Abstract
While most children receive acute myeloid leukemia (AML) chemotherapy as inpatients, there is variability in timing of discharge after chemotherapy completion. This study compared treatment-related morbidity, mortality and cumulative hospitalization in children with AML who were discharged after chemotherapy completion (early discharge) and those who remained hospitalized. Chart abstraction data for 153 early discharge-eligible patients enrolled on a Children’s Oncology Group trial were compared by discharge strategy. Targeted toxicities included viridans group streptococcal (VGS) bacteremia, hypoxia and hypotension. Early discharge occurred in 11% of courses post-Induction I. Re-admission occurred in 80–100%, but median hospital stay was 7 days shorter. Patients discharged early had higher rates of VGS (adjusted risk ratio (aRR) = 1.67, 95% CI = 1.11–2.51), hypoxia (aRR = 1.92, 95% CI = 1.06–3.48) and hypotension (aRR = 4.36, 95% CI = 2.01–9.46), but there was no difference in mortality. As pressure increases to shorten hospitalizations, these results have important implications for determining discharge practices in pediatric AML.
Keywords: Pediatrics, acute myeloid leukemia, patient discharge, morbidity
Introduction
Acute myeloid leukemia (AML) is the second most common pediatric hematologic malignancy and requires treatment with intensive chemotherapy that often causes substantial treatment-related morbidity, particularly infectious complications.[1,2] While virtually all children receive AML chemotherapy in the inpatient setting, there is substantial variability in timing of discharge following chemotherapy completion.[3] Previous studies in adults with AML have concluded that discharge after chemotherapy with close outpatient monitoring may be safe for some patients, as those re-admitted with fever, shock and bacteremia were successfully treated.[4-8] In addition, studies have also shown that adult AML patients who were discharged early have fewer inpatient hospital days [7,9] and lower expenditures.[6,10] However, these adult studies are limited by small sample sizes, single institution cohorts or lack of an appropriate control group. In addition, there may be differences in AML therapy intensity and tolerance between adults and children.
Pediatric-specific data regarding discharge after chemotherapy are limited. One study of 83 patients included pediatric patients, but grouped them with adults.[8] The Children’s Oncology Group (COG) recently used institutional surveys to evaluate the impact of hospital discharge policies on infection risk and non-relapse-related mortality (NRM) among children enrolled on COG Phase III clinical trial AAML0531.[3] This study did not detect a statistically significant benefit of hospitalization on development of Gram positive sterilesite bacterial infection (adjusted IRR = 0.95, 95% CI = 0.83–1.10) or NRM (adjusted HR =0.60, 95% CI = 0.26–1.36), suggesting that early discharge may be safe among pediatric AML patients as well. However, these analyses were limited by under-reporting of adverse events.[11] In addition, use of hospital survey-based determination of early discharge status rather than individual patient-level determination of early discharge status may have resulted in non-differential exposure (early discharge) misclassification, potentially biasing the observed association toward no effect. To address the safety of early discharge more directly, our group recently used administrative/billing data to compare inpatient mortality and resource utilization in children who were and were not discharged early. This analysis demonstrated that children discharged early had greater risk for infectious complications requiring intensive care (ICU) resources for management. However, given the absence of microbiology data and other well-described limitations of administrative/billing data, we sought to confirm our results with chart-abstracted data.
The primary objective of the current study was to compare treatment-related morbidity, mortality and length of stay in children who remained hospitalized during neutropenia and those who were discharged after chemotherapy completion using chart abstraction data collected on a sub-set of patients treated on AAML0531. Based on the results of our prior work,[12] we hypothesized that targeted toxicity rates, particularly viridans group streptococcal (VGS) bacteremia, use of vasopressors and requirement of respiratory support, would be greater in the cohort of patients who were discharged prior to absolute neutrophil count (ANC) recovery than in children who remained hospitalized, but that there would be no difference in treatment-related mortality. We also hypothesized that the number of hospital days would be fewer in patients discharged early, even if early discharge patients were re-admitted during a given course.
