53 p=0.002* |
r=−0.25 p=0.17 |
r=−0.44 p=0.018* |
- | - | - | - |
| Grooming Duration | r=−0.32 p=0.08* |
r=0.32 p=0.08 |
r=−0.003 p=0.99 |
r=−0.27 p=0.15 |
r=0.58 p=0.001* |
- | - | - |
| Anxiety-like | r=0.29 p=0.12 |
r=−0.21 p=0.26 |
r=0.24 p=0.19 |
r=0.31 p=0.09 |
r=−0.14 p=0.45 |
r=−0.14 p=0.47 |
- | - |
| Cortisol | r=−0.08 p=0.66 |
r=0.001 p=0.99 |
r=−0.12 p=0.52 |
r=−0.−13 p=0.95 |
r=0.023 p−0.91 |
r=0.19 p=0.31 |
r=0.19 p=0.31 |
- |
Asterisk (*) denote significant correlation between factors.
Effects of diet condition on social behaviors
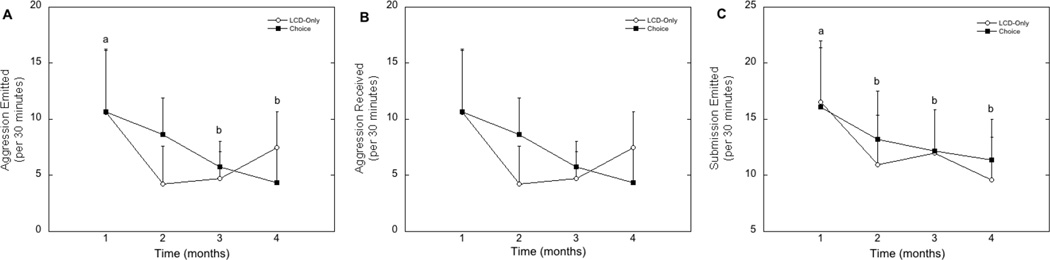
Shown in Figure 1 are the rates of agonistic behaviors for animals in both dietary conditions over the course of the four-month study. Rates of aggression directed at others (Figure 1A; F1,29=0.020, p=0.89), aggression received (Figure 1B; F1,29=0.016, p=0.90), and submissive behaviors directed towards aggressors in the group (Figure 1C; F1,29=0.026, p=0.87) were similar regardless of dietary condition. Time from group formation did, however, influence these agonist behaviors. Rates of aggression directed at others (Figure 1A; F1,29=3.18, p=0.028) and submission emitted (Figure 1C; F1,29=3.94, p=0.011) were reduced significantly over time from group formation. There was no similar effect of time on the frequency of aggression received (F1,29=2.82, p=0.085). The changes in agonistic behaviors over the four months did not vary significantly by dietary condition (Figure 1; all p>0.05).
Figure 1.
Cumulative average rates (± SEM) of aggression directed at group mates (emitted; A), aggression received from group mates (B), and submissive behaviors (C) per 30 minutes for each month from group formation. Letters denote significant changes in behaviors over time.
Collapsed across dietary condition, females that initiated proximity more frequently were more aggressive towards group mates also (r29=0.53; p=0.002) and exhibited lower rates of submissive behavior (r29=−0.44; p=0.018). These associations were also evident when analyzed for each dietary condition separately. Higher rates of proximity initiated were associated with increased frequency of aggression directed towards group mates for the LCD-only (r13=0.51; p=0.05) and dietary choice condition (r14=0.77; p=0.001). However, the negative correlation of greater proximity initiated and lower submission emitted was not significant for either the LCD-only (r13=−0.50; p=0.07) and dietary choice condition (r14=−0.46; p=0.09), likely due to reduced power. Furthermore, it is possible the grooming received from group mates may mitigate the amount of aggression a female would direct toward others. While there was not a significant correlation of grooming received and aggressiveness for females in the LCD-only condition (r13=0.22; p=0.43), females who were more aggressive in the dietary choice condition actually received more grooming group mates (r14=0.59; p=0.016). Finally, rates of submissive behavior were unrelated to how much grooming females received from group mates in both dietary conditions (both p>0.05).
