Abstract
Background
A randomised controlled trial in Fiji examined the immunogenicity and impact on nasopharyngeal carriage following 0, 1, 2 or 3 doses of pneumococcal conjugate vaccine (PCV7) in infancy followed by 23-valent pneumococcal polysaccharide (23vPPV) vaccine at 12 months of age. At 18 months of age, children given 23vPPV exhibited immune hyporesponsiveness to a micro-23vPPV (20%) challenge dose in terms of serotype-specific IgG and opsonophagocytosis, while 23vPPV had no impact on vaccine-type carriage.
Objective
This follow-up study examined the long-term impact of the 12-month 23vPPV dose by evaluating the immune response to PCV13 administration 4-5 years later.
Methods
Blood samples from 194 children (now 5-7 years old) were taken before and 28-days after PCV13 booster immunisation. Nasopharyngeal swabs were taken before PCV13 immunisation. We measured serotype-specific IgG to all 13 vaccine serotypes, opsonophagocytosis (OPA) for 8 vaccine serotypes and memory B-cell responses for 18 serotypes pre- and post-PCV13 immunisation.
Results
Paired samples were obtained from 185 children. There were no significant differences in the serotype-specific IgG, OPA or memory B-cell response at either time-point between children who did or did not receive 23vPPV at 12 months of age. Nasopharyngeal carriage of PCV7 and 23vPPV serotypes were similar among the groups. Priming with 1, 2 or 3 PCV7 doses during infancy did not impact on serotype-specific immunity or carriage.
Conclusion
Immune hyporesponsiveness induced by 23vPPV in toddlers does not appear to be sustained among preschool children in this context and does not affect the pneumococcal carriage rate in this age group.
Keywords: Pneumococcal polysaccharide vaccine, 23vPPV, hyporesponsiveness, memory B cell, opsonophagocytosis, antibody
Introduction
Streptococcus pneumoniae (the pneumococcus) is a Gram positive bacterium that is responsible for a large burden of morbidity and mortality in children less than 5 years of age worldwide1. According to the latest WHO estimates, pneumococcal diseases such as pneumonia, meningitis and septicaemia cause 826,000 child deaths per year, mostly in developing countries2.
Protection against pneumococcal diseases is achieved principally through vaccination, with two types of vaccines currently licensed and implemented in many countries. Pneumococcal conjugate vaccines (PCVs), whereby the capsular polysaccharide from up to 13 serotypes is coupled to a protein carrier, have had a remarkable impact on vaccine-type disease in every setting they have been used3. At present, PCVs containing 10 or 13 common disease-causing serotypes3 are available. In comparison, the pneumococcal polysaccharide vaccine (23vPPV) is a plain polysaccharide vaccine representing the 23 pneumococcal serotypes that are responsible for more than 90% of disease in the USA4. 23vPPV however, is not recommended for use in children less than 2 years of age due to immaturity of their immune system to respond to polysaccharide antigens5. Instead, 23vPPV is administered to older children following a primary series of PCV in some settings, to the immunocompromised and to healthy adults from the age of 65 years. In Australia, Indigenous children have a higher rate of pneumococcal disease. Until recently, in addition to pneumococcal conjugate vaccine in infancy, these children also received 23vPPV as a booster vaccine at 18 months due to its broader serotype coverage6.
Vaccine efficacy of 23vPPV for the prevention of IPD in adults was found to be 74% in a Cochrane review7 based on studies that mainly used older generation vaccines but has been reported to be much lower in some studies8, while protection against pneumonia or death has been difficult to establish7. Early trials of 23vPPV in young children in Papua New Guinea demonstrated a 59% efficacy against acute lower respiratory infections (ALRI) and a significant reduction in mortality9. In the Fiji Pneumococcal Project (FiPP), we set out to evaluate the utility of 23vPPV as a booster vaccine in children primed with various schedules of PCV7. We examined the response to a 20% challenge dose of 23vPPV at 17 months of age (to mimic infection), in children previously primed with 0-3 doses of PCV7 and randomised to receive, or not receive, a full dose of 23vPPV at 12 months. This was designed to examine the immunological effects of 23vPPV at 12 months of age. While children less than 12 months of age may not be expected to respond to a polysaccharide vaccine, the children given 23vPPV at 12 months of age in this study had good booster antibody responses to the PCV7 types that were higher than children who did not receive 23vPPV. However, these 23vPPV-vaccinated children failed to boost these responses further when given a 20% 23vPPV challenge dose at 17 months of age while children who did not receive 23vPPV at 12-months of age produced higher antibody levels to all PCV7 and almost all non-PCV7 serotypes10. This has led to safety concerns for these children given the substantial burden of pneumococcal carriage and disease in this population.
We now report the findings of a long-term follow-up investigation of immune hyporesponsiveness in these children. We investigated whether the immune hyporesponsiveness observed at 18 months of age has had a deleterious effect on immune competence, nasopharyngeal carriage of pneumococci and associated clinical outcomes measured in these children at 5-7 years of age.
Methods
Study population and samples
The study design and details of the Fiji Pneumococcal Project (FiPP, 2003-2008) have been reported previously10-12. Briefly, healthy Fijian infants (N=552) were randomised to receive a primary series of 0, 1, 2 or 3 doses of the 7-valent pneumococcal conjugate vaccine (PCV7, Prevnar®, Pfizer Inc.), with half the children randomised to receive the 23-valent pneumococcal polysaccharide vaccine (23vPPV, Pneumovax®, Merck & Co., Inc., USA) at 12 months of age. Children given 0 or 1 PCV7 during the primary series were given a catch-up PCV7 dose at 2 years of age (Table I). Between March 2011 and February 2012, families who had participated in FiPP and who had previously agreed to be contacted for follow-up studies, were invited to participate in a follow-up study. Following consent, on the first visit, all children had 10 mL of blood drawn into a sodium heparin tube, and a nasopharyngeal (NP) swab was taken according to standard methods13. In addition, they were each administered a single dose of PCV13. A second blood sample was taken 28 days later. This study was approved by the Fiji National Research Ethics Review Committee and the Royal Children's Hospital Human Research Ethics Committee in Melbourne, Australia.
Table I.
