Abstract
Background
The aging population in Latin America is characterized by not optimal conditions for good health, experiencing high burden of comorbidity, which contribute to increase the frequency of frailty; thus, identification should be a priority, to classify patients at high risk to develop its negative consequences.
Aim
The objective of this analysis was to validate the FRAIL instrument to measure frailty in Mexican elderly population, from the database of the Mexican Health and Aging Study (MHAS).
Materials and methods
Prospective, population study in Mexico, that included subjects of 60 years and older who were evaluated for the variables of frailty during the year 2001 (first wave of the study). Frailty was measured with the five-item FRAIL scale (fatigue, resistance, ambulation, illnesses, and weight loss). The robust, pre-frail or intermediate, and the frail group were considered when they had zero, one, and at least two components, respectively. Mortality, hospitalizations, falls, and functional dependency were evaluated during 2003 (second wave of the study). Relative risk was calculated for each complications, as well as hazard ratio (for mortality) through Cox regression model and odds ratio with logistic regression (for the rest of the outcomes), adjusted for covariates.
Results
The state of frailty was independently associated with mortality, hospitalizations, functional dependency, and falls. The pre-frailty state was only independently associated with hospitalizations, functional dependency, and falls.
Conclusions
Frailty measured through the FRAIL scale, is associated with an increase in the rate of mortality, hospitalizations, dependency in activities of daily life, and falls.
Keywords: Frailty, Elderly, Mortality, Hospitalizations, México
Introduction
The demographic and epidemiological transitions associated with the increase in life expectancy has had important implications for health systems worldwide, one of which is the aging of the population. In Mexico, for the year 2050 the elderly population will increase by about 26 million, and more than 75 % of this growth will occur after 2020. Due to this rapid growth of aged people, it is estimated that the population over 60 years, which actually represent one in 13 Mexicans (7.6 %), and in 2030 will represent one in six (17.1 %), but even in 2050 will be one in four (27.7 %). The average age of the population, which currently is 28 years, will increase to 37 and 43 years in 2030 and 2050, respectively [1]. This phenomenon leads to an increase in clinical conditions associated with age, such as frailty, considered a vulnerability state, with increased risk of adverse events in health when exposed to a stressor [2, 3]. Moreover, frailty is associated with higher incidence of hospitalizations, falls, and death [4–7], thus affecting the quality of life of older adults, and results in greater use of health services [8], affecting the direct and indirect costs of care.
The aging population in Latin America is characterized by suffering poverty, high burden of comorbidity, and live with social conditions not optimal for sound health, because social and health services are limited [9, 10], which contribute to increase the frequency of frailty, whose prevalence in Mexico is between 15 and 39 % [7, 8, 11]. Therefore, identification of frailty should be a priority for health institutions, in order to classify patients at high risk to develop its negative consequences, and conduct close monitoring in search to avoid them.
Actually, there are multiple tools, and standardized indexes to measure frailty [3, 4, 6, 12–22], with good results for predicting adverse outcomes in health. The FRAIL tool [3, 12–15, 23], is fast, simple, economical, and could lead to early identification, and guide treatment by clinicians [22]. The FRAIL tool has not been validated in Mexican population to identify older adults at risk for adverse events in health such as death, functional dependence, falls and hospitalizations. Therefore, the objective of this analysis was to validate the FRAIL instrument in Mexican elderly population, from the database of the Mexican Health and Aging Study (MHAS) [24], a prospective study in Mexicans born before 1951, as well as their couples, who through a survey on the processes of aging and the burden of disease that occur in this group and recording data in 2001 and 2003 (with measurement of mortality, falls, hospital days and functionality).
