Abstract
Background
Metabolic syndrome (MetS), defined by a constellation of cardiometabolic pathologies, is highly prevalent among veterans, especially those with posttraumatic stress disorder (PTSD), and poses a major risk for adverse health outcomes, including neurodegeneration and mortality. Given this, we evaluated: (a) the association between MetS and neural integrity, indexed by cortical thickness; (b) the relationship between PTSD and MetS; and (c) if PTSD was associated with cortical thickness indirectly through MetS.
Methods
The sample consisted of 346 US military veterans (89.3% male; 71.4% white) who deployed to Iraq and/or Afghanistan. Neuroimaging data were available for 274 participants.
Results
In whole-brain analyses, MetS was negatively associated with cortical thickness in 2 left and 4 right hemisphere regions: (a) bilateral temporal lobe, including temporal pole, fusiform gyrus, and insula, and extending into occipital cortex (left hemisphere) and orbitofrontal cortex (right hemisphere); (b) bilateral precuneus, posterior cingulate, calcarine, and occipital-parietal cortex; and (c) right rostral anterior cingulate cortex and central sulcus/postcentral gyrus. Path models showed that PTSD predicted MetS (β = .19, p < .001), which, in turn, was associated with reduced cortical thickness (βs from −.29 to – .43, all p <.001).
Conclusions
Results from this young veteran sample provide evidence that PTSD confers risk for cardiometabolic pathology and neurodegeneration and raise concern that this cohort may be aging pre-maturely and at risk for substantial medical and cognitive decline. This highlights the need to identify the molecular mechanisms linking PTSD to MetS and for effective interventions to reduce PTSD-related health comorbidities.
Keywords: metabolic syndrome, cortical thickness, magnetic resonance imaging, posttraumatic stress disorder, structural equation modeling, accelerated aging
Introduction
Metabolic syndrome (MetS) is a constellation of pathogenic cardiometabolic markers that collectively increase risk for cardiovascular disease (1–2), type 2 diabetes (2–3), cancer (4), cognitive decline (5–6), and death (1;7). It is defined by three or more of the following: obesity, high blood pressure, insulin resistance, and dyslipidemia (elevated triglycerides or low high-density lipoprotein) (8–9) and medical consensus is that when these symptoms co-occur, the health consequences are particularly profound (6,10). Stress, including psychological and traumatic stress (11–13), is thought to play a role in the pathogenesis and course of MetS (14–16) and various pathways have been implicated including autonomic dysregulation and cardiovascular reactivity (13;17–18), hypothalamic-pituitary-adrenal (HPA) axis dysregulation (13;16–17;19), oxidative stress (13;19–22), and immune system dysfunction (13;17–19).
Posttraumatic stress disorder (PTSD) has been linked with elevated risk for MetS (23–25). PTSD is defined by severe trauma exposure followed by reliving of the traumatic experience, avoidance of trauma-related stimuli, negative alterations in cognition and mood, and alterations in arousal and reactivity (26). It is prevalent among nearly a quarter of veterans of the conflicts in Iraq and Afghanistan (27), and poses a major public health concern (28) and financial burden (29). Chronic symptoms of PTSD, including emotional and physiological reactivity, blunted positive affect, social isolation, sleep disturbance, hypervigilance, and startle, are thought to induce dysregulation in autonomic arousal (30), HPA axis (31), and immune functioning (32–33), and may also be associated with oxidative stress and premature cellular senescence (34–36). Two recent meta-analyses found that the prevalence of MetS given PTSD was 37–39% (23–24), which was nearly double that of general population controls (23). Longitudinal studies also suggest that PTSD predicts increasing metabolic risk over time (37–38). Thus, MetS may be an important mechanism linking PTSD to a host of associated medical comorbidities, including cardiovascular disease (39–43), type 2 diabetes (44), cognitive decline (45), and pre-mature death (35;39).
MetS features have also been shown to exert widespread effects on the structural integrity of the brain through reduced blood flow which leads to suboptimal vessel perfusion and degeneration of brain tissue (46–47). Obesity is a strong predictor of decreased cortical thickness, an indicator of gray matter integrity (48–49), and this is most consistently found for temporal, frontal, and parietal regions (50–54). Blood pressure has been negatively associated with cortical thickness (55–56), as have glucose, insulin resistance, and type 2 diabetes (55;57–59). Cholesterol, in contrast, has shown less consistent patterns, with some studies supporting negative associations with cortical thickness (54) and others suggestive of positive associations(55;60). To our knowledge, only one prior study has evaluated the combined effects of the full constellation of MetS markers on cortical thickness. Specifically, Song et al. (61) found that MetS diagnosis was associated with reduced cortical thickness in the left insular, superior parietal, postcentral, entorhinal, and right superior partial cortices in a sample of 86 participants (40 with MetS).
The primary aim of this study was to examine associations between PTSD, MetS, and neural integrity of the cortex in a cohort of veterans deployed to Iraq and/or Afghanistan. We hypothesized that PTSD severity would be associated with greater MetS severity which, in turn, would be associated with reduced cortical thickness in temporal and frontal lobes.