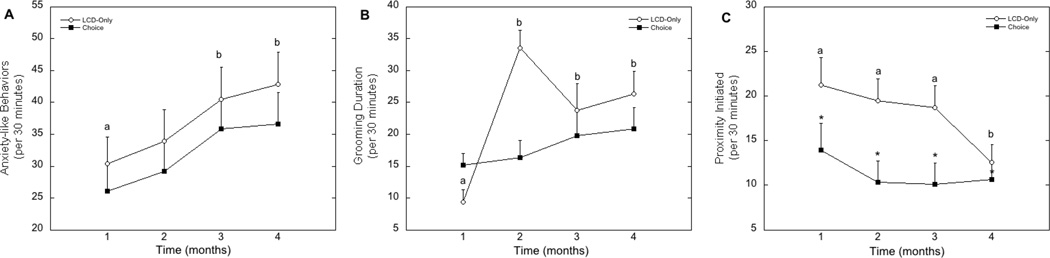
There were no main effects of dietary condition on anxiety-like behaviors or duration of grooming received (both p>0.05). However, rates of anxiety-like behaviors increased significantly over the course of the study (F1,29=7.10, p<0.001) in a similar pattern for females in both dietary condition (Figure 2A; F1,29=0.041, p=0.99). Similarly, the duration of grooming with group mates increased over time (F1,29=7.83, p<0.001). However, there was also a significant interaction between diet condition and time spent grooming (F1,29=5.26, p=0.002) such that the increase in grooming duration was evident only in females in the LCD-only dietary condition (Figure 2B). The frequency of proximity initiated towards group mates was also affected significantly by diet (F1,29=4.92, p=0.035), such that females in the choice diet condition initiated less proximity to group-mates compared to female in the LCD-only condition (Figure 2C). The frequency of proximity initiated towards group mates also decreased significantly over time (F1,29=5.24, p=0.002). However, there was no significant interaction between diet and time from group formation on initiation of proximity with group mates (F1,29=2.42, p=0.072, Figure 2C).
Figure 2.
Cumulative average rates ± (SEM) of anxiety-like behaviors (A), total time spent grooming (b) and frequency of proximity initiated towards group mates (C) per 30 minutes for each month from group formation. Letters signify a significant increase in anxiety-like behaviors over time (A), a significant increase in grooming received only in the LCD-Only females (B) and a significant decrease in the amount of initiated proximity with others in all females (C). The asterisks denote a significantly fewer proximity initiation by choice females compared to LCD-Only females (C).
Predictors of caloric intake
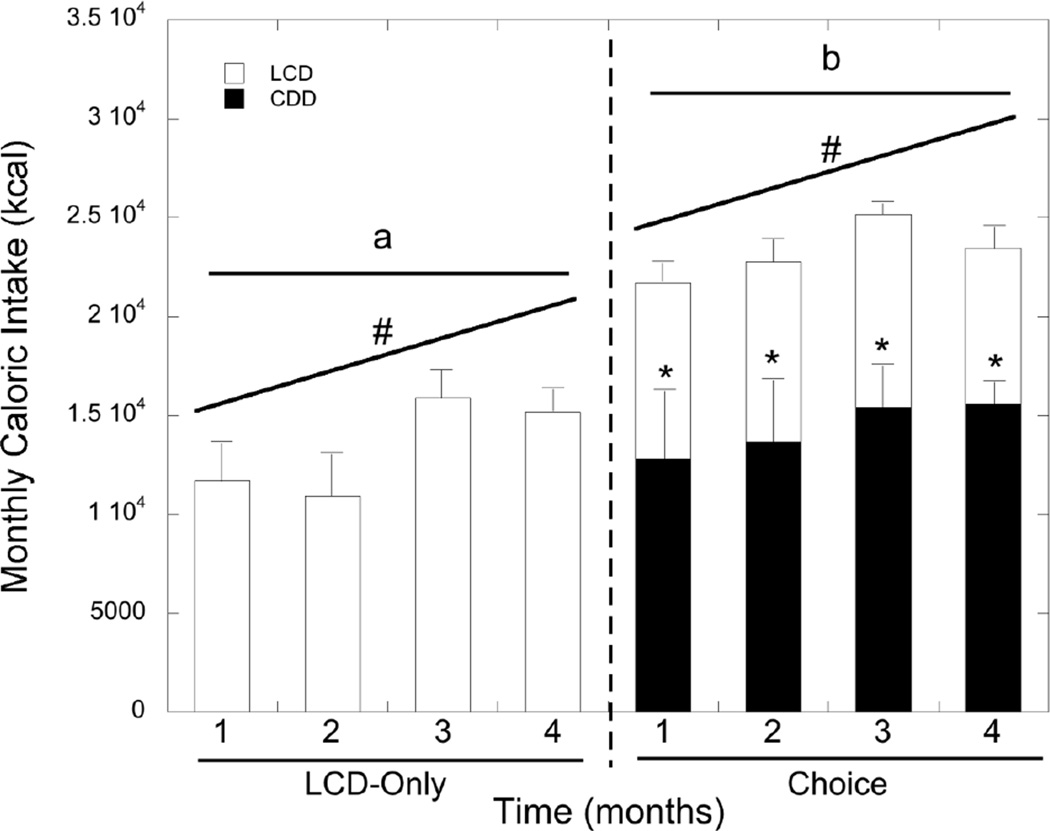