Materials and methods
Patients/demographics
AAML0531 randomized 1022 eligible children from August 2006 to June 2010 to receive standard chemotherapy with or without gemtuzumab ozogamicin.[13] Two pediatric oncologists (T.P.M and M.K.) performed retrospective chart abstraction for participants enrolled on AAML0531 at 11 hospitals.[11]
Patient characteristics were obtained from COG, including: sex; date of birth; race (white, non-white); ethnicity (Hispanic, non-Hispanic, unknown); AML risk classification (high, intermediate, low); minimal residual disease (MRD) quantification at the end of Induction I; course commencement and completion dates; and date of death. Age at enrollment was categorized as <1 year, 1–<5 years, 5–<10 years, 10–<15 years, and 15 + years. For each course, the first chemotherapy day was the AAML0531 course start date or the first hospitalization day, whichever was later. The last chemotherapy day was determined by the protocol-mandated regimen.
Early discharge was defined as discharge within 4 days of expected chemotherapy completion. The analyses were restricted to patients determined to be early discharge-eligible based on less than 5% blasts at the end of Induction I and no hypotension, hypoxia, invasive fungal infection (IFI) or VGS from 2 days before through 2 days after chemotherapy completion or the date of first discharge for the course, whichever occurred first. The analysis period for each course was from 2 days after chemotherapy completion through the start of the next course, 60 days after the course start date or the COG off-protocol date, whichever occurred first.
Data sources
Chart abstraction collected data on the presence or absence of hypotension, hypoxia, VGS and IFI on each inpatient day. Chart abstraction procedures were developed a priori and are available upon request. Data on 384 patients treated on AAML0531, including those with chart abstraction data, were previously merged with data from the Pediatric Health Information System (PHIS) administrative/billing database.[14]
Analyses
Distributions of patient characteristics were compared for the study population of abstracted patients and all patients enrolled on AAML0531. Among abstracted patients, distributions of patient characteristics for the study population, by course, and by treatment arm were compared using chi-square tests. Proportions of early-discharge patients for each course and by treatment arm were compared using chi-square tests. Rates and timing of re-admission were determined for early-discharge patients. Cumulative inpatient days after chemotherapy completion were compared by discharge strategy using the Wilcoxon rank sum test. The proportions of mortality and any VGS, IFI, hypoxia and hypotension in each course and the median time to development of VGS were compared by discharge status. Log-binomial regression models were used to estimate adjusted risk ratios (aRR) and 95% confidence intervals (CI) comparing occurrence by discharge status adjusting for course. General estimating equation (GEE) methods with an exchangeable correlation matrix were utilized to account for potential correlation between observations within an individual. For non-early discharge patients, PHIS data were used to determine the proportion that received antibiotics prior to development of VGS or prior to the median time of development of VGS if a patient did not develop VGS. All analyses were performed using SAS (version 9.2, SAS Institute, Inc., Cary, NC). Local Institutional Review Board approval was obtained at each chart abstraction site.
Results
Patients/demographics
Chart abstraction was completed for all 184 (18%) of the 1022 eligible patients enrolled on the AAML0531 trial at the 11 study sites who had medical charts available. One patient at a chart abstraction site did not have a chart available. There were no statistically significant differences in baseline characteristics between abstracted and non-abstracted patients enrolled on AAML0531 except for in insurance status (p < 0.001), but this was driven by a large percentage of patients with unknown or other insurance (Supplemental Table I). Of these 184 patients, 153 patients (84%) were determined to be early discharge-eligible in at least one treatment course. The majority of ineligible patients had > 5% leukemic blasts at the end of Induction I (Fig. 1). Although there were no statistically significant differences in baseline characteristics by discharge status across courses (Table I for Induction II, Supplemental Table II for Induction I and Intensification I–III), patients less than 1 year of age and those with a high-risk classification were generally not discharged early. There were no differences in percentages of early discharge patients overall by treatment arm assignment.
Figure 1.
