Abstract
Auditory neuropathy spectrum disorder (ANSD) is a type of hearing disorder which is challenging for assessment and rehabilitation. This disorder has been studied over a decade and prevalence of the disorder is variable. The study aimed at estimating the prevalence and audiological characteristics of ANSD in children. A retrospective study was conducted from the medical records of pediatric patients evaluated at Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai to estimate the prevalence of ANSD. Medical records of 2,624 children evaluated during the period of November 2010 to October 2012 within the age range of 6 months to 12 years were analyzed. Out of 2,624 pediatric population assessed 217 (8.26 %) of them had unilateral or bilateral sensory neural hearing loss with varying degrees. Out of 217 children with sensory neural hearing loss 5.06 % (N = 11) had ANSD. Audiological characteristics varied among the group. Children with ANSD had varied degree of hearing thresholds from normal to profound hearing impairment. All of them had ‘A’ type tympanogram with absent stapedial reflexes. DPOAEs or TEOAEs were observed in 54 % of population with ANSD. All of them had abnormal auditory brainstem responses (ABR). Replicable cochlear microphonics was observed in 46 % of children with ANSD. These results indicate that ANSD is not a rare condition among children and we emphasize the use of objective tests like tympanometry, Stapedial Reflex test, otoacoustic emissions and ABR in routine hearing assessment procedure for all children to identify ANSD.
Keywords: Otoacoustic emissions, Sensory neural hearing loss, Auditory brainstem responses, Stapedial reflexes, Cochlear microphonics
Introduction
Auditory neuropathy spectrum disorder (ANSD) is a condition in which a patient’s otoacoustic emissions (OAE) or cochlear microphonics (CM) are present, and auditory brain-stem responses (ABR) are abnormal or absent [1]. In individuals with ANSD, pure-tone audiogram may range anywhere from essentially normal hearing sensitivity to a profound hearing loss [1, 5, 10]. Perception of speech in ANSD is reported to be varying and speech recognition scores are impaired in the presence of noise [6, 11]. The combination of normal OAE responses and severely impaired ABR responses is thought to reflect normal outer hair cell function in the cochlea and abnormal auditory nerve function. The site of lesion for ANSD is often unknown, but possibilities include cochlear inner hair cells, cochlear spiral ganglia, and the auditory nerve [1]. Multiple risk factors like Hyperbilirubinemia, Anoxia/Hypoxia, Prenatal/neonatal infections, Immune disorders, Genetic and syndromal disorders [4] have been described in the literature for ANSD.
Various studies have been conducted to estimate the prevalence of ANSD in children through universal newborn screening programs at hospitals, institutes, clinical and research centers. Most of these studies have used a test battery approach including pure tone Audiometry, Speech Audiometry, Immittance Audiometry, OAE (transient and Distortion product) and ABR [1, 13]. In addition, some researchers have also used Electrocochleography in test protocol for estimating the prevalence of ANSD [14].
In the literature the prevalence of ANSD varies across the population being studied. In neonates with high risk for hearing loss the prevalence of ANSD is reported to be 40 %, especially in neonatal intensive care unit babies [12]. The prevalence rate of ANSD in infants is very high when compared deaf school children. The prevalence rate of ANSD in deaf school children is 2.46 % [7] within the age range of 6–12 years. Studies who considered infants with profound hearing loss also have estimated the prevalence of ANSD to be high as 5.26 % [13], 8.44 % [3], 13.4 % [14] and 19 % [8].
There are only few studies on prevalence of ANSD in India. A register-based study to estimate the prevalence of auditory dys-synchrony has been conducted in Mysore [6]. Results have shown that the prevalence of auditory dys-synchrony was around 1 in 183 individuals with sensory neural hearing loss which includes children, adult and geriatric population. Recently study carried out by Mittal et al. [9] have assessed 487 children and reported 183 cases with sensory neural hearing loss and estimated the prevalence of ANSD as 5.3 % (26 cases) of the total population studied at tertiary care hospital, New Delhi. Since there is limited number of studies and varying data available regarding prevalence of ANSD in India across various regions, this study aims at estimating the prevalence and audiological characteristics of ANSD in Children at Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai, Tamil Nadu.