Materials and methods
To carry out the present work was employed MHAS database [24]. The methodology was conducted by the Center for Population Studies at the University of Pennsylvania, Center for Research on Population at the University of Maryland and the Center for Demography and Ecology, University of Wisconsin, while the National Institute of Statistics, Geography and Informatics (INEGI) of Mexico performed fieldwork. Information related to various aspects, such as dynamics of health, family structure and intergenerational transfers, migratory behaviors and socioeconomic differences by income and property ownership was collected. The sample is representative of the non-institutionalized population component aged 50 years in 2000. Collecting data from the first round was conducted from May to October 2001 and a second round took place from June to September 2003 in which participants were re-interviewed in 2001 and a third round in the year 2012. The weighting factors were defined according to four criteria: (a) per household, (b) for subjects 50 years or older with a couple of 50 or older in the household, (c) for single subjects 50 years or more, and (d) for subjects younger than 50 years with a couple of 50 or more in the same household. All subjects 60 years or older, who underwent a follow-up in 2003 and determined whether they were still living were selected. We excluded participants in who we could not determine the state of frailty and/or the studied variables (missing values).
Variables of the frailty index
For the construction of the frailty index were included five questions, which correspond to the FRAIL tool [3, 12–15], validated in different populations [13–15]. The first question was: During the last 2 years have you had severe fatigue or serious exhaustion often? It was taken as positive response and was assigned one point when answered “yes”. The second question involved: Because of health problems: do you have difficulty climbing a flight of stairs without resting? Which was taken as a positive response when they answered yes, “cannot” or “do not do”, and one point was assigned in such cases. The third question was: because of health problems: Do you have any difficulty walking one block? Which was taken as a positive response when they answered yes, “can not” or “do not do it”, and in such case a point is assigned. The fourth question was, compared with 2 years ago do you weight: 5 kg more, 5 kg less or weighs more or less the same? The answer was taken as positive response and was assigned a point when answered 5 kg less. Finally, when they had 5 or more chronic diseases, (such as hypertension, diabetes, cancer, chronic respiratory disease, myocardial infarction, cerebral vascular disease, arthritis or rheumatism and kidney or liver disease) was assigned one point, as employed by Morley et al. [14]. The categorization of this frailty index was as follows: when zero points were obtained was considered as robust, with one or two points was considered pre-frail, and when three or more points were obtained the participant was cataloged as frail.
Analyzed covariates
There were also included the following variables: gender, age, comorbidities such as hypertension or self-reported high blood pressure, diabetes or high sugar level in blood, cancer, lung disease, heart disease (heart attack), brain disease (stroke, or transient ischemic attack), arthritis or rheumatism, and kidney or liver disease. For these comorbidities, we created the variable number of comorbidities, representing the sum of the latter, and have values ranging from 0 to 8. We also included: quality of vision, and hearing, frequency of smoking, and drinking. Cognitive status was evaluated was obtained through the cross-cultural cognitive examination. This test has a maximum score of 80 and includes an assessment of the following cognitive functions: (a) primary verbal memory (repeat eight words with score from 0 to 8); (b) selective attention (visual detection of a target stimulus, with a score of 0 to 60); (c) secondary verbal memory (recall of eight words, with a score ranging from 0 to 8); (d) visual-space capacity (copying two geometric figures, scoring between 0 and 2); (e) visual memory (recall of two geometric figures, with a score ranging from 0 to 2). The test has a sensitivity of 100 % and a specificity of 83 % for patients with dementia [25]. Was also adopted the depression variable, which corresponds to the total score for each subject on a questionnaire of nine questions about depressive symptoms with a score ranging from 0 to 9, and which has already been validated [26]. Functional assessment was also included with a score that was obtained by the sum of the number of basic activities of daily living (bathing, dressing, toileting, moving, eating and being continent, score 0–6) and instrumented daily living (preparing hot food, buy food, taking medications and managing their money, score 0–4) which require assistance. Missing values were considered in the case of respondents did not answer or they reply, “do not know” on each of the variables analyzed. All subjects who participated in the study gave their informed consent at the time of interview.
Dependents variables
The dependent variables were mortality, hospitalizations, presence or absence of falls in the past 2 years, and need help in at least one basic or instrumental activities of daily living. All the above variables were reported in conducting the second round of the survey in 2003. For the calculation of incidence of hospitalizations, and functional dependence, we included only those participants that in the first wave of 2001, showed no hospitalization, and were independent in all activities of daily living for each group of variables, respectively.