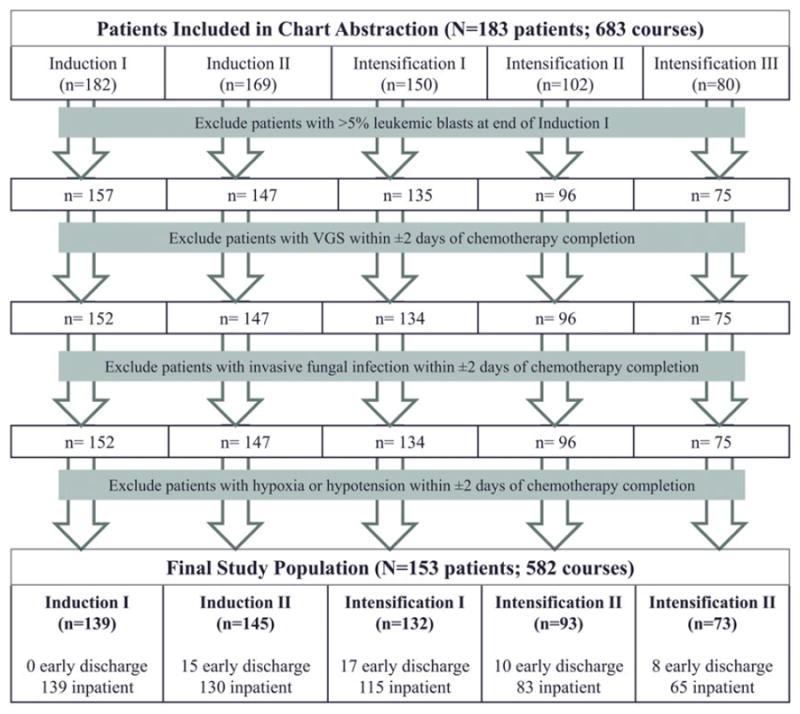
Patients determined to be discharge-eligible by course. Flow chart showing the reasons for exclusion from the discharge-eligible population by course.
Table I.
Frequency (%) of baseline demographic and clinical characteristics for the overall study population and by discharge status for Induction II.
| Overall population (N = 153) | Induction II (n = 145)
|
|||
|---|---|---|---|---|
| Early discharge (n = 15) | Non-early discharge (n = 130) | p-value* | ||
| Patient sex | ||||
| Female | 84 (54.9) | 10 (66.7) | 70 (53.8) | 0.34 |
| Male | 69 (45.1) | 5 (33.3) | 60 (46.2) | |
| Patient age | ||||
| < 1 year | 19 (12.4) | 0 (0) | 16 (12.3) | 0.26 |
| 1–< 5 years | 39 (25.5) | 3 (20.0) | 34 (26.2) | |
| 5–< 10 years | 28 (18.3) | 6 (40.0) | 22 (16.9) | |
| 10–515 years | 45 (29.4) | 4 (26.7) | 39 (30.0) | |
| 15 + years | 22 (14.4) | 2 (13.3) | 19 (14.6) | |
| Patient race | ||||
| White | 108 (70.6) | 12 (80.0) | 90 (69.2) | 0.20 |
| Black | 28 (18.3) | 1 (6.7) | 26 (20.0) | |
| Other | 7 (4.6) | 2 (13.3) | 5 (3.8) | |
| Unknown | 10 (6.5) | 0 (0) | 9 (6.9) | |
| Patient ethnicity | ||||
| Hispanic or Latino | 27 (17.6) | 1 (6.7) | 26 (20.0) | 0.28 |
| Not Hispanic or Latino | 121 (79.1) | 13 (86.7) | 100 (76.9) | |
| Unknown | 5 (3.3) | 1 (6.7) | 4 (3.1) | |
| Insurance status | ||||
| Private or self-pay | 81 (52.9) | 6 (40.0) | 68 (52.3) | 0.64 |
| Medicaid or Medicare | 40 (26.1) | 6 (40.0) | 34 (26.2) | |
| Other | 3 (2.0) | 0 (0) | 3 (2.3) | |
| Unknown | 29 (19.0) | 3 (20.0) | 25 (19.2) | |
| Risk class | ||||
| High | 19 (12.4) | 1 (6.7) | 15 (11.5) | 0.67 |
| Intermediate | 106 (69.3) | 10 (66.7) | 93 (71.5) | |
| Low | 28 (18.3) | 4 (26.7) | 22 (16.9) | |
| Treatment regimen | ||||
| Standard | 76 (49.7) | 8 (53.3) | 64 (49.2) | 0.76 |
| Standard + Gemtuzumab | 77 (50.3) | 7 (46.7) | 66 (50.8) | |
Data are presented for Induction II. There were no differences in results for Intensification I–III. See Supplemental Table II for these results.