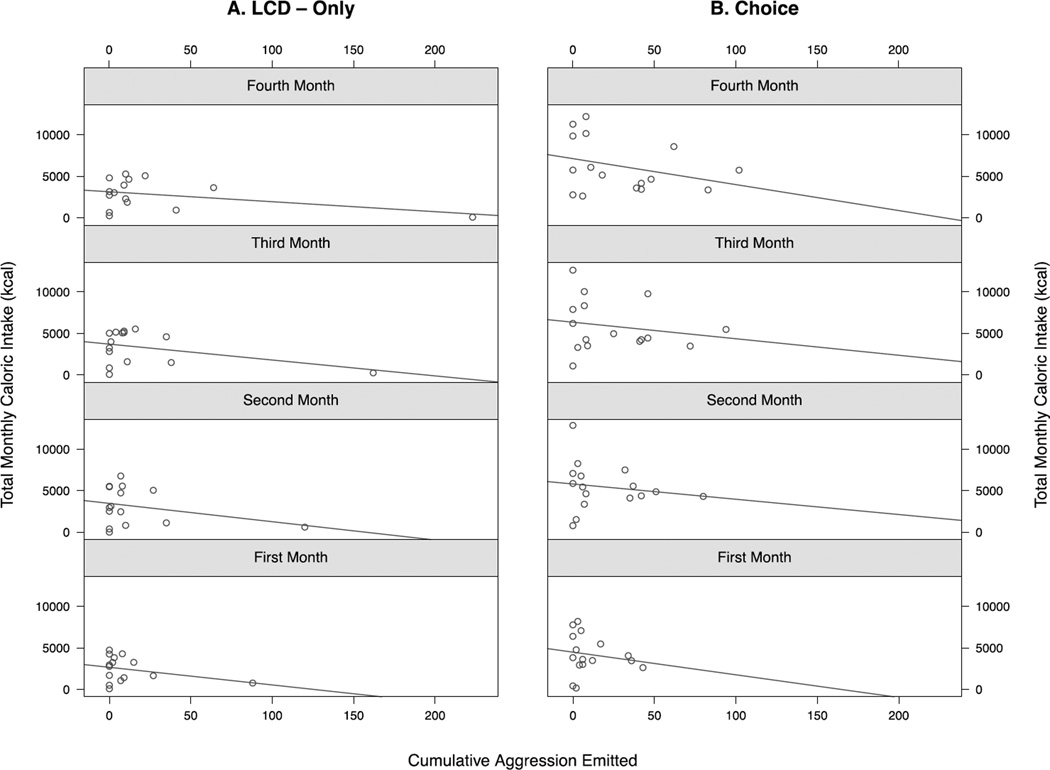
Total caloric intake expressed as monthly kcal consumed for each of the four study months is shown in Figure 3. Females in the choice dietary condition consumed significantly more total calories than females in the LCD-only condition (F1,21=5.84, p=0.025) in each of the four months following group formation, as there was no significant interaction between diet condition and time (p>0.05). Females in the choice dietary condition consumed significantly more calories from the CDD compared with the LCD that was stable across the four-month period (F1,15=8.96, p=0.009; Figure 3). The analysis of the effect of differences in agonistic behavior on caloric intake across the four-month study period and whether this was influenced by dietary condition was elevated with linear mixed models. As shown in Figure 4, females in the dietary choice but not the LCD-only condition that were less aggressive towards group mates consumed more total calories (t=2.02, p=0.047), and this effect of lower rates of aggressiveness on more caloric intake increased over time (t=−2.28, p=0.025). In contrast, caloric intake was unaffected by differences in rates of submissive behavior and aggression received from group mates in either dietary condition across the four-month period (all p>0.05). Finally, measures of morning cortisol obtained during the fourth month did not predict total caloric intake in either dietary condition (all p>0.05; data not shown).
Figure 3.
Average monthly caloric intake (SEM) for females in the LCD-only and dietary choice conditions. Females in the choice diet condition consumed significantly more overall calories than females in the LCD-Only condition (denoted by letters). Furthermore, overall caloric intake in all females increased over the course of the four months (denoted by #). Asterisks denote preference for CDD over LCD in the choice dietary condition.
Figure 4.