Study Design and Participant Groups
| Group | PCV7 Primary Series | 23vPPV | Catch-up PCV7 dose* | Total PCV7 Doses |
|---|---|---|---|---|
| A | 3 × PCV7 | No | No | 3 |
| B | 3 × PCV7 | Yes | No | 3 |
| C | 2 × PCV7 | No | No | 2 |
| D | 2 × PCV7 | Yes | No | 2 |
| E | 1 × PCV7 | No | Yes | 2 |
| F | 1 × PCV7 | Yes | Yes | 2 |
| G | 0 × PCV7 | Yes | Yes | 1 |
| H | 0 × PCV7 | No | Yes | 1 |
PCV7=7-valent pneumococcal conjugate vaccine; 23vPPV=23-valent pneumococcal polysaccharide vaccine
Catch-up dose at 24 months of age for partially-vaccinated children in FiPP
Measurement of pneumococcal serotype-specific IgG levels by ELISA
Serotype-specific IgG levels to all 23 serotypes were measured using a previously published modified WHO ELISA developed in our laboratory14 (details in Supplemental material).
Opsonophagocytosis Assay
All opsonophagocytic assays (OPA) were undertaken in our laboratory based on previously published multiplexed methods (MOPA)15 (details in Supplemental material).
Enumeration of pneumococcal-specific memory B-cells
We undertook the measurement of pneumococcal-specific memory B-cells on site in Fiji using a previously established method16 (details in Supplemental material).
Measurement of nasopharyngeal carriage
Pneumococcal carriage was measured using standard culture-based methods similar to the original FiPP study12, 13 (details in Supplemental material).
Hospitalisation data
The Colonial War Memorial Hospital in Suva, Fiji uses a computerised system for recording admissions and discharges (PATIS). All discharges were entered into a nationwide linked database and a search for admissions performed by demographic identifiers or hospital number. In addition to asking parents whether their child has been hospitalised at the time of the first study visit, a thorough search of the computerised discharge system based on the name and date of birth from the commencement of the study up to the present time to identify all hospital admissions involving all children in the original study (N=552) was undertaken. Serious adverse events in children aged over 12 months, and by receipt or not of the 12-month 23vPPV was assessed. The laboratory results of any lumbar punctures or blood cultures taken were recorded.
Statistical analysis
Serotype-specific IgG and OPA titers were presented as geometric mean concentration (GMC) or geometric mean opsonisation index (GMOI) with 95% confidence intervals (CIs), respectively. Log-transformed data were used for statistical analyses of IgG and OPA data using two-tailed paired Student's t-tests. The median and interquartile (IQR) was considered for the memory B-cell responses, and the Mann-Whitney U test was used for analysis. Rates of NP carriage were calculated using the number of total pneumococcal, PCV7 and 23vPPV vaccine type (VT), or non-vaccine type (NVT) isolates in children in each group at each time point divided by the total number of children who had an NP swab taken in each group at each time point. Analyses comparing the proportions of children with NP carriage of any pneumococcus, VT pneumococci (PCV7 and 23vPPV), and NVT pneumococci were performed using logistic regression where an interaction between 23vPPV receipt and dose of PCV7 was considered. McNemar's test was used to assess changes in binary outcomes pre- and post-PCV13 vaccination. Linear regression models were used to adjust for the influence of PCV experience on the outcomes measured, and also to adjust for the influence of pre-PCV13 levels of measurements on the post-PCV13 responses. P-values are presented in 4 decimal places unless p<0.0001 or p>0.01, the latter of which was considered non-significant.
Results
Of the 552 children in the original FiPP study, the families of 437 gave permission to be contacted for this follow-up study. There were 194 families that were contactable for this follow-up study due to the fact that the population is mobile and contact details, including phone numbers, were no longer valid. However, the families of all 194 children, now aged 5-7 years old, provided written informed consent for this study. A NP swab, and paired blood specimens were collected from 185 children who formed the basis of this study. The demographics and breakdown of study participants are summarised in Table 2 and were similar to the original study. 98 (53%) children had received 23vPPV at 12 months, with each group having approximately equal numbers of children having received either 0, 1, 2 or 3 prior doses of PCV7 in infancy (Table II). During the follow-up period, there were 31 hospital admissions among children who had been enrolled in FiPP (Table III). There were seven children who potentially had pneumococcal related disease, although none were laboratory confirmed. No differences were found between the 23vPPV groups, with two cases in the 12-month 23vPPV group and five cases in the non-23vPPV group, and all cases were culture negative. A CSF was not taken on the child with meningitis and there was no growth on blood culture. In the 12 months immediately following 23vPPV, there was no difference in hospitalisations between the groups, suggesting no early impact of 23vPPV.
Table II.
Summary data for children (N=185†) in this follow-up study
| Characteristic | Number (%) |
|---|---|
| Sex | |
| Male | 106 (57.3%) |
| Female | 79 (42.7%) |
| Ethnicity | |
| Fijian (i-Taukei) | 135 (73.0%) |
| Indo-Fijian | 45 (24.3%) |
| Other | 5 (2.7%) |
| Received 23vPPV at 12 months of age | |
| Yes | 98 (53%) |
| No | 87 (47%) |
| Prior number of PCV7 doses | |
| 0 | 41 (22.2%) |
| 1 | 39 (21.1%) |
| 2 | 52 (28.1%) |
| 3 | 53 (28.6%) |
Number represents those with paired pre- and post-PCV13 samples (Actual number of participants is 194 (103 received 23vPPV; 91 did not receive 23vPPV)
Table III.