Methods and Materials
Participants
Participants were US Military veterans deployed to Iraq and/or Afghanistan who underwent assessment at the Translational Research Center for TBI and Stress Disorders, a US Department of Veterans Affairs (VA) Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence at VA Boston Healthcare System. Exclusion criteria included history of seizures unrelated to head injury, neurological illness, current psychotic or bipolar disorder, severe depression or anxiety, active homicidal and/or suicidal ideation with intent, cognitive disorder due to general medical condition other than traumatic brain injury (TBI), and unstable psychological diagnosis that would interfere with accurate data collection. Additional MRI exclusion criteria included pregnancy and having a metal implant, shrapnel, aneurysm clip, or pacemaker. MetS-related measures were available for 346 participants. Of these, 89.3% were male and the mean age was 32.48 years (SD: 8.95). The majority of the sample (71.4%) reported their race as white; additional self-reported race and ethnicity was 15.6% Hispanic or Latino/a, 7.8% black, 1.7% Asian, 1.2% American Indian. With respect to educational attainment, 34.4% of the sample had earned up to a high school degree or equivalent and an additional 65.0% of the sample engaged in education beyond high school. Whole-brain cortical thickness data were available for a subset of 274 participants, none of whom had a history of moderate or severe TBI. Demographic characteristics for this subgroup were near identical to that of the larger sample, as detailed in Supplementary Results and Supplementary Table 1.
Procedure
Participants provided written informed consent and had early morning fasting blood samples drawn. Two standing and two seated blood pressure readings were taken at one minute intervals and height, weight, and waist-to-hip ratio was measured. Blood samples were processed immediately and shipped the same day to a commercial lab for metabolic panels. A binary MetS variable was computed following the National Cholesterol Education Program (NCEP) Adult Treatment Panel III recommendations (9) (Table 1), which requires the presence of three or more MetS criteria for the diagnosis. Participants completed diagnostic interviews performed by a PhD-level clinician and underwent MRI scans. The protocol was approved by the relevant institutional review boards.
Table 1.
Descriptive Statistics for MetS Criteria and Factor Indicators
| MetS Cluster | NCEP MetS Criterion | Mean (SD)
|
% Meeting MetS Criterion
|
||||
|---|---|---|---|---|---|---|---|
| All | PTSD+ | PTSD− | All | PTSD + | PTSD− | ||
| Obesity | |||||||
| Waist-to-Hip Ratio | Men: >102cm/40in Women: >88cm/36in |
0.88 (0.07) |
0.88 (0.07) |
0.88 (0.06) |
30.1% | 33.6% | 23.4% |
| BMI | 28.34 (4.69) |
28.53 (4.89) |
27.72 (3.91) |
||||
| Blood Pressure (mmHg) | 14.7% | 16.3% | 10.3% | ||||
| Diastolic | ≥85 | 77.56 (9.55) |
77.92 (9.72) |
76.35 (8.91) |
|||
| Systolic | ≥130 | 117.63 (12.79) |
118.02 (13.02) |
116.33 (11.96) |
|||
| HDL Cholesterol (mg/dL) | Men: < 40 Women: < 50 |
47.51 (12.64) |
47.13 (12.81) |
48.78 (12.06) |
28.0% | 30.9% | 23.4% |
| Triglycerides (mg/dL) | ≥ 150 | 144.12 (133.08) |
147.81 (141.32) |
131.82 (100.70) |
28.0% | 29.6% | 25.6% |
| Glucose | |||||||
| Fasting Glucose (mg/dL) | ≥ 110 | 89.93 (29.19) |
90.90 (32.57) |
86.64 (11.58) |
4.0% | 4.6% | 2.6% |
| A1C (% of hemoglobin) | 5.43 (0.71) |
5.45 (0.79) |
5.35 (0.31) |
||||
Note. There were no significant group differences as a function of lifetime PTSD diagnosis for any of the MetS-related variables listed above. However, individuals with lifetime PTSD met the threshold for a greater numbers of MetS criteria overall compared to those without PTSD (p = .027; see Supplementary Materials and Results). Criteria listed above are based on the NCEP Adult Treatment Panel III (9) definition of MetS. Criteria based on the International Diabetes Federation (103) are shown in the Supplementary Materials. BMI was defined as (weight [lbs])/height [in])2*703. MetS = metabolic syndrome; PTSD = posttraumatic stress disorder; NCEP = National Cholesterol Education Program; SD = standard deviation; BMI = body mass index; HDL Cholesterol = high-density lipoprotein cholesterol; A1C = glycated hemoglobin. SI conversion factors: to convert cholesterol to mmol/L, multiply values by 0.0259; to convert triglycerides to mmol/L, multiply values by 0.0113; to convert fasting glucose to mmol/L, multiply values by 0.0555.
MRI Acquisition and Processing
Structural imaging scans were completed in a 3-Tesla Siemens TIM TRIO whole-body MRI scanner. Two T1-weighted anatomical scans (voxel size = 1mm3, TR = 2530ms, TE = 3.32ms, FOV = 256×256, # of slices = 176) were acquired and averaged to create a single high contrast-to-noise image. The FreeSurfer v5.1 (http://surfer.nmr.mgh.harvard.edu/) morphometric pipeline was applied, including reconstruction of the cortical mantle and spatial smoothing of 20mm FWHM. Cortical surface models were manually checked slice-by-slice and edited for accuracy. Measurement of cortical thickness was calculated using procedures described previously (49;55;62). See Supplementary Materials for additional MRI processing details.