N, number of early discharge eligible patients; n, number of patients within each exposure group, early discharge or non-early discharge; Early discharge, discharge within 4 days after the last chemotherapy administration within the course.
p-values from Chi-square or exact test comparing the distribution of covariates among the early discharge group vs non-early discharge group.
Rates of early discharge
Three hospitals discharged some patients early and eight hospitals did not discharge patients early. No hospital discharged all eligible patients; the three hospitals discharged 11.1%, 32.6% and 56.3% of discharge-eligible patients. The distributions for the timing of discharge for each course are presented in Supplemental Figure 1. Overall, the median total time to discharge after chemotherapy completion varied by course, ranging from 16 days for Induction II to 26 days for Intensification II. There were no patients discharged early following Induction I. The proportion of patients discharged early was similar in each course (Induction II: 10.3%, Intensification I: 12.9%, Intensification II: 10.8%, Intensification III: 11.0%, p = 0.920, Table II). Among the patients discharged early, rates of re-hospitalization were 80% following Induction II and 100% for each Intensification course. Median times to re-admission following early discharge were similar across courses and ranged from 5–8 days after initial discharge (Table II).
Table II.
Frequency of early discharge following chemotherapy administration and rate of re-admission by course.
| Course | Total patients, n | Early discharge, n (%) | Re-admission among early discharge patients, n (%) | Time to re-admission after early discharge, median (range) |
|---|---|---|---|---|
| Induction I | 139 | 0 (0) | NA | NA |
| Induction II | 145 | 15 (10.3) | 12 (80.0) | 7.5 (4.0–11.0) |
| Intensification I | 132 | 17 (12.9) | 17 (100.0) | 8.0 (2.0–15.0) |
| Intensification II | 93 | 10 (10.8) | 10 (100.0) | 7.0 (4.0–11.0) |
| Intensification III | 73 | 8 (11.0) | 8 (100.0) | 5.0 (2.0–11.0) |
NA, not applicable.
Cumulative hospital days
Cumulative number of hospital days after chemotherapy completion are tabulated in Table III for each course by discharge status. Despite high rates of re-admission, early discharge resulted in a reduction of hospital days after chemotherapy completion by ~7 days for Induction II (p < 0.0001), Intensification I (p < 0.0001) and Intensification II (p = 0.080) (Table III). Cumulative hospital days did not differ by discharge status for Intensification III (p = 0.589). Previously published estimates of inpatient cost on AAML0531 report a mean of $2564 per inpatient day.[15] Based on that estimate and the observed median difference in cumulative inpatient days per course, non-early discharge patients would on average incur an increase in daily inpatient costs of $19 230 for Induction II, $17 948 for Intensification I, $17 948 for Intensification II and $3846 for Intensification III.
Table III.
Median (range) cumulative inpatient days per chemotherapy course by discharge status.
| Early discharge | Non-early discharge | p-value* | |
|---|---|---|---|
| Induction II | 8.5 (2–15) | 16.0 (10–36) | <0.0001 |
| Intensification I | 13.0 (4–32) | 20.0 (10–50) | <0.0001 |
| Intensification II | 21.0 (11–42) | 28.0 (2–49) | 0.080 |
| Intensification III | 24.5 (18–35) | 26.0 (6–46) | 0.589 |
p-value for Wilcoxon rank sum test comparing the early discharge group to the non-early discharge group.