Hospitalisations during the follow-up period*
| Hospitalisation | Prior 23vPPV | No prior 23vPPV |
|---|---|---|
| Pneumonia | 2** | 3 |
| Meningitis | 0 | 1a |
| Staphylococcus aureus septic arthritis | 0 | 1 |
| Febrile convulsion | 1 | 1 |
| Acute gastroenteritis | 2 | 5 |
| Otherb | 7 | 8 |
| TOTAL | 12 | 19 |
hospitalisations reported during the study follow-up period: one year of age until March 2012
One Staphylococcus aureus blood culture positive
unknown aetiology
other includes: laceration, superficial abscesses, burns, kerosene ingestion, fractures, skin rash, foreign body inhalation, dehydration, jaundice, viral illness
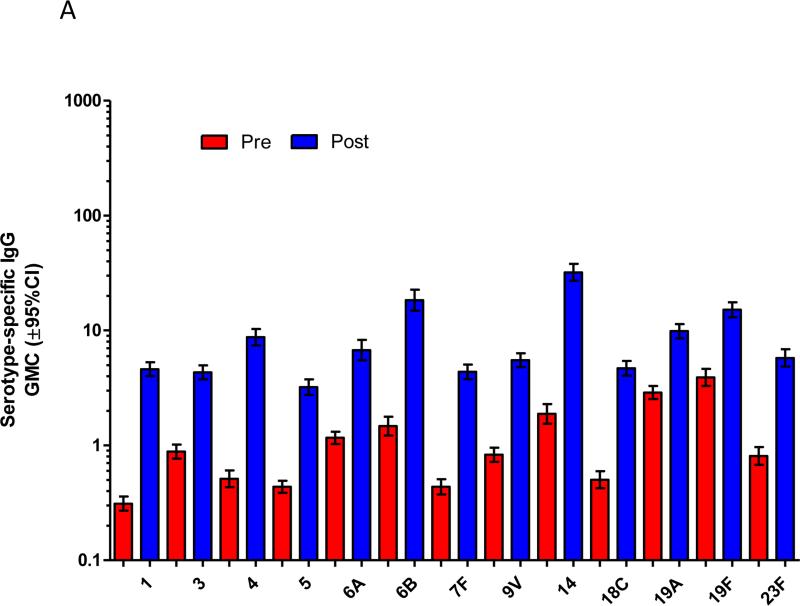
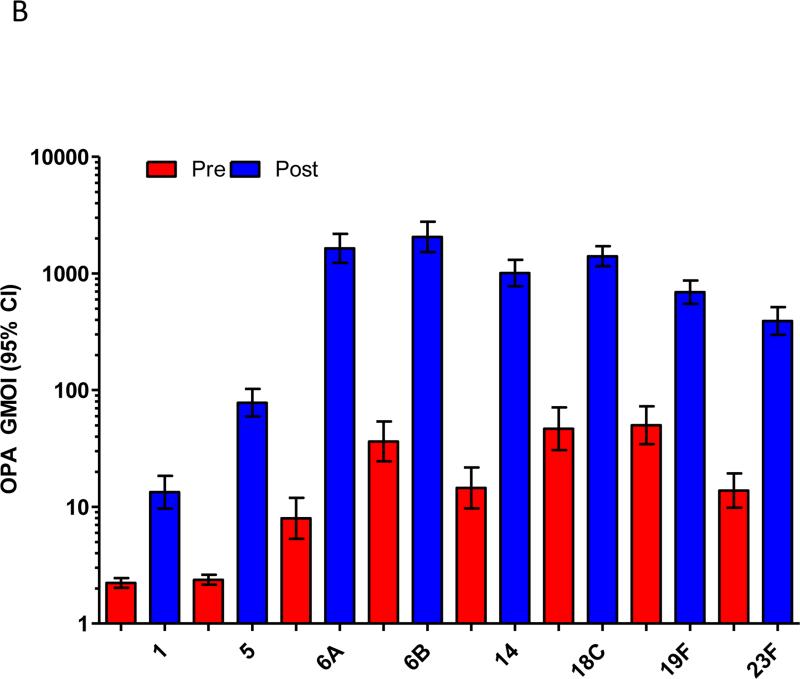
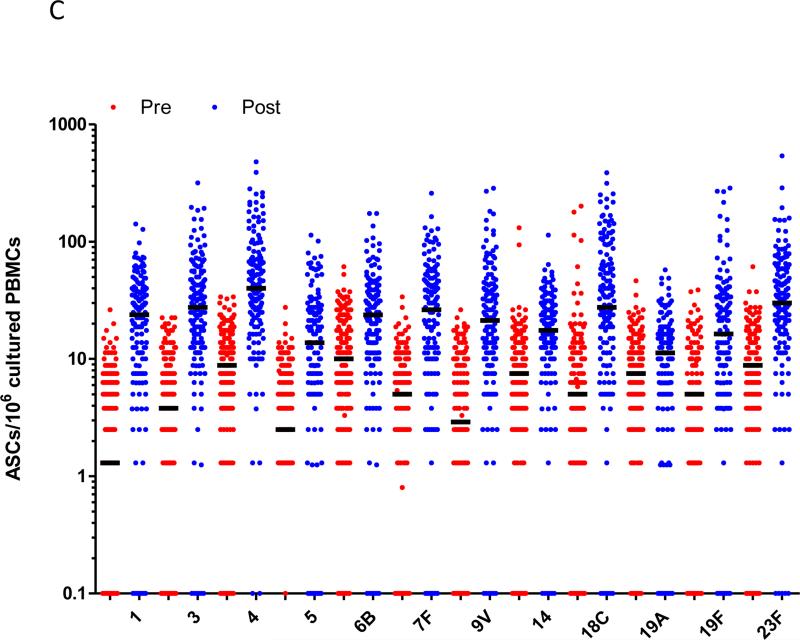
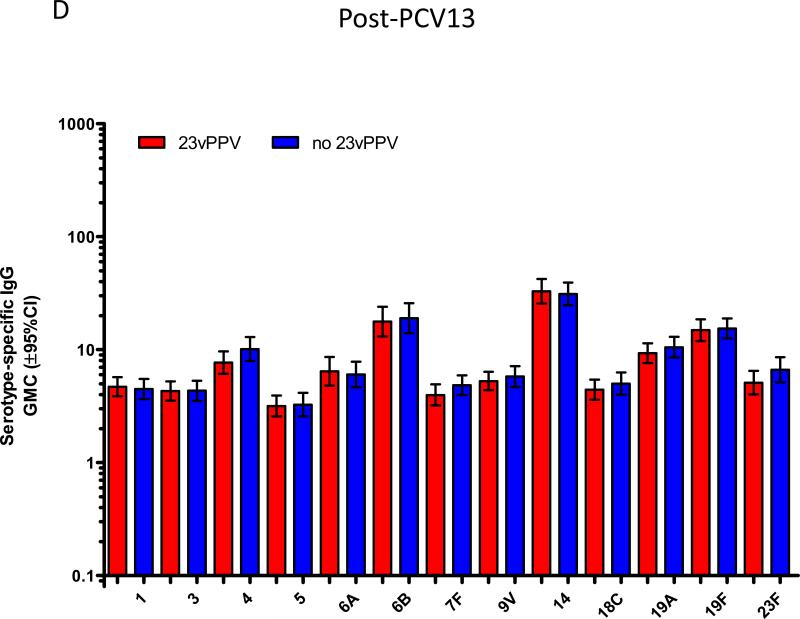
In the study children who were now aged 5-7 years old, immunisation with a single dose of PCV13 produced strong immune responses for all PCV13 vaccine serotypes compared to baseline (Figure 1). This was observed for serotype-specific IgG and OPA (p≤0.001 for all serotypes; Figures 1A and 1B). The mean fold rises in serotype-specific IgG ranged from 6.1 (serotype 19A) to 48.6 (serotype 4), while for OPA, the mean fold rise ranged from 33.6 (serotype 1) to 1,025 (serotype 6A). Significant rises in the proportion of children with serotype-specific IgG levels ≥0.35μg/ml and ≥1.0μg/ml and OI ≥8 were also observed for all serotypes (Supplemental Figure E1). Consistent with these findings, there was also a significant increase in the number of pneumococcal-specific memory B-cells post-immunisation for all PCV13 serotypes examined (p<0.001 for all serotypes; Figure 1C). Responses to non-vaccine serotypes were negligible, as expected, following PCV13 immunisation (Supplemental Tables EI-IV).