Measures
PTSD diagnosis and symptom severity was assessed with the Clinician Administered PTSD Scale for DSM-IV (CAPS) (63), the gold-standard PTSD structured diagnostic interview. The CAPS was administered for three time periods: past-month (current PTSD), pre-deployment (if relevant pre-deployment trauma), and post-deployment (worst post-deployment symptoms). The frequency and intensity of symptoms were summed to form a severity score and the highest value of these three assessments was used in analyses as an index of maximum lifetime PTSD severity. Psychiatric diagnoses were reviewed by an expert consensus team.
Data Analysis
Primary analyses modeled MetS using raw lab values and physiological measurements which were submitted to a confirmatory factor analysis (CFA) to form an overall index of the severity of MetS features (i.e., “MetS Severity”). CFA generates latent variables that capture the variance in common across multiple indicators to better reflect an underlying trait, such as an unobserved syndrome. Higher scores on the latent variable reflect greater pathology and co-occurrence of multiple MetS indicators; CFA is preferable from a statistical and ecological validity standpoint to the use of arbitrary diagnostic thresholds. Our model included three lower-order factors to represent MetS components: latent Blood Pressure was indicated (defined) by diastolic and systolic blood pressure measurements; latent Lipid/Obesity was indicated by waist-to-hip ratio, BMI, HDL, and triglycerides; and latent Blood Sugar was indicated by fasting glucose and A1c values. These three factors were specified to load together on a higher-order latent MetS Severity variable, with age and sex included in all analyses as covariates of MetS Severity.a All We then extracted factor scores for the higher-order MetS Severity factor, reflecting individual differences in MetS severity, and used those in the whole-brain analyses.
Whole-cortex vertex-wise analyses examined associations between MetS severity and cortical thickness using the FreeSurfer general linear model application in Qdec with factor scores on latent MetS entered as the predictor, and age and sex as covariates. The vertex-wise significance threshold was set at p < .05. We applied a Monte Carlo simulation with 10,000 iterations to correct for multiple comparisons with a cluster-wise threshold of p < .001. We extracted cortical thickness scores from significant clusters for use in subsequent structural equation modeling (SEM).
In each SEM (Figures 2 and 3), maximum PTSD severity was specified to predict latent MetS Severity and latent MetS was specified to predict scores on the cortical thickness clusters with clusters from the right and left hemisphere examined in separate models. Age and sex were included as covariates of MetS Severity and of each cluster and residual covariances among the clusters were estimated. The indirect effects of maximum PTSD severity on each cluster via MetS severity were tested using the ‘model indirect’ command in Mplus 7.11 (64), which was the software employed for all structural models evaluated (e.g., CFAs and SEMs). Models were evaluated using standard fit indices and guidelines (65) (see Supplementary Materials) by focusing first on overall model fit before evaluating the strength and significance of each path; as such, no p-value thresholds were applied.
Figure 2.
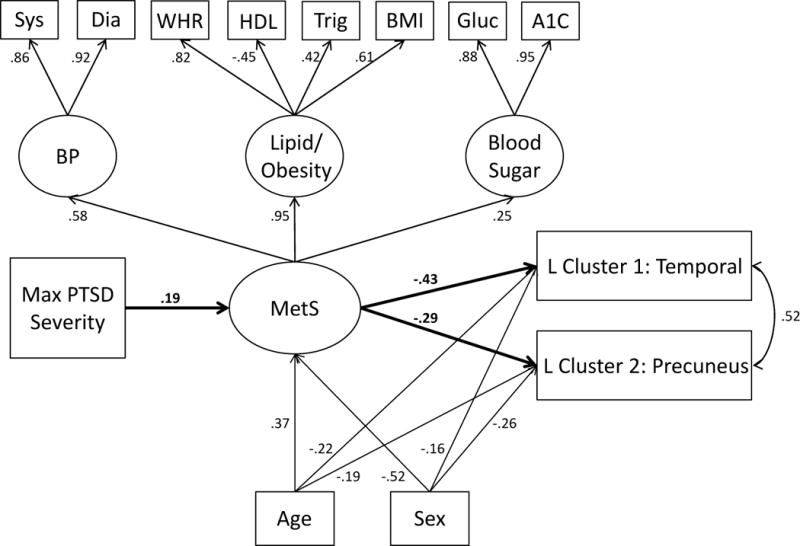
The Figure shows the higher-order measurement model (confirmatory factor analysis) of MetS and the structural associations between maximum PTSD severity, MetS severity, and the left hemisphere cortical thickness clusters that emerged in the whole-brain analyses. Latent variables are denoted by circles and observed indicators by squares. The primary structural paths of interest are bolded for clarity. All lower- and higher-order factor loadings were significant at the p < .001 level. The primary paths of interest were significant at the p < .001 level except for MetS→ Cluster 2 where p = .001. For covariates, age and sex were significantly associated with MetS (p < .001) and with cluster 1 (ps =.004 and .02, respectively) and cluster 2 (ps = .004 and < .001, respectively). PTSD = posttraumatic stress disorder; MetS =metabolic syndrome; sys = systolic; dia = diastolic; WHR = waist-hip-ratio; HDL = high density lipoprotein; trig = triglyceride; BMI = body mass index; gluc = glucose; BP = blood pressure; L = left.
Figure 3.