Scatter plots of total caloric intake for females in the LCD-only (A. left panel) and dietary choice condition (B. right panel) in each of the four months from group formation (first month in the bottom panel). Lower rates of aggression emitted (more subordinate social ranking) were associated with increased overall caloric intake over time specifically in females with access to a dietary choice.
Relation between caloric intake, agonistic and anxiety-like behavior on D2R-BP
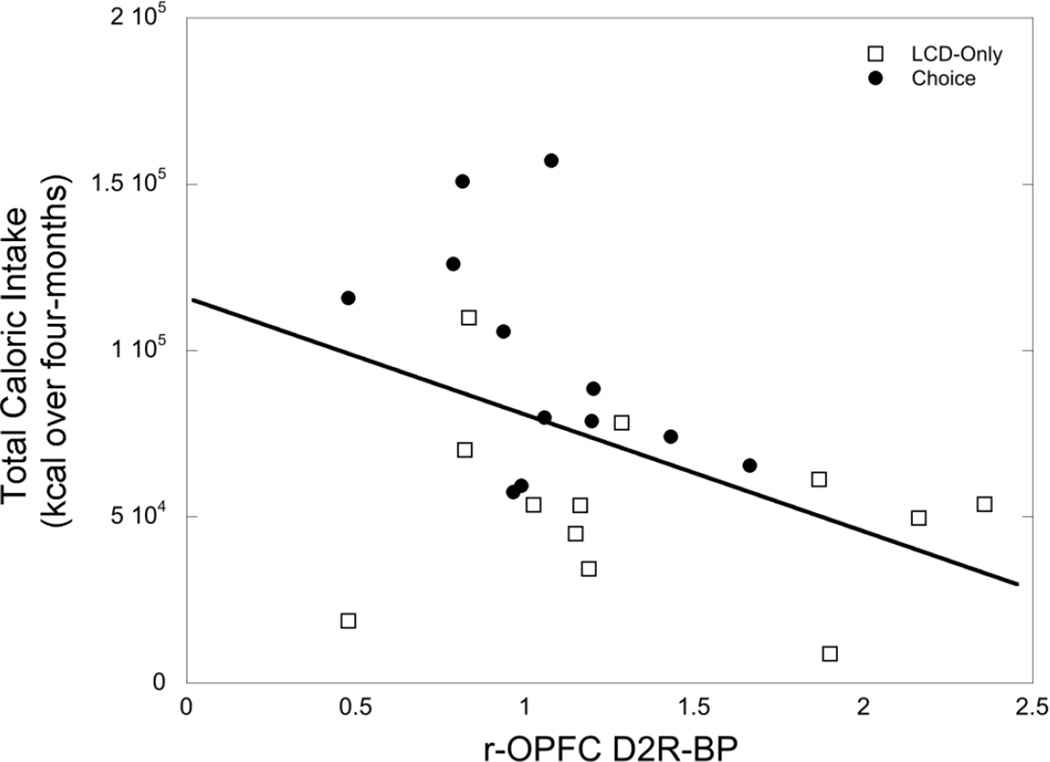
Table 2 summarizes bivariate correlations between D2R-BP in specific ROIs obtained during month four, the cumulative total of agonistic behavior and total caloric intake at the end of the four-month study in all females independent of the dietary environment. D2R-BP in the r-OPFC was significantly associated with submission emitted (r=0.42; p=0.045), and predicted less total caloric intake across both dietary conditions (Figure 5; p=0.05). Finally, there were no associations between striatal D2R-BP (NACC, caudate, putamen) with parameters of agonistic behavior and total caloric intake (all p>0.05).
Table 2.
Correlations between regions of interest (ROI) D2R-BP, total frequencies of agonistic behavior, and total caloric intake in all females (regardless of dietary condition) four months following group formation.
| D2R-BP by ROI |
Total Aggression Emitted |
Total Submission Emitted |
Total Caloric Intake |
|||
|---|---|---|---|---|---|---|
| r22 | p | r22 | p | r22 | p | |
| r-OPFC | 0.09 | 0.68 | 0.42 | 0.045* | −0.40 | 0.05* |
| l-OPFC | −0.10 | 0.64 | 0.47 | 0.025* | −0.29 | 0.17 |
| r-ACC | 0.18 | 0.40 | 0.09 | 0.68 | −0.32 | 0.13 |
| l-ACC | 0.01 | 0.97 | 0.10 | 0.65 | −0.14 | 0.53 |
| r-NACC | −0.26 | 0.22 | 0.07 | 0.75 | 0.12 | 0.57 |
| l-NACC | −0.09 | 0.67 | −0.11 | 0.61 | 0.13 | 0.53 |
Asterisks denote significant bivariate Pearson correlation coefficients.