Figure 1.
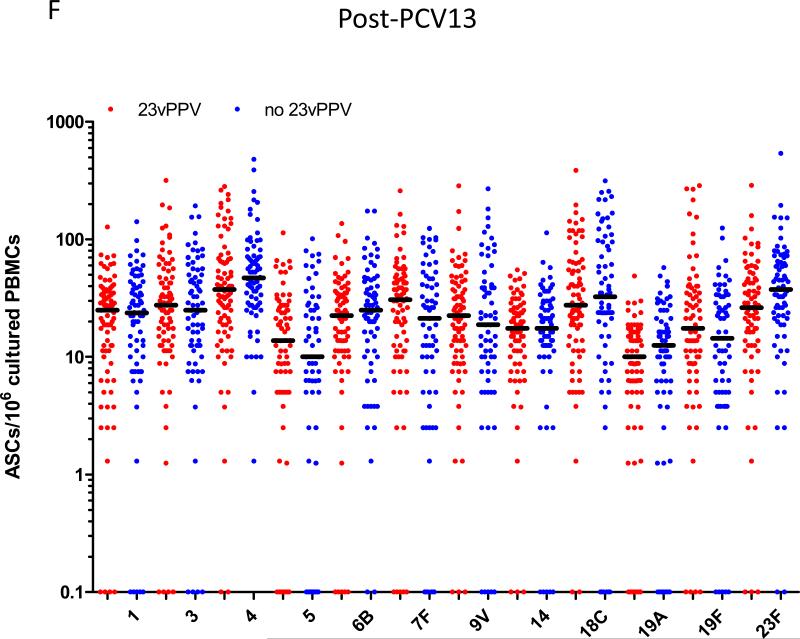
PCV13 induces a strong immune response in children previously vaccinated with 23vPPV at 12 months of age. Measurement of serotype-specific IgG levels (μg/mL; A), opsonophagocytic response (B) and the memory B-cell response (C) in children pre- and post-PCV13 (N=185 paired samples). ASC = antibody-secreting cell; ***p<0.0001 comparing pre and post-PCV13 response.
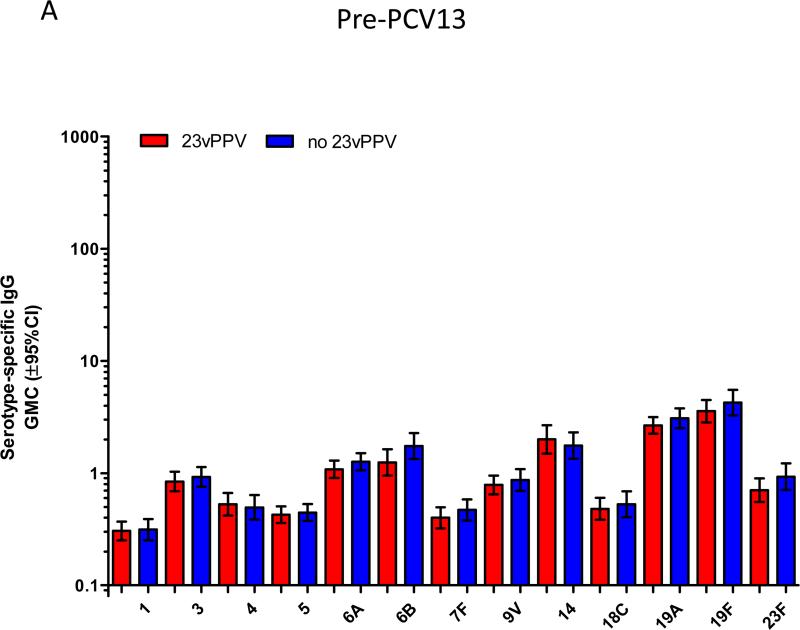
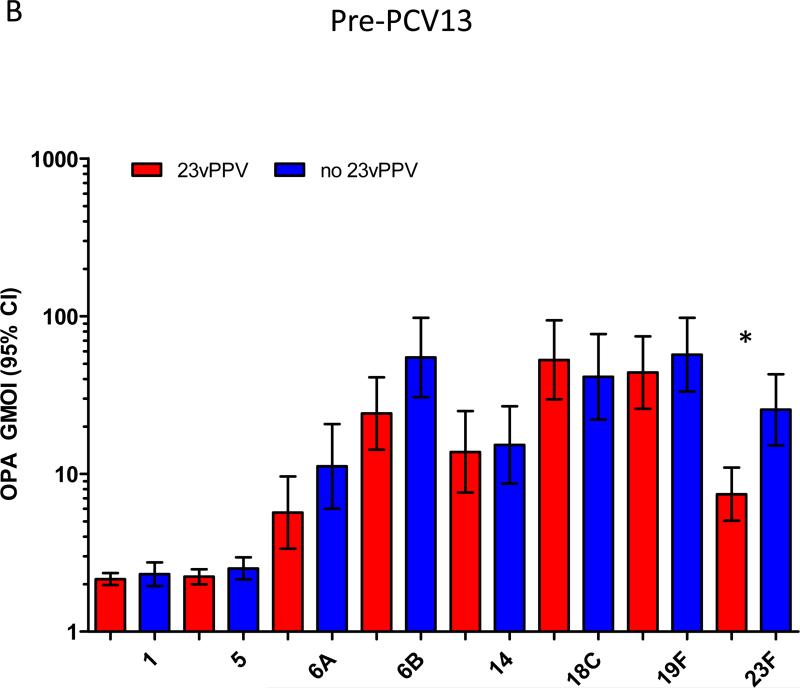
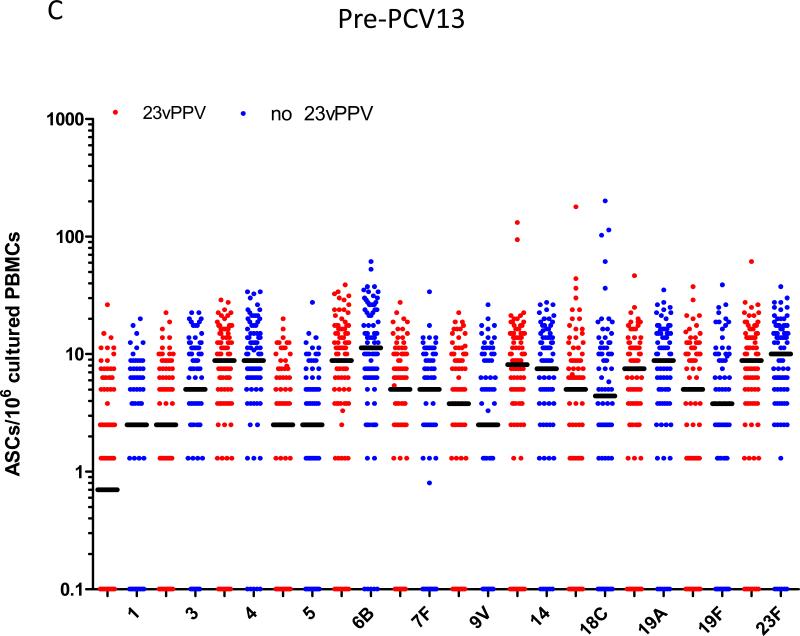
Figure 2A-C shows the pre-PCV13 immune response at 5-7 years of age comparing children that did or did not receive the 12-month 23vPPV dose. The pre-PCV13 levels of serotype-specific IgG and number of memory B-cells were similar between children who had or had not received 23vPPV for most PCV7 and non-PCV7 serotypes, indicating that the titers generated at 17 months of age by children who received the 23vPPV had waned by age 5 years (Supplemental Table E1). For OPA, responses were also similar in the two groups, except for 23F for which children who had received the 23vPPV had lower OPA titers (p<0.001; Figure 2B). There was also a trend towards lower IgG and B-cell responses for serotype 23F in the 23vPPV group. No differences were also observed in the proportions of children with IgG titres ≥0.35 or 1.0μg/ml or with an OI≥8 (Supplemental Figure E2 and Tables EIV-V).
Figure 2.