Statistical analysis
The analysis was performed by the method of complete cases (only those subjects without missing values in the variables analyzed) [27]. Participants were characterized using descriptive statistics, and included median, and interquartile range for quantitative variables, and absolute frequencies, and percentages in the case of qualitative variables. Chi square tests were used to determine differences between qualitative variables, and Kruskal–Wallis test to demonstrate the difference between quantitative variables. p values lower than 0.05 were considered statistically significant. The variable survival time was calculated from the time in months between the date of the first clinical evaluation and the second evaluation or by the date of death. The degree of association of variables was measured with hazard ratio through Cox regression model for the case of mortality, and odds ratio through logistic regression for the variables: hospitalization, falls and dependence in at least a basic or instrumented activities of daily living. A minimum of 852 participants in each group was estimated to identify statistically significant difference in proportions between 0.02 and 0.05 through Chi square two-tailed test, power of 0.9 and alpha of 0.05 for mortality variable. All statistical analyses were performed using Stata/SE, version 12 (Stata Corporation, College Station, TX, USA).
Results
General characteristics of the participants
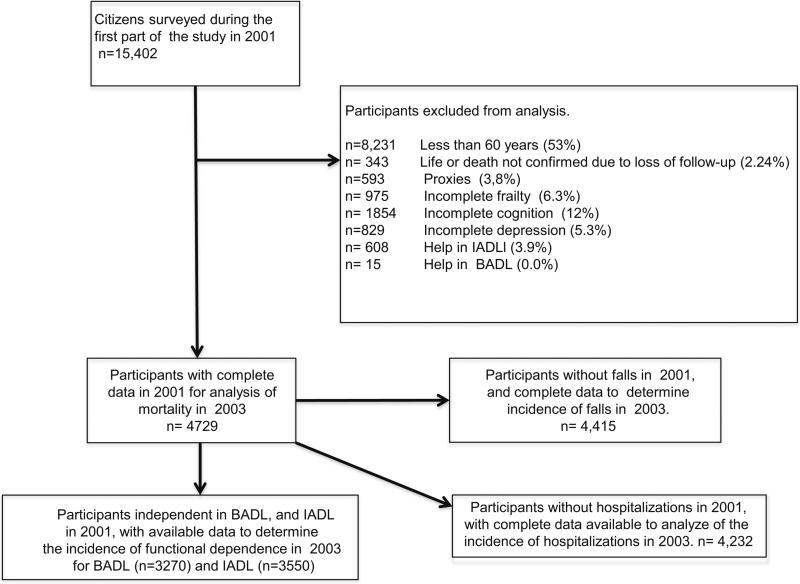
Baseline clinical and demographic characteristics of 4729 selected participants were grouped and analyzed according to frailty status (see Fig. 1; Table 1). The groups of frail, pre frail and robust individuals included 493 (10.4 %), 2121 (44.8 %) and 2115 (44.7 %), respectively. With regard to their general characteristics, those identified as frail were older, more often women, suffered from more depressive symptoms, required more aid for activities of daily living, reported higher number of chronic diseases, lower scores on cognition, and fewer years of schooling, they experienced poorer quality vision and hearing.
Fig. 1.
Flowgram of the study
Table 1.