Method
A retrospective study was conducted from the medical records of pediatric patients evaluated at Upgraded Institute of Otorhinolaryngology—Institute for the Rehabilitation of the Speech and Hearing Handicapped, Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai to estimate the prevalence of ANSD and its audiological characteristics. Medical records of 2,624 children (Male = 1,840, Female = 784) assessed during the period of November 2010 to October 2012 within the age range of 6 months to 12 years (mean age of 3.2 years) were analyzed. All of them were infants and children with high risk of hearing loss and with the complaint of congenital or acquired deafness. Medical records of all the children consisted of detailed case history including demographic data, gender, age, family history, developmental and audiological history, pediatric, neurological, and ENT examination and comprehensive hearing, speech, language and communication assessment.
Hearing sensitivity was assessed for all the children using Audiological test battery by a qualified Audiologist. Audiological test battery used for identifying hearing loss included Behavioral observation audiometry, Free field audiometry, Play conditioned audiometry, and Objective tests using Impedance audiometry, OAE testing and ABR testing. MA-52 Dual Channel Digital audiometer (Maico Diagnostics GmbH, Germany) was used to assess approximate behavioral pure tone thresholds using Speakers or earphones, Impedance Audiometry testing using Maico MI 34 instrument (Maico Diagnostics GmbH, Germany), DPOAE, TEOAE and ABR testing using IHS smart EP systems (Intelligent Hearing systems, U.S.A).
Free Field Audiometry was used for the age group of 6 months to 3 years, Play conditioned Audiometry was carried out for the age group of 3–6 years, and conventional audiometry procedures were performed for children above age group of 6 years. Tympanometry was carried out using 226 Hz probe tone and ipsilateral and contralateral reflexes were recorded at 500, 1000, 2000 and 4000 Hz. TEOAE’s were recorded using click stimuli with a stimulus intensity of 85 dB SPL. The presence of transient OAE were considered normal if response was 3 dB higher than the background noise and its reproducibility was greater than 75 % in at least three tested frequencies. DPOAE’s were recorded using varying F1 and F2 frequencies short duration pure tone stimuli and considered as normal when the signal to noise ratio was greater than 6 dB at three frequencies. ABR were recorded using click stimuli at 90 dBnHL at 11.1/s or 30.1/s repetition rate with the analysis time of 12 ms. Intensities were varied in 10 dB steps to estimate threshold if responses were observed. If CM were observed in ABR, change in polarity of the stimuli was also made to observe change in CM.
The child is diagnosed as ANSD if the child meets the diagnostic criteria with normal tympanogram, absence of stapedial reflexes, normal DPOAE or TEOAE and abnormal ABR.
Results
Out of 2,624 pediatric population assessed 217 (8.26 %) of them had unilateral or bilateral sensory neural hearing loss with varying degrees. Prevalence of ANSD estimated from the total population tested was 0.42 % (N = 11). Out of 217 children with sensory neural hearing loss 5.06 % (N = 11) had ANSD. Table 1 shows the number of infants identified with sensorineural hearing loss classified with respect to their gender and unilateral or bilateral hearing loss. Out of 217 children with sensory neural hearing loss 3.2 % (N = 7) of children had unilateral sensory neural hearing loss and 96.8 % (N = 210) of them had bilateral sensory neural hearing loss.
Table 1.
Number of children identified with sensory neural hearing loss from total population
| Total number of children assessed | Number of children with sensory neural hearing loss | ||||
|---|---|---|---|---|---|
| Unilateral hearing loss | Bilateral hearing loss | ||||
| Male | Female | Male | Female | Male | Female |
| 1,840 | 784 | 5 | 2 | 121 | 89 |
Out of 217 children with sensory neural hearing loss 5.06 % (N = 11) of them met the diagnostic criteria of ANSD. Interestingly there were no children who had unilateral ANSD. All of them identified were children with bilateral ANSD. Table 2 shows the number of children identified with ANSD with respect to their gender.
Table 2.
Number of Children identified with ANSD
| Number of children with auditory neuropathy spectrum disorder | |||
|---|---|---|---|
| Unilateral hearing loss | Bilateral hearing loss | ||
| Male | Female | Male | Female |
| – | – | 7 | 4 |
The audiological characteristics of children with ANSD varied within the group. Table 3 shows the audiological characteristics in each individual child with ANSD identified in the present study.
Table 3.