Administration of 23vPPV at 12 months of age did not lead to sustained hyporesponsiveness in children now aged 5-7 years old. Measurement of serotype-specific IgG levels (μg/mL), opsonophagocytic response, and the memory B-cell response in children that did (N=98) or did not (N=87) receive 23vPPV at 12-months of age pre- (Panels A-C) and post- (Panels D-F) PCV13 (N=185 paired samples). **p<0.001 comparing children who did or did not receive 23vPPV at 12 months of age.
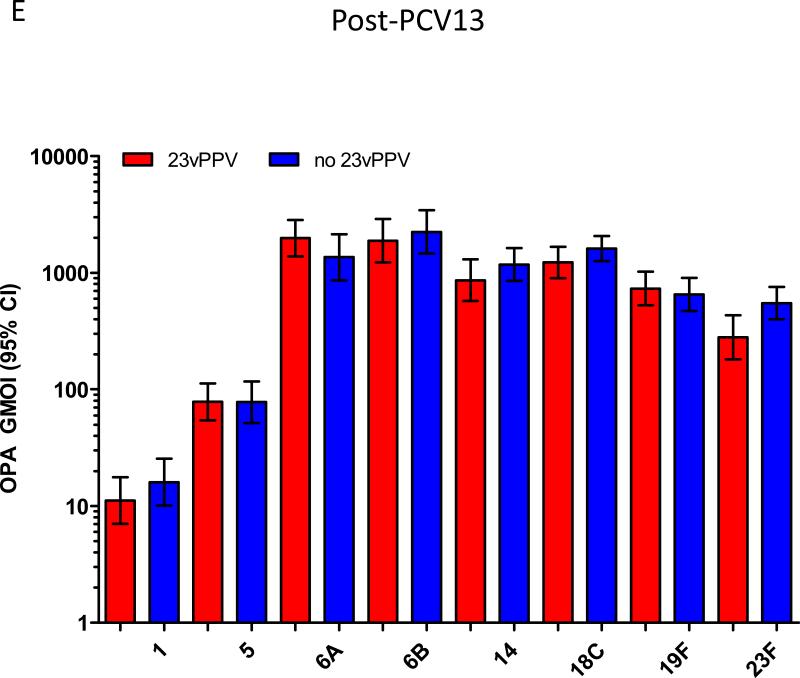
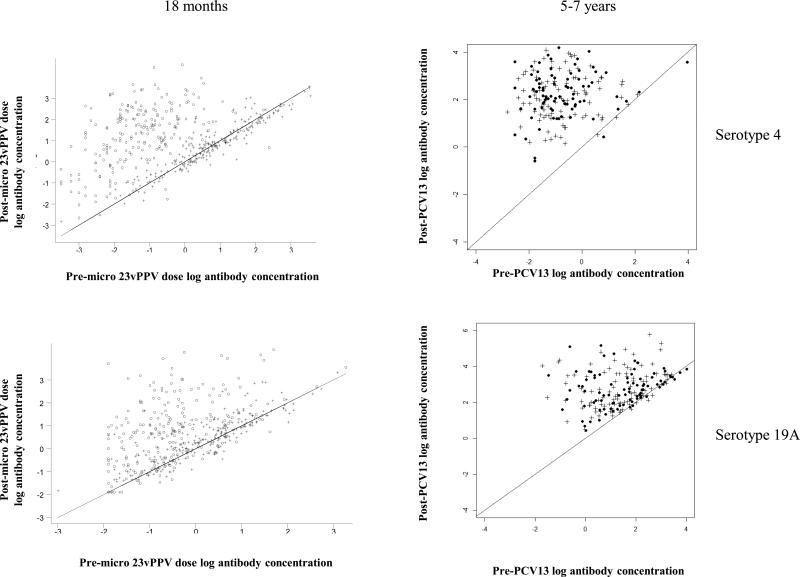
Following a dose of PCV13, children who had received 23vPPV at 12 months of age responded comparably with those children who had not received 23vPPV (Figure 2C-E). No significant differences in the serotype-specific IgG, OPA or memory B-cell levels were detected, even after adjustment for the number of prior PCV7 doses received and pre-PCV13 levels of pneumococcal immunity (Supplemental Figure E3). Figure 3 demonstrates that, for serotype-specific IgG, all children responded similarly to PCV13 serotypes (with adjustment for the pre-PCV13 IgG level and the effect of the catch-up PCV7 dose at 2 years of age), regardless of 23vPPV receipt at 12 months of age, compared to their responses at 18 months of age (representative data are shown for serotypes 4 and 19A; Supplemental Figure E4 for all other serotypes). Similarly, for non-PCV13 serotypes, no children responded to PCV13 immunisation (Supplemental Tables EI-IV).