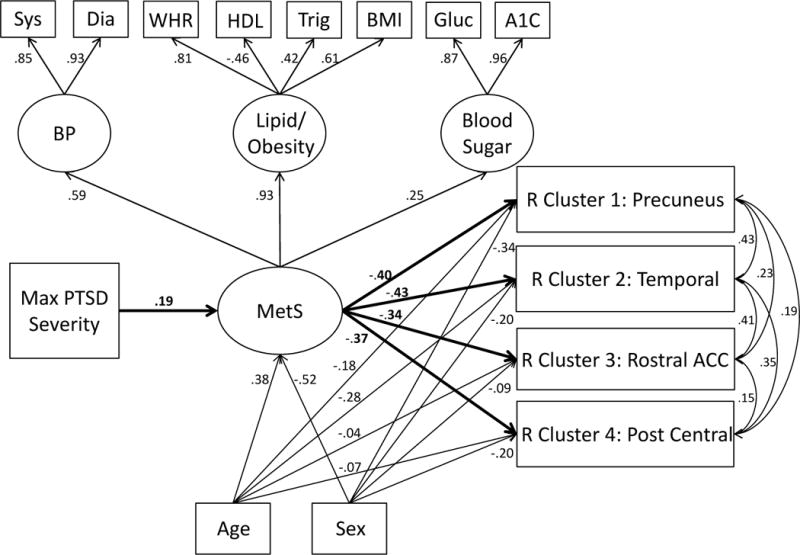
The Figure is identical to Figure 2 except right hemisphere clusters are depicted. MetS predicted clusters 1 and 2 at the p < .001 level and predicted clusters 3 and 4 at the p = .001 level. Age and sex were significant covariates of cluster 1 (ps = .005 and < .001, respectively), and cluster 2 (ps < .001 and = .002, respectively), but not of cluster 3 (ps = .68 and .26, respectively) and only partially of cluster 4 (ps = .40 and .02, respectively). R = right; all other abbreviations as in Figure 2.
Results
Prevalence of MetS, Medication Use, and PTSD
The prevalence of MetS in the overall sample was 12.7%; an additional 19.4% had 2 MetS criteria (just short of the diagnostic threshold; see Table 1). A minority of participants was on cholesterol lowering (4.6%), diabetes management (0.6%), or antihypertensive (11.0%) medications; 26.9% were on antidepressants. The prevalence of current PTSD was 61.0% and of lifetime PTSD was 77.2%; the mean maximum PTSD severity across the three assessment time frames was 69.18 (SD: 32.72). The prevalence of MetS among those with a lifetime diagnosis of PTSD was 14.3% compared with 7.6% among controls (i.e., those who never met criteria for PTSD). Veterans with a lifetime diagnosis of PTSD met the threshold for a greater number of MetS criteria (M = 1.12, SD = 1.13) than controls (M = .84, SD = .94, p = .027; see Supplementary Results and also Supplementary Table 2 for an alternative MetS definition).
MetS Severity Latent Variable
The CFA with covariates provided adequate fit to the data (see Supplementary Table 3) with all indicators showing significant associations with their respective lower-order factors at the p < .001 level and in the range of β = .41 – .95 (see Supplementary Figure 1). The three lower-order factors were associated with the higher-order MetS Severity factor at the p < .001 level, with the Lipid/Obesity factor showing the strongest association with MetS. Age was positively associated with MetS and women tended to have lower MetS scores (see Supplementary Figure 1).
Cortical Thickness Analyses
The whole-cortex vertex-wise analysis revealed two left and four right hemisphere clusters that survived correction for multiple comparisons wherein cortical thickness was reduced given greater MetS severity (see Table 2 and Figure 1). Bilateral reductions were observed in temporal lobe, including temporal pole, fusiform gyrus, and insula, and extending into occipital cortex (left hemisphere) and orbitofrontal cortex (right hemisphere). Both hemispheres also showed MetS severity-related reductions in portions of precuneus, posterior cingulate, calcarine, and occipital-parietal cortex. Two additional clusters emerged in right hemisphere, the first located in rostral anterior cingulate cortex and the second in central sulcus/postcentral gyrus. Cortical thickness values for these six clusters were extracted and included in the subsequent SEMs. Analyses examining potential confounders of the MetS-cortical thickness associations, including medication use, cigarette and alcohol use, major depression, traumatic brain injury, educational attainment, head size, and an index of inflammation, are described in Supplementary Materials (none altered our primary results).
Table 2.
Reductions in Cortical Thickness Associated with MetS
| Cluster | Peak F-Value | Peak (x,y,z) | No. of Vertices | Cluster Size (mm2) |
|---|---|---|---|---|
| L Temporal | −8.0 | −30.6, 10.9, −32.5 | 18629 | 10864.37 |
| L Precuneus | −4.7 | −9.8, −56.2, 18.5 | 1234 | 566.98 |
| R Precuneus | −9.3 | 17.1, −53.7, 12.5 | 2003 | 850.95 |
| R Temporal | −7.0 | 55.1, −10.1, 1.6 | 24730 | 12887.62 |
| R Rostral ACC | −5.3 | 8.1, 35.9, 12.1 | 1490 | 717.06 |
| R Postcentral | −4.6 | 43.8, −15.7, 32.6 | 1329 | 569.07 |
Note. L = left; R = right. A description of the regions that comprise each cluster follows: (1) L Temporal: temporal superior/middle/inferior; temporal pole, fusiform, insula, occipital; (2) L Precuneus: precuneus, occipital-parietal, posterior cingulate, calcarine; (3) R Precuneus: precuneus, occipital-parietal, posterior cingulate, calcarine; (4) R Temporal: orbital medial, orbital middle/lateral, inferior frontal gyrus (pars triangularis, pars opercularis), insula, lateral fissure, temporal superior/middle/inferior; temporal pole, temporal medial/lingual; (5) R Rostral ACC: rostral anterior cingulate, pericallosal, mid-anterior cingulate; (6) R Postcentral: central sulcus, postcentral.