Figure 5.
Lower r-OPFC D2R-BP was predictive of greater overall caloric intake across both LCD-only and choice dietary conditions (p=0.05).
Because rates of social behavior varied between the choice and the LCD-only condition, we examined the relation of behavioral rates and caloric intake with D2R-BP separately for each diet group. For females in the LCD-only diet condition, D2R-BP in specific ROIs was not associated with total LCD intake at the end of the four-month study (Table 3; all p>0.05). However, total rates of submission emitted were positively associated with D2R-BP in the r-OPFC (p=0.032), r-dlPFC (p=0.045), and l-dlPFC (p=0.047) in females in the LCD-only diet condition (Table 3). Overall rates of aggression directed at group mates by females in the LCD-only diet condition were not associated with D2R-BP within the ROIs assessed in the current study (Table 3; all p>0.05).
Table 3.
Correlations between regions of interest (ROI) D2R-BP, total of agonistic behavior, and total (LCD) caloric intake at four months following group formation for females in the LCD-only condition.
| LCD-Only | ||||||
|---|---|---|---|---|---|---|
| D2R-BP by ROI |
Total Aggression Emitted |
Total Submission Emitted |
Total (LCD) Intake |
|||
| r10 | p | r10 | p | r10 | p | |
| r-OPFC | 0.13 | 0.69 | 0.64 | 0.032* | −0.16 | 0.61 |
| l-OPFC | −0.15 | 0.64 | 0.11 | 0.75 | −0.26 | 0.42 |
| r-dlPFC | −0.16 | 0.62 | 0.61 | 0.045* | 0.22 | 0.49 |
| l-dlPFC | −0.16 | 0.62 | 0.61 | 0.047* | −0.001 | 0.99 |
| r-NACC | −0.51 | 0.09 | 0.50 | 0.12 | 0.37 | 0.24 |
| l-NACC | −0.30 | 0.34 | 0.23 | 0.49 | 0.26 | 0.42 |
Asterisks denote significant bivariate Pearson correlation coefficients.
Table 4 summarizes bivariate correlations between specific ROIs, D2R-BP and total caloric intake of each diet type as well as total calories from both diets in females with access to a dietary choice. In females with a dietary choice, D2R-BP in the l-OPFC was significantly associated with the frequency of submissive behavior (r10=0.72; p=0.009). No other ROIs were associated with total rates of aggression and submission emitted (data not shown; all p>0.05). In females exposed to a dietary choice, greater D2R-BP in the l-OPFC was associated with increased intake of the LCD (Table 4; p=0.018). There were, however, no other significant correlations between D2R-BP in other ROIs and caloric intake (Table 4; all p>0.05).
Table 4.
Correlations between regions of interest (ROI) D2R-BP, total caloric intake, and caloric intake of LCD and CDD at four months following group formation for females in the dietary choice condition.
| Choice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D2R-BP by ROI |
Total Aggression Emitted |
Total Submission Emitted |
LCD Intake | CDD Intake | Total Caloric Intake |
|||||
| r10 | p | r10 | p | r10 | p | r10 | p | r10 | p | |
| r-OPFC | −0.24 | 0.45 | 0.45 | 0.14 | 0.28 | 0.39 | −0.54 | 0.07 | −0.49 | 0.11 |
| l-OPFC | −0.06 | 0.85 | 0.72 | 0.009* | 0.67 | 0.018* | −0.38 | 0.23 | −0.32 | 0.31 |
| r-dlPFC | 0.20 | 0.53 | 0.15 | 0.65 | 0.16 | 0.62 | −0.19 | 0.55 | −0.23 | 0.47 |
| l-dlPFC | 0.09 | 0.79 | −0.06 | 0.86 | 0.04 | 0.90 | 0.12 | 0.71 | 0.11 | 0.75 |
| r-NACC | 0.28 | 0.38 | −0.17 | 0.59 | 0.24 | 0.44 | 0.04 | 0.91 | 0.05 | 0.88 |
| l-NACC | 0.26 | 0.42 | −0.24 | 0.46 | 0.43 | 0.16 | 0.26 | 0.41 | 0.30 | 0.35 |
Asterisks denote significant bivariate Pearson correlation coefficients.