Figure 3.
Children responded similarly to all PCV13 serotypes regardless of 23vPPV receipt. The change in serotype-specific IgG level (μg/mL) following PCV13 immunisation is shown for serotype 4 and 19A as representative of all serotypes. The diagonal line represents no change from pre-PCV13 (baseline) levels. The data at 18 months of age is from Russell et al.10 and at 5-7 years, the data represents the antibody response in children who did (cross) or did not (dark circles) receive the 23vPPV at 12 months of age.
Nasopharyngeal carriage was also assessed in these children (Table IV). Overall 45% (n=88) of children carried pneumococci, with 13 (15%) having a PCV7 type, 37 (42%) having a 23vPPV type (including PCV7 types), and 51 (58%) having non-vaccine types. Overall, 102 isolates were identified, with the most frequently isolated serotypes being 11A (9.7%), 6A (7.8%) and 16F (6.8%) with non-typeable pneumococci accounting for 12.6% of all carriage positive isolates. For children who received 23vPPV at 12 months of age, there was more carriage of PCV7 serotypes (9%) and 23vPPV serotypes (21%) compared with children who had not received 23vPPV (4% and 17%, respectively), while the overall pneumococcal carriage rates were similar across the two groups (40% vs 51%, respectively).
Table IV.
Nasopharyngeal carriage of S. pneumoniae (%)
| Group | Carriage of any pneumococci | Carriage of PCV7 serotypes | Carriage of 23vPPV serotypes |
|---|---|---|---|
| 3 PCV dose group (n=54) | |||
| 23vPPV+ (n=28) | 13 (46) | 3 (11) | 6 (21) |
| 23vPPV- (n=26) | 12 (46) | 0 (0) | 2 ( 8) |
| 2 PCV dose group (n=95) | |||
| 23vPPV+ (n=55) | 20 (36) | 4 (7) | 10 (18) |
| 23vPPV- (n=40) | 21 (53) | 2 (5) | 8 (20) |
| 1 PCV dose group (n=45) | |||
| 23vPPV+ (n=19) | 8 (42) | 2 (11) | 5 (26) |
| 23vPPV- (n=26) | 14 (54) | 2 (8) | 6 (23) |
| Total carriage (n=194) | |||
| 23vPPV+ (n=102) | 41 (40) | 9 (9) | 21 (21) |
| 23vPPV- (n=92) | 47 (51) | 4 (4) | 16 (17) |
23vPPV+ = children who received 23vPPV at 12 months of age
23vPPV- = children who did not receive 23vPPV at 12 months of age
Discussion
This is the first study to comprehensively characterise the long-term effects of immune hyporesponsiveness following 23vPPV immunisation in children at high risk of pneumococcal disease. In a vaccine trial in which 552 Fijian infants were vaccinated with various dose schedules of PCV7, and half were boosted at 12 months with 23vPPV, we demonstrated that, at 18 months of age, 23vPPV recipients were non-responsive to a small 20% dose of 23vPPV, while children who had not received 23vPPV had good responses. In the present study, 194 of the 552 children enrolled in the original trial were followed up 4-5 years later. While pneumococcal carriage rates in the present study were similar in the groups who had, or had not received 23vPPV at 12 months (41/102 versus 47/92 in 23vPPV+ and 23vPPV− groups respectively), carriage of PCV7 serotypes was slightly higher in those who had received 23vPPV, and had a subsequent very strong response to the PCV711. We undertook a detailed immunological investigation of these children including assessing pneumococcal antibodies to PCV13 serotypes (by ELISA and OPA) and pneumococcal B-cell responses, before and after a dose of PCV13. No differences between the groups were seen in any of the immunological measurements before or after PCV13 and responses to PCV13 were normal. Although unknown at the time, the similar response to PCV13 immunisation in infants who did or did not receive 23vPPV at 12 months of age was not surprising given the similar pre-PCV13 levels. Moreover, PCV13 was given to ensure all children were able to respond equally well to vaccination and that the hyporesponsiveness observed at 18 months did not persist. There was no evidence of residual hyporesponsiveness in the 23vPPV-vaccinated children. We also reviewed hospital records for all children in the original study and found no difference in hospitalisations related to pneumococcal disease, although this study was not sufficiently powered for this analysis.
Vaccine-induced hyporesponsiveness was first reported for meningococcal polysaccharide-based vaccines, where prior immunisation resulted in reduced antibody titres for both children and adults17, 18. For pneumococcal polysaccharide vaccines, studies to detect hyporesponsiveness in adults receiving repeated doses of 23vPPV have produced variable results19-22, while making results difficult to interpret due to issues of possible bias from the non-randomised study design of a number of these 23vPPV trials. Recent studies have shown that 23vPPV-induced hyporesponsiveness in adults and children was not completely restored by additional doses of PCV723 or PCV1324, suggesting a sustained impact of 23vPPV vaccination. The FiPP study was the first to demonstrate true hyporesponsiveness in infants following the use of 23vPPV. At that time, Australian policy recommended that Indigenous children (who are at high risk of pneumococcal disease) who had received PCV in infancy should be boosted with 23vPPV at 18 months of age to provide broader serotype coverage, although only 57% of eligible children were receiving this booster dose25. An observational study found that children who had received 23vPPV were at higher risk of pneumonia than children who had not, even when controlled for potential confounders26. Following that study, and the Fiji study demonstrating hyporesponsiveness, Australian policy was changed, replacing 23vPPV with a fourth dose of PCV for Indigenous children. Other countries considering a 23vPPV booster policy for children did not proceed.