Rates of treatment-related mortality and targeted toxicities
Three deaths occurred among the discharge-eligible patients who remained hospitalized; one (1.2%) death was during Intensification II and two (3.1%) deaths were during Intensification III. None of these deaths were among patients discharged early. The incidences of targeted toxicities are presented in Table IV by discharge status. Across all courses, the risks for VGS (aRR = 1.67, 95% CI = 1.11–2.51) and hypotension (aRR = 4.36, 95% CI = 2.01–9.46) were significantly higher among patients discharged early compared to those who remained hospitalized. All episodes of VGS in the early discharge group were documented on the day of hospital re-admission. There was no statistically significant difference in the median time to VGS overall (early discharge =8.0 days, range =6.0–12.0; non-early discharge =9.0 days, range =3.0–32.0) or by course, except for in Intensification I (early discharge =8.0 days, range =6.0–12.0 days; non-early discharge =11.0 days, range =3.0–16.0 days, p = 0.0215). The overall risk for hypoxia requiring supplemental oxygen, respiratory support or intubation was also higher in the early discharge cohort (aRR = 1.92, 95% CI = 1.06–3.48). While the percentages of patients who required CPAP/BiPAP or intubation were higher among patients who were discharged early, the number of patients requiring such support was small in both groups and precluded comparative analyses (data not shown). In the early discharge cohort, in 50% of courses where patients developed VGS, patients also had hypotension or hypoxia and, in the non-early discharge cohort, in 38% of courses where patients developed VGS, patients also had hypotension or hypoxia; this difference was not statistically significant (p = 0.3730). IFIs were rare (early discharge: 2.0% of courses, non-early discharge: 1.3% of courses).
Table IV.
Rates of toxicities by discharge status.
| Early discharge % | Non-early discharge % | aRR (95% CI) | |
|---|---|---|---|
| VGS | 31.9 | 15.8 | 1.67 (1.11–2.51) |
| IFI | 2.0 | 1.3 | 1.59 (0.20–12.5) |
| Hypoxia, any | 26.6 | 12.3 | 1.92 (1.06–3.48) |
| Hypotension | 17.9 | 4.4 | 4.36 (2.01–9.46) |
aRR, Adjusted Risk Ratio, adjusted for course; VGS, Viridans group streptococcal bacteremia; IFI, Invasive fungal infection.
All 153 patients and 531 courses of chemotherapy were identified in PHIS. Of these 531 courses, patients developed VGS in 84 (15.8%) courses. Antibiotic resource utilization could only be evaluated in patients who remained hospitalized because PHIS only includes inpatient data. In those non-early discharge patients who developed VGS, 32.1% of courses had antibiotic utilization prior to development of VGS. In those non-early discharge patients who did not develop VGS, 74.0% of courses had antibiotic utilization in the 10 days after chemotherapy completion. Ten days was chosen due to the median number of days until development of VGS in the cohort of patients who remained hospitalized throughout the period of neutropenia.
Discussion
To our knowledge, this is the first study that uses individual patient medical record data to compare mortality, chemotherapy-associated toxicity rates and length of stay in pediatric patients with AML who were discharged after completing chemotherapy to patients who remained hospitalized. In this study there were only a small number of events, but early discharge of pediatric AML patients did not lead to an increase in mortality. However, patients who were discharged early were more likely to develop VGS bacteremia, hypoxia and hypotension than those who remain hospitalized. The increases in these toxicities were substantial, with an ~67% greater risk of VGS, 90% greater risk of hypoxia and a 4-fold greater risk of hypotension. Despite these increased risks and the fact that 80–100% of patients were re-admitted across all courses, early discharge patients spent on average 1 week less in the hospital.