Demographic and health variables of respondents in the Mexican Health and Aging Study, according to the variable frailty
| Variable | Frail (n = 493) | Prefrail (n = 2121) | Robust (n = 2115) | |
|---|---|---|---|---|
| Age (years) | 70 (65-76) | 67 (63-73) | 66 (62-71) | <0.001 |
| Grouped age (years) | ||||
| 60-69 | 227 (46 %) | 1297 (61.12 %) | 1430 (67.6 %) | <0.001 |
| 70-79 | 187 (37.9 %) | 669 (31.5 %) | 576 (27.2 %) | |
| 80 or more | 79 (16 %) | 155 (7.3 %) | 109 (5.2 %) | |
| Gender | ||||
| Man | 166 (33.7 %) | 936 (44.1 %) | 1100 (52.3 %) | <0.001 |
| Woman | 327 (66.3 %) | 1185 (55.9 %) | 1015 (48 %) | |
| Years of scholarity | ||||
| 0 | 191 (38.8 %) | 607 (28.6 %) | 455 (21.5 %) | <0.001 |
| 1-4 | 191 (38.8 %) | 831 (39.2 %) | 721 (34.1 %) | |
| 5-9 | 90 (18.3 %) | 554 (26.1 %) | 752 (35.6 %) | |
| 10 or more | 20 (4.1 %) | 129 (6.1 %) | 185 (8.8 %) | |
| Chronic diseases | ||||
| Number of chronic diseases | 2 (1-3) | 1 (0-2) | 1 (0-1) | <0.001 |
| Hypertension | 272 (55.2 %) | 965 (46.2 %) | 703 (33.7 %) | <0.001 |
| Diabetes mellitus | 145 (29.4 %) | 408 (19.2 %) | 248 (11.7 %) | <0.001 |
| Cancer | 13 (2.6 %) | 47 (2.2 %) | 19 (0.9 %) | 0.001 |
| Pulmonary disease | 69 (14.0 %) | 165 (7.8 %) | 100 (4.7 %) | <0.001 |
| Myocardial infarction | 51 (10.3 %) | 86 (4.1 %) | 39 (1.8 %) | <0.001 |
| Cerebrovascular disease | 26 (5.3 %) | 67 (3.2 %) | 27 (1.3 %) | <0.001 |
| Rheumatism | 198 (40.2 %) | 608 (28.7 %) | 332 (15.7 %) | <0.001 |
| Kidney or liver disease | 91 (18.5 %) | 219 (10.3 %) | 133 (6.3 %) | <0.001 |
| Vision | ||||
| Use of glasses | 224 (45.4 %) | 949 (44.7 %) | 936 (44.3) | 0.211 |
| Quality of vision with glasses | ||||
| Excellent, very good or good | 185 (37.5 %) | 1054 (49.7 %) | 1340 (63.4 %) | <0.001 |
| Hearing | ||||
| Use of hearing aid | 8 (1.6 %) | 28 (1.3 %) | 33 (1.6 %) | 0.716 |
| Quality of hearing | ||||
| Excellent, very good or good | 295 (59.8 %) | 1440 (67.9 %) | 1618 (76.5 %) | <0.001 |
| At least one fall | 284 (57.7 %) | 929 (43.8 %) | 676 (32 %) | <0.001 |
| Currently smokes | 62 (30.4 %) | 310 (31.3 %) | 342 (35 %) | 0.295 |
| Ever smoked | 204 (41.4 %) | 991 (46.7 %) | 976 (46.1 %) | 0.096 |
| Having consumed alcohol | 189 (52.9 %) | 723 (53.2 %) | 682 (55.8 %) | 0.607 |
| Cross-cultural cognitive examination score | 24 (16-33) | 30 (21-11) | 34 (24-46) | <0.001 |
| Number of depressive symptoms | 6 (4-8) | 4 (2-6) | 2 (1-1) | <0.001 |
| Need help in at least one BADL | 65 (13.2 %) | 36 (1.7 %) | 4 (0.2 %) | <0.001 |
The data represent absolute frequencies and percentages or median (interquartile range). Data were compared with Chi squared or Kruskal-Wallis
The number of chronic diseases is the sum of hypertension, diabetes, cancer, respiratory disease, acute myocardial infarction, cerebrovascular disease, rheumatism, and kidney or liver disease
Frailty and its association with mortality at the year 2003
After a mean follow up of 29.2 months (95 % CI 29.1–29.3), the death rate was 52 (10.5 %), 98 (4.6 %) and 62 (2.9 %) participants in frail, pre frail and robust respectively (p < 0.001). The unadjusted relative risk was 3.59 (95 % CI 2.52–5.13, p < 0.001) and 1.57 (95 % CI 1.15–2.15, p = 0.001) for the state of frail and pre frail, respectively, which after adjusting for covariates, only the frail state remained statistically significant (see Table 2).
Table 2.