Figure 1.
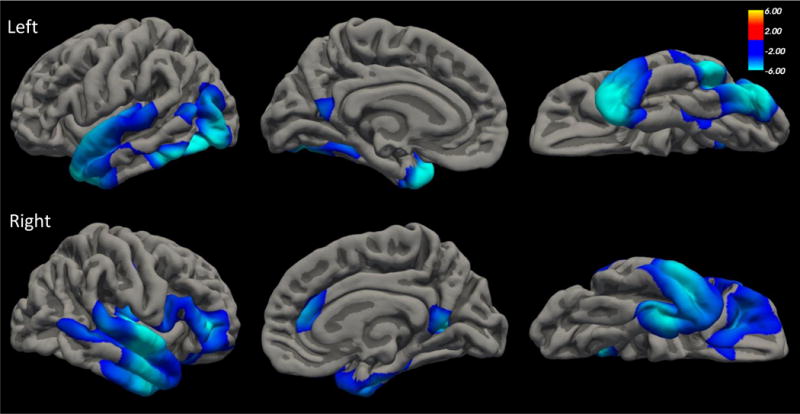
The top and bottom panels show the regions of the left and right cortices, respectively, that were whole-brain associated with MetS factors scores, controlling for age and sex. The figures show lateral (left), medial (middle), and ventral (right) slices.
Structural Equation Models
SEMs were used to test our hypothesis that PTSD severity influences cortical thickness via its association with MetS. The left hemisphere model yielded good fit (Supplementary Table 3) and revealed a significant association between PTSD severity and MetS severity (β = .19, p < .001) and between MetS and both clusters (β = −.43, p < .001 for temporal cluster and β = −.29, p = .001 for precuneus cluster; see Figure 2). The indirect effect of PTSD on left temporal cluster via MetS severity was significant (β = −.08, p = .01) as was the indirect effect on left precuneus cluster (β = −.06, p = .02). The model explained 27% of the variance in temporal cluster and 17% in precuneus. The right hemisphere model also yielded good fit (Supplementary Table 3) and revealed an equivalent PTSD-MetS association as that for left hemisphere and significant associations between MetS and precuneus (β = −.40, p < .001), temporal (β = −.43, p < .001), rostral anterior cingulate cortex (β = −.34, p = .001), and postcentral (β = −.37, p = .001) clusters (see Figure 3). All indirect effects of PTSD on the cortical thickness clusters via MetS severity were significant: precuneus, β = −.08, p = .01; temporal, β = −.08, p = .007; rostral anterior cingulate cortex, β = −.07, p = .02; postcentral, β = −.07, p = .02. The model explained 25% of the variance in precuneus cluster, 32% in temporal, 11% in rostral anterior cingulate cortex, and 14% in postcentral.
Discussion
PTSD is a serious and often disabling condition that affects approximately 23% of veterans of the Iraq and Afghanistan conflicts (27), with an estimated one-third of individuals with PTSD exhibiting chronic symptoms (28;66). Medical comorbidity and pre-mature cognitive decline and mortality are common among those with the disorder (39;43–45). In this sample of returning veterans with a high prevalence of military-related PTSD, we examined associations between PTSD severity, MetS severity, and neural integrity and found PTSD to be associated with both MetS and broad bilateral reductions of cortical thickness, primarily in temporal and parietal regions. That these associations were observed in a relatively young sample, with an average age in their early thirties, is remarkable and underscores the need for new interventions that target not only the psychiatric features of PTSD, but the co-occurring metabolic and disease processes that are potentiated by PTSD.
The prominence of temporal lobe cortical alterations observed in this sample is consistent with prior studies showing obesity to be associated with decreased microstructural integrity of white matter frontal-temporal tracts (67–68), shorter temporal (and frontal) fiber bundle length (69), decreased cerebral blood flood in temporal and frontal regions (70), decreased functional connectivity in the temporal lobe network (71), and longitudinally associated with temporal lobe atrophy (72). Reduced cortical thickness and other forms of temporal and frontal neurodegeneration may be one mechanism underlying the association between cardiometabolic problems and cognitive decline, including onset of dementing disorders like Alzheimer’s disease (70;73–75). Effects observed for parietal regions may bear on research suggesting that parietal cortex atrophy is a prodromal marker for Alzheimer’s disease (76): associations between MetS and this region raise questions regarding if MetS contributes to this initial Alzheimer’s-related decline in some cases. This is speculative and requires additional research.