While the biologic mechanism for the increased rates of VGS in early discharge patients is uncertain, this study provides further support of our group’s prior work, suggesting increased rates of infectious complications in patients discharged after chemotherapy for AML by replicating the results with documented VGS infections in chart data.[12] These findings differ, however, from another recent COG analysis using data from patients enrolled on AAML0531 that reported no statistically significant difference in infections in children discharged early.[3] Several possible explanations exist for these discordant results. The prior COG analysis categorized discharge status based on a hospital survey response regarding early discharge practices and did not ascertain early discharge status for individual patients. Thus, patients classified as ‘discharged early’ included all children at hospitals that self-identified as sometimes or always discharging patients early and may, therefore, have included children who remained hospitalized. This exposure (early discharge) misclassification may have obscured a positive association between early discharge and VGS bacteremia. In addition, VGS has been shown to be under-reported on AAML0531.[11] Such under-reporting should be non-differential between discharge groups and would, thus, bias towards the null (no difference in VGS rates).
The marked increase in VGS observed in this pediatric population of early discharge patients has not been previously reported in adult studies.[3-6,8,9,16] While unproven as an explanation, the intensity of pediatric AML chemotherapy regimens is higher than adult AML regimens, and this may increase the risk of infection in outpatient management of children. In addition, this study used a larger, more representative sample than many prior adult studies and directly compared VGS infection rates in patients who were discharged early and those who remained hospitalized.[9,16]
Variation in antibiotic utilization between early discharge and hospitalized patients may also explain the different VGS rates. Patients who remain hospitalized may receive antibiotics for various indications, which may lead to reduced VGS rates. Additionally, antibiotic prophylaxis given to discharged patients may vary by hospital or between clinicians and may affect rates of infectious complications. The differing rates of infections reported in prior studies may be due to the use of antibiotic prophylaxis. Halim et al. [5] compared inpatient and ambulatory chemotherapy administration and follow-up of adults with AML. There were higher rates of sepsis (28% vs 22%) in those who received inpatient chemotherapy and were discharged early compared with those who remained inpatient. However, there were lower rates of sepsis in patients who received outpatient chemotherapy. Among patients who developed sepsis, there was an increased proportion of Gram positive organisms compared to Gram negative organisms and Halim et al. [5] conclude that this was potentially due to the fact that the outpatients were given prophylactic ciprofloxacin. Unfortunately, data on outpatient antibiotic administration were not available and, thus, we could not directly compare the impact of antibiotic administration on VGS rates in the two study groups.
While COG survey data indicates the rate of prophylactic antibiotic use is low on AAML0531,[3] the absence of data on this covariate is an important limitation of the current study. A single institution study previously concluded that early discharge in pediatric AML was safe due to lower rates of VGS in children who were given antibiotic prophylaxis compared to those who were not,[17] but this study did not directly compare rates of VGS infection in early discharge patients and those who remained hospitalized. Some institutions may have a policy of initiating antibiotic prophylaxis at development of severe neutropenia or may continue antibiotics started empirically for a fever throughout ANC nadir and recovery. Recently, a single institution study showed that prophylactic intravenous antibiotic use during neutropenia was associated with no deaths from infections in pediatric AML patients, but this study also did not directly compare cohorts of patients who received prophylaxis and those who did not.[18]
Analyses using PHIS antibiotic resource utilization data for the sub-set of patients who remained hospitalized showed that there was a higher rate of antibiotic utilization in patients who did not develop VGS than those who did. The reasons for initiation of antibiotics cannot be determined from the PHIS data-set and PHIS only includes inpatient data so could not be used to evaluate antibiotic exposure in early discharge patients. However, these data indicate that the patients who remained hospitalized may have had fewer VGS infections due to more antibiotic use.
Despite greater risks of infection and of vasopressor and respiratory support, the cumulative number of hospital days was shorter for patients who were discharged early for most courses. There were no differences in cumulative number of hospital days for Intensification III, but this was likely due to a shorter time to re-admission of 5.0 days for early discharge patients in Intensification III compared to 7.0–8.0 days in other courses. While definitive comparison of standardized costs was outside the scope of this work, previously published data in adults and pediatric data from the AAML0531 trial suggest that an early discharge strategy is likely less costly than hospitalization.[6,10,15] A comprehensive assessment of inpatient and outpatient costs for both early and standard discharge approaches will be a critical component of a comprehensive evaluation of discharge strategies for pediatric AML patients.