Frailty in participants from the Mexican Health and Aging Study, and its association with death, hospitalization, dependency in basic and instrumental ADL, and new falls during the 2 years in the multivariate analysis, Mexico, 2001-2003
| Variable | Death (N = 4729) |
Hospitalization (N = 4232) |
Need help in at least one BADL (N = 3270) |
Need help in at least one IADL (N = 3550) |
New falls (N = 2536) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | HR (CI 95.0 %)a | p | OR (CI 95.0 %)b | p | OR (CI 95.0 %)c | p | OR (CI 95.0 %)d | p | OR (CI 95.0 %)e | |
| Frail | 0.005 | 1.862 (1.205-2.878) | <0.001 | 1.741 (1.289-2.351) | <0.001 | 5.295 (3.174-8.831) | <0.001 | 2.389 (1.633-3.494) | <0.001 | 2.726 (1.718-4.325) |
| Prefrail | 0.182 | 1.254 (0.899-1.747) | 0.019 | 1.284 (1.061-1.554) | 0.003 | 1.888 (1.240-2.874) | 0.010 | 1.420 (1.089-1.853) | 0.014 | 1.462 (1.078-1.982) |
| Robust | 1 | 1 | 1 | 1 | 1 | |||||
HR hazard ratio, OR odds ratio
Adjusted for age, sex, number of depressive symptoms, cognitive score and help in at least one BADL
Adjusted for age, sex, cognitive score and help BADL
Adjusted for age, sex, number of depressive symptoms, number of chronic diseases, and cognitive score
Adjusted for age, sex, number of chronic diseases, number of depressive symptoms, and cognitive score
Adjusted for age, sex, number of depressive symptoms, cognitive score, and help in BADL
Frailty and its association with incidence of hospitalization by year 2003
To evaluate the association between frailty and incidence of hospitalizations 4232 participants were included. During the 2 years of follow up 588 hospitalizations occurred. The incidence of hospitalizations was 83 (21.8 %), 277 (14.8 %) and 228 (11.5 %) participants in frail, pre frail and robust, respectively (p = 0.003). The unadjusted relative risk was 1.89 (95 % CI 1.513–2.378, p = 0.001) and 1.285 (95 % CI 1.09–1.51, p < 0.001) for frail and pre frail states, respectively, which after adjusting for covariates, statistical significance remained (see Table 2).
Frailty and its association with incidence of dependence on at least one basic activity of daily living to the year 2003
We selected 3270 participants to evaluate the incidence of dependency on at least one basic activity of daily living (BADL). During the follow up 165 (5 %) cases occurred. Frequency dependence on at least one BADL was 48 (16.7 %), 80 (5.4 %) and 37 (2.5 %) participants in the frail, pre-frail and robust groups, respectively (p < 0.001). The unadjusted relative risk was 6.71 (95 % CI 4.45–10.12, p < 0.001) and 2.16 (95 % CI 1.47–3.17, p < 0.001) for the frail and pre frail state, respectively, which after adjusting for covariates remained statistically significant (see Table 2).
Frailty and its association with incidence of dependence on at least one instrumented activity of daily living to the year 2003
We selected 3550 participants to evaluate dependency on at least one instrumental activity of daily living (IADL). There were 365 incident cases of dependence on at least one IADL by year 2003. Frequency of dependence on at least one IADL was 67 (25.3 %), 186 (13.5 %) and 112 (8.2 %) in participants frail, pre frail and robust pre respectively (p < 0.001). The unadjusted relative risk was 3.09 (95 % CI 2.36–4.07, p < 0.001) and 1.65 (95 % CI 1.32–2.06, p < 0.001) for the frail and pre frail states, respectively, which remained after adjusting its significance statistics (see Table 2).
Frailty and its association with incidence of falls to the year 2003
To evaluate the association between frailty and the incidence of falls, 2536 participants were included. The general incidence of falls is 794 (31.3 %). The incidence of falls for frail, prefrail and robust participants is 70 (37.4 %), 351 (31 %) and 373 (26.9 %). The unadjusted relative risk was 1.39 (95 % CI 1.13–1.70, p < 0.001) and 1.15 (95 % CI 1.01–1.30, p < 0.001) for frail and pre frail states, respectively, which after being adjusted for covariates, remained statistically significant (see Table 2).