Total caloric intake across all females was also not associated with rates of anxiety-like behavior (r29=−0.14; p=0.44). The importance of the dietary condition on the expression of behavior again emerges when these relations are examined for each diet condition separately. In the LCD-only condition, more anxious females also received more aggression from group mates (r13=0.55; p=0.036) and also showed higher rates of submissive behavior towards group mates (r13=0.70; p=0.006). Caloric intake in females with access to the LCD-only diet condition was not associated with rates of anxiety-like behavior (r13=−0.17; p=0.56). Rates of agonistic behavior in females in the dietary choice condition were not associated with rates of anxiety-like behaviors (all p>0.05). However, more anxious females in this choice condition consumed significantly more calories from the LCD (r14=0.68; p=0.004), but not of the CDD (r14=−0.14; p=0.61) or total caloric intake (r14=0.07; p=0.81). With respect to anxiety-like behaviors, more anxious females in the LCD-only condition had higher D2R-BP in the l-dlPFC (r10=0.67; p=0.017) but no other ROIs. There were no significant relations between anxiousness in the dietary choice condition and D2R BP in our ROIs (all p>0.05; data not shown).
Discussion
The results of the current study using adult female rhesus monkeys show that a dietary environment that includes a choice between a lab chow diet and CDD significantly promotes caloric intake beyond that observed when only the chow diet is available. These data corroborate the notion that access to palatable foods, high in sugars and fats, can result in excessive food intake, a phenotype linked to increased risk for the development of obesity [4]. Furthermore, because the dietary intervention was begun coincident with the formation of new social groups, the data show that less aggressive females in the dietary choice but not the chow only condition showed progressively more caloric intake over the four-month study period. While reduced rates of aggression directed at group mates are often considered a characteristic of more subordinate animals [63], rates of submissive behavior during the group formation process, a defining feature of subordinate status, did not predict caloric intake. Because previous data from long established social groups of adult female rhesus monkeys show that subordinate group members consume significantly more calories in a dietary choice environment than do more dominant group mates [38–40], observations from the present study suggest that this rank-related phenotype may emerge more slowly as the consequences of subordinate status becomes more chronic. Finally, examination of D2R BP in specific cortico-striatal regions shows that attenuated levels of D2R-BP in the orbitofrontal cortex was predictive of increased overall caloric intake in all females regardless of dietary environment.
Our previous characterization of stress-induced effects on appetite in different dietary environments studied groups of female rhesus monkeys that had been living together in stable groups for at least four years. In these conditions, social subordination is associated with impaired LHPA function, characterized by diminished glucocorticoid negative feedback [33] and significantly greater caloric intake in a dietary choice environment that was predicted by greater activation of the LHPA axis [38–40]. These data are in line with rodent and human data indicating that exposure to psychosocial stress in a complex dietary environment leads to increased caloric intake [10–12, 41, 42]. However, the present study assessing the consequences of the imposition of a social stressor, in the form of establishing new social groups and emergence of new social ranks, did not replicate these observations. Not only did measures of morning cortisol fail to differentiate females on the basis of rank or agonistic behavior, but rates of submissive behavior, the defining feature of subordinate status, did not predict caloric intake in either dietary environment. Rather, females with access to a diet choice that were less aggressive towards group mates progressively consumed more calories over the course of the first four months following group formation. While less aggressive females also showed more submissive behavior and also had a more subordinate rank (see Table 1), rates of submissive behavior did not significantly account for caloric intake in the choice dietary environment. Based on these observations, our hypothesis is that the rank-related differences in food intake in an obesigenic environment will emerge with time as the consequences of subordinate status become more chronic.
Subordinate social status in the current study, reflected by more submissive and less aggressive behavior emitted and more aggression received from group mates, was also not associated with an attenuation of D2R-BP within the striatum, as previously described in socially-housed subordinate cynomolgus macaques [27, 36]. Previous studies in female cynomolgus monkeys show attenuated levels of D2R-BP in the striatum of subordinate females living in stable social groups of three years [36] and in females who became subordinate three-months after group formation [64]. Contrary to these previous results in cynomolgus females, greater rates of submission emitted, which is related to and defines lower social status, over the course of the current four-month study were associated with significantly greater D2R-BP in extra-striatal regions involved in the top-down control of behavior, such as the OPFC and dlPFC [65]. There were also no effects of agonistic behaviors on striatal D2R-BP in the current study, including the NACC that is involved in reward processing [66]. Reconciling these study related differences in rank effects on D2R BP is difficult. While it is possible that the imposition of social status in newly formed groups of female rhesus monkeys may require longer periods of time to influence D2R-BP that previously described in female cynomolgus monkeys [36, 64], a macaque species difference seems unlikely. Differences in ROI identification and analytical methods used to identify radioligand binding seem more probable. Importantly, continuing assessment of these females will determine how status-related differences in D2R BP changes as time form group formation lengthens.