This study builds on prior work showing associations between PTSD and reduced cortical thickness in temporal and frontal regions (62;77–79) by suggesting that MetS may be one path to such degeneration. Though precise molecular mechanisms linking the stress of PTSD to MetS are still unknown, various well-known processes are likely involved, including heightened autonomic activity, glucocorticoid activation, immunological dysregulation, and oxidative stress (13;17;80). For example, chronic activation of glucocorticoid pathways secondary to emotional and physiological arousal may yield impaired negative feedback inhibition of the HPA-axis with downstream effects on systemic inflammation (31). This, in turn, may give rise to weight gain and dyslipidemia, which together may provide a pathogenic milieu that leads to MetS (13). Behavioral factors also likely play a role as PTSD has been linked to poor diet and insufficient exercise (81) and these may directly contribute to MetS. Just how psychological symptoms and cardiometabolic processes affect the brain is also not fully understood. One possibility is that they exert epigenetic changes that are expressed in the brain (82–83). A second, emerging possibility based on animal research is that PTSD (84) and MetS components (85–86) (i.e., obesity) might have effects on the integrity of the blood-brain barrier such that it becomes more permeable, which allows inflammation to spread, resulting in neuroinflammation and oxidative stress, and ultimately, cognitive and neuronal decline.
PTSD-related MetS and neurodegeneration may be clinical manifestations of a broader accelerated aging process (30;34–35) wherein normal age-related decline in cellular integrity and function is enhanced due to the cumulative burden of chronic PTSD-related stress. Recent work using DNA methylation data (87) and telomere degradation (88–92) as indices of cellular age provide support for PTSD-related accelerated cellular aging. Emerging research suggests that accelerated aging may be evident even in young adults, coordinated across multiple biological systems, including those implicated in MetS, and predictive of age-dependent abilities such as motoric and cognitive function (93). This highlights the importance of identifying those at risk for accelerated aging as early as possible and developing targeted interventions to prevent, attenuate, or reverse age acceleration and associated morbidities. To that end, there is preliminary evidence that exercise interventions for PTSD may ameliorate psychological symptoms (94–95) and improve physical fitness and health (94).
Results should be interpreted in light of study limitations. First, these were cross-sectional data and we are unable to draw causal conclusions. Our models were based on hypothesized directional associations, grounded in evidence that PTSD longitudinally predicts MetS (37) and that obesity shows longitudinal associations with neurodegeneration (72). However, reductions in cortical thickness may reflect a risk factor rather than a consequence of MetS and PTSD and/or these variables may exert bidirectional effects on each other. Support for the former idea comes from research suggesting that neuropsychological deficits, including lower IQ, pre-date the development of obesity (96), MetS (97), and PTSD (98), raising the possibility that reduced cortical thickness may similarly pre-date PTSD and MetS. Thus, additional, prospective research is necessary to address this question. Second, generalizability is limited to predominately male military veterans from the conflicts in Iraq and Afghanistan and it is unclear if the strength of these associations might differ in substantially older (or younger) populations. Third, we did not assess insulin, which would help to inform insulin resistance and would complement our assessment of blood sugar. Related to this, there was limited variability in the blood sugar indicators and this could contribute to attenuated associations with the MetS Severity factor, and indirectly, with PTSD and cortical thickness. Fourth, other structural models of MetS were not evaluated and could also be supported by the data, though the model we employed overlaps substantially with prior higher-order latent variable models of MetS (99). Replication and longitudinal research will be required to address these concerns. These limitations are offset by the strengths of the study which include the large sample size, the evaluation of MetS-related gray matter integrity throughout the cortex, with conservative corrections for multiple testing, and the use of multivariate data analyses to more accurately and parsimoniously reflect MetS in association with PTSD and cortical thickness.
The prevalence of PTSD is growing among our nation’s veterans (100) and the same is true for MetS, with some estimates suggesting that at least a quarter of VA health care users meet MetS criteria (101). Together, these two conditions reflect a tremendous cost to the individual and society (28–29;102). Results suggest that PTSD may serve as a catalyst for the association between MetS and widespread alterations to cortical integrity in temporal, parietal, and frontal regions. This speaks to the burden of PTSD and highlights the need to better understand the molecular mechanisms linking PTSD to MetS and for effective interventions to stem the tide of PTSD-related neurodegeneration and pre-mature medical comorbidities.
Supplementary Material
Acknowledgments
This research was supported in part by NIMH grant R21MH102834 “Neuroimaging Genetics of PTSD” and the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B9254-C), and the Cooperative Studies Program, Department of Veterans Affairs, and NINDS grant R01NS086882. This research is the result of work supported with resources and the use of facilities at the Pharmacogenomics Analysis Laboratory, Research and Development Service, Central Arkansas Veterans Healthcare System, Little Rock, Arkansas. This work was also supported by a Career Development Award to Erika J. Wolf from the United States (U.S.) Department of Veterans Affairs, Clinical Sciences Research and Development Program. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Footnotes
Although BMI and A1c are not components of the NCEP MetS definition, they reflect similar metabolic profiles as do the core MetS criteria and are included here to provide additional indicators of the latent variables. This helps to avoid factor under-identification (i.e., negative df). Age and sex were included to identify the higher-order portion of the model (i.e., ensure positive df so the regression equations can be solved simultaneously).
Disclosures
All authors report no financial conflicts of interest.