Discussion
The objective of this analysis was to validate the FRAIL instrument for identifying frail older adults at risk for adverse health events from the database of the National Study on Health and Aging, Mexico (MHAS).
In reviewing the clinical characteristics of the members of the groups, it was found that frailty is associated with older age, female sex, more depressive symptoms, lower scores on cognition, required more aid in activities of daily living, worse quality of vision and hearing, and higher number of chronic diseases. Which agrees with the findings of Fried et al. [4], as well as in Mexican–American population, described by Snih, and colleagues [28] and in American women [29].
About mortality, we found an association between pre frail and frail states with an increased risk of death. This association remained even after adjusting for confounding variables such as age, sex, cognition score, and help in basic activities of daily living, and depression scale score. These results are consistent with findings from other studies that used the same definition used in the present study [13–15, 23] as well as the one used by Fried and colleagues, both in the Cardiovascular Health Study [4], as in others previously discussed [30–32]. The explanation for this, is that the vulnerability could be a marker of another underlying disorder, and is associated with social or environmental factors that may increase the risk of mortality [33].
Regarding the incidence of hospitalizations, the pre frail and frail states are associated with the incidence of hospitalizations. However, only the frail state remained significant after adjusting for confounding variables such as age, sex, score on cognition and function. These results are consistent with the association found in other studies [4, 34], unlike in the study of Fried et al. [4], an association for both pre frail and the frail states were found. These differences could be attributed to the different populations studied, since Caucasian and French populations were included in these studies, not to mention that the definition used in the present study differs from that used in them. A larger sample size would be required to find that association.
With regard to the incidence of dependence on at least one BADL or IADL, the frail, and pre frail status were significantly associated in the bivariate analysis, retaining their statistical significance after being adjusted for confounding variables. These results are consistent with the association found by other studies using the FRAIL instrument [14, 23], as well as through other instruments in different populations [4, 28, 29, 35].
In evaluating the association of frailty with the incidence of falls, we found that frailty, and pre frailty, are associated with higher incidence of falls, even after adjusting for covariates. Therefore, our results are consistent with those published by other authors in different populations, and operative definitions of frailty [4, 19, 20].
The present study has some limitations. First, the medical conditions of the study population and the activities of daily living are self-reports on the state of health, although several studies have found consistency in self-reports and direct measurements [36, 37]. A recent systematic review about variability in frailty measures, showed that it has an impact on reported prevalence, of frailty phenotype reported [22]. Second, the loss of subjects during follow-up, and analysis of complete cases may have influenced the study results, and produced selection bias [38]. It is well known that subjects who do not complete the performance measures in population studies, and not included in the present analysis, are expected to be less healthy, and more likely to die [39], increasing the possibility of survival bias. Third, an additional reason for our results, could also be that power was calculated only for mortality as an outcome. Therefore, a type II error is expected to be higher for the rest of the outcomes. Despite these limitations, this study has many strengths, including its large sample size of men and women living in the community (which makes it generalizable), its prospective design, the ability to evaluate multiple medical conditions and factors that previously reported an association with adverse events such as death, disability, falls and hospitalizations.
Conclusion
FRAIL instrument [3, 12–15, 23] constitutes a simple and inexpensive way to show the risk of dependence on at least one instrumental or basic activity of daily life, hospitalization, falls and mortality in Mexican elderly people at the community level. Therefore, the evaluation of frailty is recommended through this index as a screening tool in older adults, in order to identify subjects at risk of dependency, hospitalization, falls, death, and sent immediately by the primary care physician for a complete geriatric assessment to implement appropriate interventions in this geriatric syndrome.
Footnotes
Compliance with ethical standards
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent All the participants of the study gave their informed consent at the time of the interview.