The primary limitation of this study is that chart abstraction did not collect data on prophylactic antibiotic use or on all types of bacteremia. The relative incidence of clinically important infections such as Gram negative rod bacteremia could further impact the safety of early discharge and should be evaluated. The possibility of Gram negative rod bacteremia explaining the increased hypoxia and hypotension not associated with VGS in early discharge patients also needs further exploration. In addition, since no hospital discharged all patients early, there may be selection bias in the decision to discharge patients early. However, such selection should bias towards clinicians selecting patients at less presumed risk for complications from early discharge. As noted previously, chart abstraction was only performed at 11 large, free-standing children’s hospitals. Therefore, the results of this study may not be generalizable to all centers where children are treated for AML, as there could be differences in rates of successful treatment of complications based on hospital volume.[19]
Understanding these limitations, this study shows that, in a large sample of children with AML who were eligible for early discharge based on remission status and lack of evidence of active VGS infection, IFI or organ dysfunction concerning sepsis, those who were discharged early had an ~67% increased risk of developing a VGS blood stream infection, a nearly 2-fold increased risk of developing hypoxia and a 4-fold increased risk of developing hypotension. These increased risks were not associated with increased mortality or hospitalization duration and, thus, preclude simple conclusions regarding the appropriateness of either discharge strategy. However, the increased risks of VGS and cardiopulmonary complications warrant additional supportive care and educational strategies for patients discharged early. Specifically, healthcare providers should provide patients and families with guidance regarding the increased risk of VGS infections and secondary complications, informing families that across courses there was a 31.9% risk of developing VGS blood stream infection if children were discharged early and only a 15.8% risk if they remained hospitalized in this study. Providers need to carefully evaluate whether any impediments to rapid access of the healthcare system exist for patients and families. Furthermore, careful consideration should be given to prophylactic antibiotic coverage for patients discharged prior to count recovery and to developing plans for close outpatient follow-up.[3,17,20,21]
Since hospitals are increasingly being encouraged to minimize the length of hospital admissions, further research is needed to comprehensively and precisely define the risks and benefits of each discharge strategy. This work should include accurate ascertainment of bacterial and fungal infections as well as the use of prophylactic antimicrobial agents and will require a sample size adequate to accurately estimate the risks of secondary medical complications from invasive infections and prophylactic therapies. In addition to robust cost estimates, these analyses should include patient quality-of-life, family preference and hospital-level factors. An integrative analysis of these variables will provide patients, healthcare providers and payers with a more nuanced understanding of the patient-specific risks and benefits of each discharge strategy.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health R01 CA165277 and the Pediatric Pharmacoepidemiology Training Grant 5T32HD064567-04. James Feusner, Naomi Winick, William Roberts, Michael Kelly and Samir Kahwash also contributed data for this manuscript.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.3109/10428194.2015.1088652.
Supplemental data for this article can be accessed http://dx.doi.org/10.3109/10428194.2015.1088652.