Authors Wolf and Sadeh had full access to data and take responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21(2):167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 7.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive summary. Crit Pathw Cardiol. 2005;4(4):198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Executive summary of the third report of the National Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108(13):1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 11.Räikkönen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51(12):1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- 12.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64(3):418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127(1):1–19. doi: 10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- 14.Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res. 2007;39(6):430–438. doi: 10.1055/s-2007-981462. [DOI] [PubMed] [Google Scholar]
- 15.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Kibler JL, Tursich M, Ma M, Malcolm L, Greenbarg R. Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World J Cardiol. 2014;6(6):455–461. doi: 10.4330/wjc.v6.i6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ. Autonomic and inflammatory consequences of posttraumatic stress disorder (PTSD) and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):R315–R321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 20.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treviño S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, et al. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69(9):421–433. doi: 10.1002/syn.21832. [DOI] [PubMed] [Google Scholar]
- 22.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19(8):491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64(8):926–933. doi: 10.1016/j.metabol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli F, Carrà G, Crocamo C, Caretta D, Clerici M. Metabolic syndrome in people suffering from posttraumatic stress disorder: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2013;11(5):301–308. doi: 10.1089/met.2013.0010. [DOI] [PubMed] [Google Scholar]
- 25.Heppner PS, Lohr JB, Kash TP, Jin H, Wang H, Baker DG. Metabolic syndrome: relative risk associated with post-traumatic stress disorder (PTSD) severity and antipsychotic medication use. Psychosomatics. 2012;53(6):550–558. doi: 10.1016/j.psym.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 27.Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, et al. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. [PubMed] [Google Scholar]
- 29.Tanielian T, Jaycox LH. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008. [Google Scholar]
- 30.Williamson JB, Porges EC, Lamb DG, Porges SW. Maladaptive autonomic regulations in PTSD accelerates physiological aging. Front Psychol. 2015;5:1571. doi: 10.3389/fpsyg.2014.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52(7):583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- 32.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 33.Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19(11):1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23(7):709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Villanueva M, Morath J, Vanhooren V, Elbert T, Kolassa S, Libert C, et al. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl Psychiatry. 2013;3:e320. doi: 10.1038/tp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr OM, Ko BJ, Joung KE, Zaichenko L, Usher N, Tsoukas M, et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovasc Dis. 2015;25(5):479–488. doi: 10.1016/j.numecd.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis MM, Nikulina V, Widom CS. A prospective examination of the mechanisms linking childhood physical abuse to body mass index in adulthood. Child Maltreat. 2015;20(3):203–213. doi: 10.1177/1077559514568892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 40.Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, et al. Post-traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiol Rev. 2013;21(1):16–22. doi: 10.1097/CRD.0b013e318265343b. [DOI] [PubMed] [Google Scholar]
- 41.Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol Psychiatry. 2013;74(11):861–866. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health. 2015;105(4):757–763. doi: 10.2105/AJPH.2014.302342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132(4):251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, et al. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72(3):203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Stephan BC, Ciullo M. Angiogenesis and biomarkers of cardiovascular risk in adults with metabolic syndrome. J Intern Med. 2010;268(4):338–347. doi: 10.1111/j.1365-2796.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 47.Taguchi A. Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):859–864. doi: 10.3233/JAD-2009-0975. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 50.Kaur S, Gonzales MM, Strasser B, Pasha E, McNeely J, Tanaka H, Haley AP. Central adiposity and cortical thickness in midlife. Psychosom Med. 2015;77(6):671–678. doi: 10.1097/PSY.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 51.Veit R, Kullmann S, Heni M, Machann J, Häring HU, Fritsche A, Preissl H. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307–311. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marqués-Iturria I, Pueyo R, Garolera M, Segura B, Junqué C, García-García I, et al. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res. 2013;214(2):109–115. doi: 10.1016/j.pscychresns.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Hassenstab JJ, Sweet LH, Del Parigi A, McCaffery JM, Haley AP, Demos KE, et al. Cortical thickness of the cognitive control network in obesity and successful weight loss maintenance: a preliminary MRI study. Psychiatry Res. 2012;202(1):77–79. doi: 10.1016/j.pscychresns.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walhovd KB, Sorsve AB, Westlye LT, Drevon CA, Fjell AM. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 2014;35(5):1055–1064. doi: 10.1016/j.neurobiolaging.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54(4):2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens. 2014;8(8):561–570. doi: 10.1016/j.jash.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299(1–2):126–130. doi: 10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Sun J, Yang Y, Lou X, Wang Y, Wang Y, Ma L. Cortical thinning in type 2 diabetes mellitus and recovering effects of insulin therapy. J Clin Neurosci. 2015;22(2):275–279. doi: 10.1016/j.jocn.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Tchistiakova E, Anderson ND, Greenwood CE, MacIntosh BJ. Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. Neuroimage Clin. 2014;5:36–41. doi: 10.1016/j.nicl.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krishnadas R, McLean J, Batty DG, Burns H, Deans KA, Ford I, et al. Cardio-metabolic risk factors and cortical thickness in a neurologically healthy male population: Results from the psychological, social and biological determinants of ill health (pSoBid) study. Neuroimage Clin. 2013;2:646–657. doi: 10.1016/j.nicl.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song SW, Chung JH, Rho JS, Lee YA, Lim HK, Kang SG, et al. Regional cortical thickness and subcortical volume changes in patients with metabolic syndrome. Brain Imaging Behav. 2015;9(3):588–596. doi: 10.1007/s11682-014-9311-2. [DOI] [PubMed] [Google Scholar]