Striatal D2R-BP was not associated with either total caloric intake or CDD intake for females in the dietary choice condition or LCD intake for females in the no choice condition, but decreased D2R-BP in the r-OPFC was predictive of increased total caloric intake collapsed across dietary conditions. This seemingly contradictory observation is likely due to our analysis being underpowered. The insignificant r-values of the correlation of reduced r-OPFC D2R-BP and intake of the CCD or total calories for choice females (Table 4) were greater than the r-value for all females combined (Table 2). Regardless of this statistical nuance, the association between decreased D2R-BP in the r-OPFC and increased overall caloric intake is consistent with the notion that a down-regulation of D2R within the PFC increases risk for overeating in obese humans [67]. The OPFC is involved in goal-directed behavior, reward coding, and the inhibitory control of behavior [68]. The OPFC is also critical for the computation of subjective value of a good (such as food) as opposed to an action (e.g. eating) [69]. Furthermore, the activation of the OPFC in response to food receipt is less activated in women with food addiction [70]. Importantly, dopaminergic transmission via D2R in the OPFC has been implicated in the inhibitory control of behavior [67]. States associated with decreased D2R levels in the PFC, including the OPFC, are associated with deficiencies in impulse control and regulation of goal-directed behaviors [67, 71]. Taken together, these data along with the current finding that decreased D2R-BP in the r-OPFC was predictive of augmented overall caloric intake, suggest that decreased D2R in the OPFC is a risk factor for uncontrolled eating regardless of dietary environment. Indeed, bilateral lesions of the OPFC in male rhesus monkeys results in unrestrained eating even after satiation [72]. It is also important to note that intake of the CDD specifically in females with a dietary choice was not associated with D2R-BP in any ROI we assessed, suggesting that longer exposure to palatable diets might be necessary to induce CDD-specific changes in reward neurochemistry.
The dietary environment did not have any effects on overall rates of agonistic behavior (aggression and submission) during the four-months following group formation. While rates of agonistic behavior did decrease over time in the current study, the overall frequency of these behaviors was approximately seven times greater than our previous reports of the frequency of agonistic behavior emission in long-standing groups of rhesus females in small groups of rhesus monkeys that had been together for >4 years [33]. The increase in anxiety-like behaviors over time in all females regardless of dietary condition is consistent with our previous studies [43] and suggests that the imposition of new social group is anxiogenic in female rhesus monkeys. However, lower social status was only associated with increased rates of anxiety-like behavior in females expose to the LCD-only diet condition. Anxiety-like behavior in the choice dietary environment was not associated with agonistic behavior, but rather with increased intake of the LCD, corroborating our previous study showing that a dietary choice on a background of stressor exposure is associated with stress-induced hyperphagia [38].
In summary, the findings from the current study indicate that a dietary choice environment comprised of a CDD and a more prudent, laboratory chow diet is sufficient to increase caloric intake in female rhesus monkeys experiencing a change in their social environment. Increased caloric intake regardless of dietary condition was significantly associated with decreased D2R-BP specifically in the r-OPFC, implicating alterations in prefrontal dopaminergic pathways in the etiology of unrestrained caloric intake that has been linked to obesity and food addiction [4, 73]. In the four months following the new group formations, females in the choice dietary environment directing less aggression towards group mates consumed more calories. While this is contrary to our previous findings [38–40], it is likely differences reflect the recency of group formation and reduced time from exposure to the chronic social stressor of subordination. These findings underscore the importance of social context and the duration of stressor exposure on stress-related health outcomes. Ongoing studies of these females will determine how adverse social experience, reflected by rank related differences in agonistic behaviors, and a dietary environment with access to a CDD influence feeding behavior, LHPA function, and reward neurochemistry over a prolonged period of time.
Research Highlights.
Social subordination is a model to study the etiology of stress-induced hyperphagia.
Exposure to a calorically dense diet (CDD) increases overall caloric intake.
Exposure to CDD increases preference for a CDD over a standard low calorie diet (LCD).
Less aggression emitted was associated with increased caloric intake with CDD exposure.
Increased caloric intake was associated with decreased D2R binding in OPFC.