References
- 1.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children’s Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith FO, Alonzo TA, Gerbing RB, et al. Long-term results of children with acute myeloid leukemia: a report of three consecutive Phase III trials by the Children’s Cancer Group: CCG 251, CCG 213 and CCG 2891. Leukemia. 2005;19:2054–2062. doi: 10.1038/sj.leu.2403925. [DOI] [PubMed] [Google Scholar]
- 3.Sung LAR, Alonzo TA, Gerbing RB, et al. Effectiveness of supportive care measures to reduce infections in pediatric AML: a report from the Children’s Oncology Group. Blood. 2013;121:3573–3577. doi: 10.1182/blood-2013-01-476614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sopko L, Sabty FA, Rimajova V, et al. The feasibility of an early hospital discharge following chemotherapy for the acute myeloid leukemia. Bratisl Lek Listy. 2012;113:298–300. doi: 10.4149/bll_2012_069. [DOI] [PubMed] [Google Scholar]
- 5.Halim TY, Song KW, Barnett MJ, et al. Positive impact of selective outpatient management of high-risk acute myelogenous leukemia on the incidence of septicemia. Ann Oncol. 2007;18:1246–1252. doi: 10.1093/annonc/mdm112. [DOI] [PubMed] [Google Scholar]
- 6.Eisele L, Gunther F, Ebeling P, et al. Outpatient management of acute myeloid leukemia after intensive consolidation chemotherapy is feasible and reduces hospital treatment costs. Onkologie. 2010;33:658–664. doi: 10.1159/000322209. [DOI] [PubMed] [Google Scholar]
- 7.Allan DS, Buckstein R, Imrie KR. Outpatient supportive care following chemotherapy for acute myeloblastic leukemia. Leuk Lymphoma. 2001;42:339–346. doi: 10.3109/10428190109064590. [DOI] [PubMed] [Google Scholar]
- 8.Naithani R, Kumar R, Mahapatra M, et al. Early discharge from hospital after consolidation chemotherapy in acute myeloid leukemia in remission: febrile neutropenic episodes and their outcome in a resource poor setting. Haematologica. 2008;93:1416–1418. doi: 10.3324/haematol.11696. [DOI] [PubMed] [Google Scholar]
- 9.Gillis S, Dann EJ, Rund D. Selective discharge of patients with acute myeloid leukemia during chemotherapy-induced neutropenia. Am J Hematol. 1996;51:26–31. doi: 10.1002/(SICI)1096-8652(199601)51:1<26::AID-AJH5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Arguelles GJ, Apreza-Molina MG, Aleman-Hoey DD, et al. Outpatient supportive therapy after induction to remission therapy in adult acute myelogenous leukaemia (AML) is feasible: a multicentre study. Eur J Haematol. 1995;54:18–20. doi: 10.1111/j.1600-0609.1995.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller TP, Kavcic M, Li Y, et al. Accuracy of adverse event reporting compared to patient chart abstraction on a Phase III NCI-Funded clinical trial for pediatric acute myeloid leukemia: a report from The Children’s Oncology Group. Blood. 2013:122–931. Abstract 931. [Google Scholar]
- 12.Getz KD, Miller TP, Seif AE, et al. A comparison of resource utilization following chemotherapy for acute myeloid leukemia in children discharged versus children that remain hospitalized during neutropenia. Cancer Med. 2015;4:1356–1364. doi: 10.1002/cam4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with De Novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aplenc R, Fisher BT, Huang YS, et al. Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: a report from the Children’s Oncology Group. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 2):37–43. doi: 10.1002/pds.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getz KD, Li Y, Alonzo TA, et al. Comparison of in-patient costs for children treated on the AAML0531 clinical trial: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:1775–1781. doi: 10.1002/pbc.25569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini L, Rostein C, Atenafu EG, et al. Ambulatory consolidation chemotherapy for acute myeloid leukemia with antibacterial prophylaxis is associated with frequent bacteremia and the emergence of fluoroquinolone resistant E. Coli. BMC Infect Dis. 2013;13:284. doi: 10.1186/1471-2334-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inaba H, Gaur AH, Cao X, et al. Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia. Cancer. 2014;120:1985–1992. doi: 10.1002/cncr.28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolt D, Lindemulder S, Meyrowitz J, et al. Preventive antibiotics in pediatric patients with acute myeloid leukemia (AML) Pediatr Blood Cancer. 2015;62:1149–1154. doi: 10.1002/pbc.25463. [DOI] [PubMed] [Google Scholar]
- 19.Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190:665–674. doi: 10.1164/rccm.201402-0289OC. [DOI] [PubMed] [Google Scholar]
- 20.Gamis AS. Earlier initiation of antibiotic therapy: does prophylaxis offer greater benefit in AML? Pediatr Blood Cancer. 2015;62:1121–1122. doi: 10.1002/pbc.25512. [DOI] [PubMed] [Google Scholar]
- 21.Savoie ML, Nevil TJ, Song KW, et al. Shifting to outpatient management of acute myeloid leukemia: a prospective experience. Ann Oncol. 2006;17:763–768. doi: 10.1093/annonc/mdl011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