- 62.Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601–611. doi: 10.1016/j.nicl.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 64.Muthèn LK, Muthèn BO. Mplus user’s guide. Los Angeles, CA: Muthèn & Muthèn; 2012. [Google Scholar]
- 65.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- 66.Solomon SD, Davidson JR. Trauma: prevalence, impairment, service use, and cost. J Clin Psychiatry. 1997;58(Suppl 9):5–11. [PubMed] [Google Scholar]
- 67.Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obes Rev. 2015;16(4):273–281. doi: 10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- 68.Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, et al. Brain structure and cognitive correlates of body mass index in healthy older adults. Behav Brain Res. 2015;278:342–347. doi: 10.1016/j.bbr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, et al. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging Behav. 2013;7(3):300–306. doi: 10.1007/s11682-013-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willeumier K, Taylor DV, Amen DG. Elevated body mass in National Football League players linked to cognitive impairment and decreased prefrontal cortex and temporal pole activity. Transl Psychiatry. 2012;2:e68. doi: 10.1038/tp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring HU, et al. The obese brain: association of body mass index and insulin sensitivity with resting state: network functional connectivity. Hum Brain Mapp. 2012;33(5):1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 73.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63(5):652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stranahan AM. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience. 2015;309:125–139. doi: 10.1016/j.neuroscience.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okabayashi S, Shimozawa N, Yasutomi Y, Yanagisawa K, Kimura N. Diabetes mellitus accelerates Aβ pathology in brain accompanied by enhanced GAβ generation in nonhuman primates. PLoS One. 2015;10(2):e0117362. doi: 10.1371/journal.pone.0117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bing X, Ming-Guo Q, Ye Z, Jing-Na Z, Min L, Han C, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013;1490:225–232. doi: 10.1016/j.brainres.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 78.Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66(12):1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- 79.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41(3):675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39(1):61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall KS, Hoerster KD, Yancy WS., Jr Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103–115. doi: 10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- 82.Zannas AS, Provençal N, Binder EB. Epigenetics of posttraumatic stress disorder: current evidence, challenges, and future directions. Biol Psychiatry. 2015;78(5):327–335. doi: 10.1016/j.biopsych.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Yara S, Lavoie JC, Levy E. Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics. 2015;7(2):283–300. doi: 10.2217/epi.14.84. [DOI] [PubMed] [Google Scholar]
- 84.Yang R, Daigle BJ, Jr, Muhie SY, Hammamieh R, Jett M, Petzold L, Doyle FJ., 3rd Core modular blood and brain biomarkers in social defeat mouse model for posttraumatic stress disorder. BMC Syst Biol. 2013;7:80. doi: 10.1186/1752-0509-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, et al. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69(10):1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mauro C, De Rosa V, Marelli-Berg F, Solito E. Metabolic syndrome and the immunological affair with the blood-brain barrier. Front Immunol. 2015;5:677. doi: 10.3389/fimmu.2014.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolf EJ, Logue MW, Hayes JP, Sadeh N, Schichman SA, Stone A, et al. Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. doi: 10.1016/j.psyneuen.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jergovic M, Tomicevic M, Vidovic A, Bendelja K, Savic A, Vojvoda V, et al. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:275–283. doi: 10.1016/j.pnpbp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8(7):e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology [published online January 15, 2015] Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L, Hu XZ, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, et al. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Mol Psychiatry. 2014;19(8):855–856. doi: 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- 93.Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112(30):E4104–4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenbaum S, Sherrington C, Tiedemann A. Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta Psychiatr Scand. 2015;131(5):350–359. doi: 10.1111/acps.12371. [DOI] [PubMed] [Google Scholar]
- 95.Fetzner MG, Asmundson GJ. Aerobic exercise reduces symptoms of posttraumatic stress disorder: a randomized controlled trial. Cogn Behav Ther. 2015;44(4):301–313. doi: 10.1080/16506073.2014.916745. [DOI] [PubMed] [Google Scholar]
- 96.Belsky DW, Caspi A, Goldman-Mellor S, Meier MH, Ramrakha S, Poulton R, Moffitt TE. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol. 2013;179(9):1461–1468. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Batty GD, Gale CR, Mortensen LH, Langenberg C, Shipley MJ, Deary IJ. Pre-morbid intelligence, the metabolic syndrome and mortality: The Vietnam Experience Study. Diabetologia. 2008;51(3):436–443. doi: 10.1007/s00125-007-0908-5. [DOI] [PubMed] [Google Scholar]
- 98.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol Med. 2007;37(2):181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Povel CM, Beulens JW, van der Schouw YT, Dollé ME, Spijkerman AM, Verschuren WM, et al. Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care. 2013;36(2):362–368. doi: 10.2337/dc11-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Institute of Medicine. Treatment for Posttraumatic Stress Disorder in military and veteran populations: final assessment. Institute of Medicine; 2014. [DOI] [PubMed] [Google Scholar]
- 101.Keane J, Meier JL, Noth RH, Swislocki AL. Computer-based screening of veterans for metabolic syndrome. Metab Syndr Relat Disord. 2009;7(6):557–561. doi: 10.1089/met.2009.0021. [DOI] [PubMed] [Google Scholar]
- 102.Sullivan PW, Ghushchyan V, Wyatt HR, Hill JO. The medical cost of cardiometabolic risk factor clusters in the United States. Obesity (Silver Spring) 2007;15(12):3150–3158. doi: 10.1038/oby.2007.375. [DOI] [PubMed] [Google Scholar]
- 103.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