Immune hyporesponsiveness is believed to be due to memory B-cell depletion18, 27. Immunisation with polysaccharide-based vaccines does not induce memory B-cell formation but triggers the activation and recruitment of the memory B-cell compartment to differentiate into antibody-secreting plasma cells in the circulation and secondary lymphoid tissue. Adults immunised with 23vPPV had a reduced memory B-cell frequency associated with decreased B1b-cells, a critical subset known to protect mice against pneumococcal infection28. Similarly, reduced numbers of murine splenic and bone marrow-derived memory B-cells were observed following a pneumococcal polysaccharide booster29 while for capsular group C meningococcal polysaccharide vaccine, hyporesponsiveness was associated with increased apoptosis of polysaccharide-specific B-cells30. Importantly, despite this effect of 23vPPV, while there has been no direct evidence for any clinical impact of hyporesponsiveness in a large scale setting, a recent review concluded that 23vPPV-booster vaccination should be discouraged in line with WHO recommendations31.
In our study, we found that there was no immunological evidence of continuing hyporesponsiveness to PCV13 serotypes 5 years later in children who were demonstrated to be hyporesponsive following 23vPPV vaccination. However, there may still be ongoing hyporesponsiveness to unconjugated serotypes, although we believe this is unlikely. It is likely that the phenomenon is transient, despite the continual exposure of Fijian children to pneumococcal polysaccharide due to the high natural carriage rate. Clearly, carriage does not induce or maintain hyporesponsiveness, probably because it does not present pure polysaccharide to the immune system, but rather presents polysaccharide along with pneumococcal proteins, in a manner similar to conjugate vaccines. However, in a highly vaccinated population with strong herd immunity and no circulating serotypes, it could be speculated that the hyporesponsiveness due to 23vPPV may persist for a longer period.
To undertake this study, we implemented WHO-recommended pneumococcal immunological assays (ELISA and OPA) as well as augmenting these methods with a novel B-cell assay. This assay was based on a similar assay developed for meningococcal antigens, but was modified to evaluate numbers of memory B-cells recognising pneumococcal serotypes. We were able to demonstrate that this assay identifies immunological responses following pneumococcal immunisation in a manner similar to the standard assays. We also believe that it will prove to be a more sensitive marker of long term immunity, enabling a more precise evaluation of the long term impact of various pneumococcal vaccination strategies. The data demonstrating that 23vPPV-vaccinated children have returned to normal immune responses to pneumococcal antigens is compelling and consistent across all serotypes measured and assays used. The carriage data suggests that 23vPPV-vaccinated children may have more susceptibility to carriage of PCV7 vaccine serotypes, although our small sample size makes this result difficult to draw conclusions. We have previously shown that the 23vPPV dose at 12 months stimulated very strong antibody responses in PCV7-immunised children11, but that these responses were not associated with any measurable impact on carriage12. It is possible that hyporesponsiveness to these serotypes was more prolonged, and although immunological measures had returned to normal, subtle changes may have persisted leading to increased susceptibility to carriage. We also did not identify any increased susceptibility to disease in 23vPPV-vaccinated children. Unfortunately we were only able to identify less than half of the children for the follow-up study, in part because many did not agree to be recontacted, and in part due to movement to other areas of Fiji. It is possible that we missed important disease events or even deaths, but Fiji has an excellent health service and very low child mortality, so we believe this is very unlikely.
Taken together, our findings have important implications for vaccine policy. The lack of sustained hyporesponsiveness in this population some 5-7 years post-immunisation is reassuring given the serious safety concerns that the 12-month 23vPPV dose raised. However, caution must be applied to the use of 23vPPV vaccines given the demonstrated hyporesponsiveness that occurred within 6 months of vaccination in these children, at an age when the risk of pneumococcal disease is greatest. The fact that the Australian government has since replaced the use of 23vPPV with PCV13 as a booster vaccine for all Indigenous children and Fiji have introduced a 3+0 PCV10 schedule into their national childhood immunisation program in 2012 reinforces the importance of rigorous vaccine programmes at a population level to reduce pneumococcal disease burden.
Conclusion
Our work has shown that, while 23vPPV boosting of Fijian children previously vaccinated with PCV7 vaccine resulted in immune hyporesponsiveness, we were not able to identify any evidence of clinical consequences for this, and by 5 years, immune responses to PCV13 vaccine were normal. While our work does not support the use of 23vPPV in children, we have also shown that in those settings where natural boosting due to circulating serotypes is high, any negative immunological effects on vaccinated children are transient which may be different in a highly vaccinated population.
Supplementary Material
Clinical Implications.
While this study found no long-term evidence of 23vPPV-induced hyporesponsiveness in children, continued use of 23vPPV in early childhood is not recommended based on the potential increase in disease susceptibility following 23vPPV use.
Acknowledgements
We would like to thank all the study staff and families of the children who participated in this study. We thank the study nurses Tupou Ratu and Meredani Gunavailu for the invaluable support on this project. Our sincere thanks also to Dr Mike Kama and Dr Eric Rafai for their help and use of the facilities at Mataika House. We also acknowledge and thank Bereket Mulholland for his assistance with the PATIS database searches. Thanks also to Dr David Klein and Dr Farukh Khambaty for their advice and assistance in establishing the FiPP and FiPP follow-up studies. PVL is a recipient of an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship. YBC was supported by the National Research Foundation, Singapore, under its Clinician Scientist Award (Award No. NMRC/CSA/039/2011) administered by the Singapore Ministry of Health's National Medical Research Council. EC was supported by the NIHR Oxford Biomedical Research Centre. AJP is a Jenner Institute Investigator and a James Martin Senior Fellow. The study was funded by NIAID Grant 1R01AI085198-01A1. We also acknowledge the support of the Victorian Government's Operational Infrastructure Support Program.