Acknowledgments
The current study would not have been possible without the expert technical assistance of Jennifer Whitley, Angela Tripp, Brandon Hughes, Jessica Johnson, Patrick Ulam, Rebecca Herman, Robert Johnston and Gregory Henry. These studies would not have been possible without the dedication of the animal husbandry and veterinary staff at the YNPRC, and support by NIH grants DK096983 and ORIP/OD P51OD011132. Further support was provided by the Center for Behavioral Neuroscience through the STC Program of the National Science Foundation IBN-9876754. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocrine reviews. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR. Interactions between the "cognitive" and "metabolic" brain in the control of food intake. Physiology & behavior. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 6.Gibson EL. Emotional influences on food choice: sensory, physiological, and psychological pathways. Physiology & behavior. 2006 doi: 10.1016/j.physbeh.2006.01.024. in press. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MF, Bhatnagar S. Chronic stress and energy balance: role of the HPA axis. In: McEwen B, editor. Handbook of physiology, section 7: the endocrine system. Oxford University Press; 2001. [Google Scholar]
- 8.Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiology & behavior. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiology & behavior. 2004;80:683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiology & behavior. 2006;89:536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Molecular and cellular endocrinology. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 13.Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS Neurol Disord Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- 14.Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhatnagar S, Dallman MF, Roderick RE, Basbaum AI, Taylor BK. The effects of prior chronic stress on cardiovascular responses to acute restraint and formalin injection. Brain research. 1998;797:313–320. doi: 10.1016/s0006-8993(98)00382-5. [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Hormones and behavior. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. Journal of neuroendocrinology. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of clinical investigation. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 25.Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacology, biochemistry, and behavior. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, et al. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature neuroscience. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 28.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- 30.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM. The influence of social hierarchy on primate health. Science (New York, N.Y. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 32.Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 33.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jokinen J, Nordstrom AL, Nordstrom P. ROC analysis of dexamethasone suppression test threshold in suicide prediction after attempted suicide. Journal of affective disorders. 2008;106:145–152. doi: 10.1016/j.jad.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Coryell W, Fiedorowicz J, Zimmerman M, Young E. HPA-axis hyperactivity and mortality in psychotic depressive disorder: preliminary findings. Psychoneuroendocrinology. 2008;33:654–658. doi: 10.1016/j.psyneuen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse (New York, N.Y. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiology & behavior. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012;37:1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CJ, Johnson ZP, Higgins M, Toufexis D, Wilson ME. Antagonism of corticotrophin-releasing factor type 1 receptors attenuates caloric intake of free feeding subordinate female rhesus monkeys in a rich dietary environment. Journal of neuroendocrinology. 2015;27:33–43. doi: 10.1111/jne.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Adam TC, Epel ES. Stress, eating and the reward system. Physiology & behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female 'protection' among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 45.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 46.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 47.Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- 48.Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, et al. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biological psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse (New York, N.Y. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 50.Krajnak K, Rosewell KL, Duncan MJ, Wise PM. Aging, estradiol and time of day differentially affect serotonin transporter binding in the central nervous system of female rats. Brain research. 2003;990:87–94. doi: 10.1016/s0006-8993(03)03441-3. [DOI] [PubMed] [Google Scholar]
- 51.Michopoulos V, Perez Diaz M, Embree M, Reding K, Votaw JR, Mun J, et al. Oestradiol alters central 5-HT1A receptor binding potential differences related to psychosocial stress but not differences related to 5-HTTLPR genotype in female rhesus monkeys. Journal of neuroendocrinology. 2014;26:80–88. doi: 10.1111/jne.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Embree M, Michopoulos V, Votaw JR, Voll RJ, Mun J, Stehouwer JS, et al. The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: effects of social status and 5HTT genotype. Neuroscience. 2013;228:83–100. doi: 10.1016/j.neuroscience.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2:181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. London; Burlington, MA: Academic Press; 2007. [Google Scholar]
- 55.Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in sterotaxic coordinates. San Diego: Academic Press; 2000. [Google Scholar]
- 56.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li N, Gao L, Wang XL, Chen L, Fang W, Ge SN, et al. Deep brain stimulation of the bilateral nucleus accumbens in normal rhesus monkey. Neuroreport. 2013;24:30–35. doi: 10.1097/WNR.0b013e32835c16e7. [DOI] [PubMed] [Google Scholar]
- 58.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse (New York, N.Y. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 60.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 61.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 62.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 63.Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37:1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, et al. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biological psychiatry. 2012;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee D, Rushworth MF, Walton ME, Watanabe M, Sakagami M. Functional specialization of the primate frontal cortex during decision making. J Neurosci. 2007;27:8170–8173. doi: 10.1523/JNEUROSCI.1561-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, et al. A unique adolescent response to reward prediction errors. Nature neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Padoa-Schioppa C, Cai X. The orbitofrontal cortex and the computation of subjective value: consolidated concepts and new perspectives. Annals of the New York Academy of Sciences. 2011;1239:130–137. doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of general psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science (New York, N.Y. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 72.Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: the effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gearhardt AN, Boswell RG, White MA. The association of "food addiction" with disordered eating and body mass index. Eat Behav. 2014;15:427–433. doi: 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]