References
- 1.Diaz de Leon Gonzalez E, Barragan Berlanga AJ, Gutierrez Hermosillo H, et al. Cognitive performance and mortality in people over 50 in Mexico. Rev Panam Salud Publica. 2010;27:368–375. doi: 10.1590/s1020-49892010000500007. [DOI] [PubMed] [Google Scholar]
- 2.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Abeysundera MJ, Mitnitski A. How should we grade frailty in nursing home patients? J Am Med Dir Assoc. 2007;8:595–603. doi: 10.1016/j.jamda.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Graham JE, Mogilner AJ, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Leon Diaz, Gonzalez E, Tamez Perez HE, et al. Frailty and its association with mortality, hospitalization and functional dependence in Mexicans aged 60-years or older. Med Clin (Barc) 2012;138:468–474. doi: 10.1016/j.medcli.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Garcia S, Sanchez-Arenas R, Garcia-Pena C, et al. Frailty among community-dwelling elderly Mexican people: prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatr Gerontol Int. 2014;14:395–402. doi: 10.1111/ggi.12114. [DOI] [PubMed] [Google Scholar]
- 9.Palloni A, Pinto-Aguirre G, Pelaez M. Demographic and health conditions of ageing in. Latin America and the Caribbean. Int J Epidemiol. 2002;31:762–771. doi: 10.1093/ije/31.4.762. [DOI] [PubMed] [Google Scholar]
- 10.Palloni A, McEniry M. Aging and health status of elderly in Latin America and the Caribbean: preliminary findings. J Cross Cult Gerontol. 2007;22:263–285. doi: 10.1007/s10823-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado BE, Zunzunegui MV, Beland F, et al. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63:1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- 12.Abellan van Kan G, Rolland YM, Morley JE, et al. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Hyde Z, Flicker L, Almeida OP, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010;95:3165–3172. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- 14.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez D, Flicker L, Dobson A. Validation of the frail scale in a cohort of older Australian women. J Am Geriatr Soc. 2012;60:171–173. doi: 10.1111/j.1532-5415.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 16.Kulminski AM, Ukraintseva SV, Kulminskaya, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, et al. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009;9:47. doi: 10.1186/1471-2318-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 22.Theou O, Cann L, Blodgett J, et al. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res Rev. 2015;21:78–94. doi: 10.1016/j.arr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Theou O, Brothers TD, Mitnitski A, et al. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 24.MHAS, Mexican Health and Aging Study [Feb 2014];Data files and documentation (public use): Mexican Health and Aging Study. 2014 http://www.MHASweb.org.
- 25.Mejia-Arango S, Miguel-Jaimes A, Villa A, et al. Cognitive impairment and associated factors in older adults in Mexico. Salud Publica Mex. 2007;49:S475–S481. doi: 10.1590/s0036-36342007001000006. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar-Navarro SG, Fuentes-Cantu A, Avila-Funes JA, et al. Validity and reliability of the screening questionnaire for geriatric depression used in the Mexican Health and Age Study. Salud Publica Mex. 2007;49:256–262. doi: 10.1590/s0036-36342007000400005. [DOI] [PubMed] [Google Scholar]
- 27.Haukoos JS, Newgard CD. Advanced statistics: missing data in clinical research—part 1: an introduction and conceptual framework. Acad Emerg Med. 2007;14:662–668. doi: 10.1197/j.aem.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Al Snih S, Graham JE, Ray LA, et al. Frailty and incidence of activities of daily living disability among older Mexican Americans. J Rehabil Med. 2009;41:892–897. doi: 10.2340/16501977-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 30.Graham JE, Snih SA, Berges IM, Ray LA, Markides KS, Ottenbacher KJ. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55:644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 32.Shamliyan T, Talley KM, Ramakrishnan R, et al. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Woo J, Goggins W, Sham A, et al. Social determinants of frailty. Gerontology. 2005;51:402–408. doi: 10.1159/000088705. [DOI] [PubMed] [Google Scholar]
- 34.Ãvila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the Three-City Study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyd CM, Xue QL, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 36.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 37.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Lazcano-Ponce E, Fernandez E, Salazar-Martinez E, et al. Cohort studies. Methodology, biases, and application. Salud Publica Mex. 2000;42:230–241. [PubMed] [Google Scholar]
- 39.Rockwood K, Jones D, Wang Y, et al. Failure to complete performance-based measures is associated with poor health status and an increased risk of death. Age Ageing. 2007;36:225–228. doi: 10.1093/ageing/afl160. [DOI] [PubMed] [Google Scholar]