Funding Statement
The funders had no role in the study design, the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Abbreviations
- 23vPPV
23-valent pneumococcal polysaccharide vaccine (Pneumovax®)
- PCV
Pneumococcal Conjugate Vaccine
- CFU
Colony-forming unit
- CI
Confidence Interval
- CPS
Cell wall polysaccharide
- ELISA
Enzyme linked immunosorbent assay
- FCS
Foetal Calf Serum
- FiPP
Fiji Pneumococcal Project
- GMC
Geometric Mean Concentration
- IPD
Invasive pneumococcal disease
- IQR
Interquartile range
- MOPA
Multiplexed opsonophagocytic assay
- NP
Nasopharyngeal
- NVT
Non-vaccine type carriage
- OD
Optical density
- OI
Opsonization index
- OPA
Opsonophagocytic assay
- PBS
Phosphate buffered saline
- VT
Vaccine-type
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors statement
PVL undertook most of the experiments, was responsible for data collection and contributed to data analysis and interpretation as well as drafted the manuscript, ZQT undertook the experiments and contributed to the manuscript, EAC performed the memory B-cell assays and contributed to the manuscript, AB undertook the ELISAs and contributed to the manuscript, RM and LT undertook the OPAs and contributed to the manuscript, KEL undertook the statistical analysis and contributed to the manuscript, KB was the study co-ordinator and was involved in analysis of clinical data, UR assisted with the laboratory work in Fiji, LT was the Fiji study paediatrician, LKB undertook the NP carriage analysis and contributed to the manuscript, EMD assisted with the NP carriage work and contributed to the manuscript; CS oversaw the NP carriage work and contributed to the manuscript, YBC undertook the statistical analysis and contributed to the manuscript, AJP provided expertise in the memory B-cell assays and analysis and contributed to the manuscript, FMR undertook the clinical analysis and contributed to the manuscript, EKM designed the study, was involved in data analysis and interpretation and contributed to the manuscript.
Declaration of interests
AJP reports grants from Pfizer, grants from GSK, outside the submitted work; and chair of the UK Department of Health's (DH) Joint Committee on Vaccination and Immunisation (JCVI). The views expressed in this manuscript do not necessarily reflect the views of DH or JCVI. EKM reports other from Merck & Co, from null, outside the submitted work. All other authors declare they have no conflicts of interest.
References
- 1.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Infect. 2014;69:309–25. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 5.Publication WHO. Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine. 2012;30:4717–8. doi: 10.1016/j.vaccine.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 6.Moberley S, Krause V, Cook H, Mulholland K, Carapetis J, Torzillo P, et al. Failure to vaccinate or failure of vaccine? Effectiveness of the 23-valent pneumococcal polysaccharide vaccine program in Indigenous adults in the Northern Territory of Australia. Vaccine. 2010;28:2296–301. doi: 10.1016/j.vaccine.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 7.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–8. doi: 10.1016/j.vaccine.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Riley ID, Lehmann D, Alpers MP, Marshall TF, Gratten H, Smith D. Pneumococcal vaccine prevents death from acute lower-respiratory-tract infections in Papua New Guinean children. Lancet. 1986;2:877–81. doi: 10.1016/s0140-6736(86)90409-5. [DOI] [PubMed] [Google Scholar]
- 10.Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine. 2010;28:3341–9. doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell FM, Licciardi PV, Balloch A, Biaukula V, Tikoduadua L, Carapetis JR, et al. Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy. Vaccine. 2010;28:3086–94. doi: 10.1016/j.vaccine.2010.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell FM, Carapetis JR, Satzke C, Tikoduadua L, Waqatakirewa L, Chandra R, et al. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clin Vaccine Immunol. 2010;17:1970–6. doi: 10.1128/CVI.00117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32:165–79. doi: 10.1016/j.vaccine.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Balloch A, Licciardi PV, Leach A, Nurkka A, Tang ML. Results from an inter-laboratory comparison of pneumococcal serotype-specific IgG measurement and critical parameters that affect assay performance. Vaccine. 2010;28:1333–40. doi: 10.1016/j.vaccine.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clutterbuck EA, Oh S, Hamaluba M, Westcar S, Beverley PC, Pollard AJ. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2008;15:182–93. doi: 10.1128/CVI.00336-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow R, Joseph H, Andrews N, Acuna M, Longworth E, Martin S, et al. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine. 2000;19:1129–32. doi: 10.1016/s0264-410x(00)00317-0. [DOI] [PubMed] [Google Scholar]
- 18.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- 19.Mufson MA, Hughey DF, Turner CE, Schiffman G. Revaccination with pneumococcal vaccine of elderly persons 6 years after primary vaccination. Vaccine. 1991;9:403–7. doi: 10.1016/0264-410x(91)90126-q. [DOI] [PubMed] [Google Scholar]
- 20.Manoff SB, Liss C, Caulfield MJ, Marchese RD, Silber J, Boslego J, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J Infect Dis. 2010;201:525–33. doi: 10.1086/651131. [DOI] [PubMed] [Google Scholar]
- 21.Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin. 2011;7:919–28. doi: 10.4161/hv.7.9.15996. [DOI] [PubMed] [Google Scholar]
- 22.Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–602. doi: 10.1016/j.vaccine.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis. 2011;52:736–42. doi: 10.1093/cid/cir003. [DOI] [PubMed] [Google Scholar]
- 24.Sigurdardottir ST, Center KJ, Davidsdottir K, Arason VA, Hjalmarsson B, Elisdottir R, et al. Decreased immune response to pneumococcal conjugate vaccine after 23-valent pneumococcal polysaccharide vaccine in children. Vaccine. 2014;32:417–24. doi: 10.1016/j.vaccine.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Hull B, Dey A, Menzies R, McIntyre P. Annual immunisation coverage report, 2010. Commun Dis Intell Q Rep. 2013;37:E21–39. doi: 10.33321/cdi.2013.37.2. [DOI] [PubMed] [Google Scholar]
- 26.O'Grady KA, Lee KJ, Carlin JB, Torzillo PJ, Chang AB, Mulholland EK, et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian indigenous infants 5-23 months of age following pneumococcal vaccination: a cohort study. Clin Infect Dis. 2010;50:970–8. doi: 10.1086/651079. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? The Lancet infectious diseases. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 28.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–16. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjarnarson SP, Benonisson H, Del Giudice G, Jonsdottir I. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS One. 2013;8:e72588. doi: 10.1371/journal.pone.0072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis. 2012;205:422–30. doi: 10.1093/infdis/jir750. [DOI] [PubMed] [Google Scholar]
- 31.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10:307–22. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.