Audiological findings in each child with ANSD
| S.No. | Age/sex | Audiological characteristics | |||||
|---|---|---|---|---|---|---|---|
| Degree of hearing loss | Tympanogram | Reflex | DPOAE/TEOAE | ABR | CM | ||
| 1 | 1 year 8 months/male | Moderate SNHL | A | Absent | Present | Absent | Absent |
| 2 | 2 years/male | Profound SNHL | A | Absent | present | Absent | Absent |
| 3 | 7 months/male | Normal Hearing | A | Absent | Present | Absent | Absent |
| 4 | 1 year 6 months/female | Profound SNHL | A | Absent | present | Absent | Absent |
| 5 | 2 years 6 months/male | Profound SNHL | A | Absent | Absent | Prolonged wave V | present |
| 6 | 5 years/female | Profound SNHL | A | Absent | present | Absent | Absent |
| 7 | 7 years/male | Moderate SNHL | A | Absent | Present | Absent | Absent |
| 8 | 3 years/male | Profound SNHL | A | Absent | Absent | Absent | present |
| 9 | 3 years 6 months/male | Moderate SNHL | A | Absent | Absent | Absent | Present |
| 10 | 11 months/male | Profound SNHL | A | Absent | Absent | Absent | present |
| 11 | 8 months/male | Normal hearing | A | Absent | Absent | Prolonged wave V | Present |
DPOAE distortion product otoacoustic emissions, TEOAE transient evoked otoacoustic emissions, ABR auditory brainstem responses, CM cochlear microphonics, SNHL sensory neural hearing loss
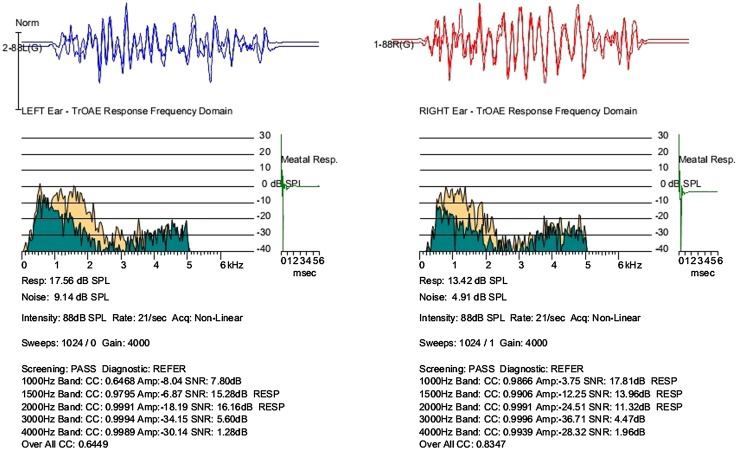
Children with ANSD had variable degrees of hearing thresholds ranging from normal to profound hearing impairment. 82 % of them had significant hearing loss and 18 % had normal hearing sensitivity. All of them had ‘A’ type tympanogram with absent stapedial reflexes. DPOAE or TEOAE observed were greater than 6 dB signal to noise ratio in 54 % of population with ANSD. Figure 1 shows TEOAE response recorded from child 2 with ANSD showing high amplitude of otoacoustic emission.
Fig. 1.
TEOAE responses of Child 2 with ANSD
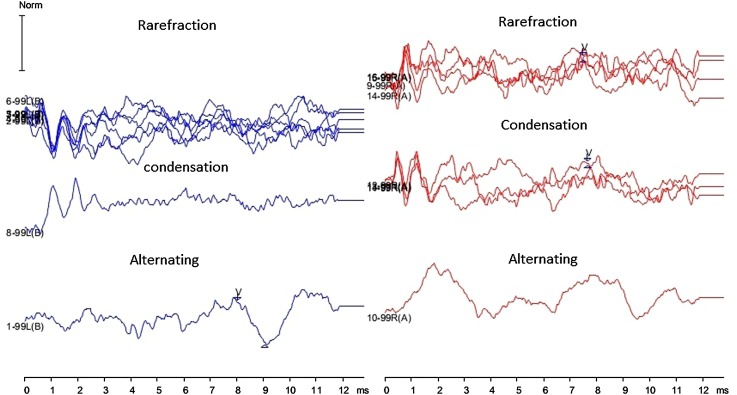
ABR were significantly abnormal and no replicable wave I, III and V were observed in 82 % and prolonged wave V in 18 % of population with ANSD at lower repetition rates of 11.1/s. In ABR, recordable and replicable CM were observed in 46 % of children with ANSD. Figure 2 shows the ABR recordings of Child 5 with CM which is reversible with the change in the polarity of the stimuli. CM was observed in condensation and rarefaction polarity and absent in alternating polarity.
Fig. 2.
Auditory Brainstem responses of Child 5 with ANSD showing cochlear microphonics that changes with stimulus polarity and absent wave I, III and prolonged wave V. Upper, middle and lower tracing shows auditory brainstem response recorded with rarefaction, condensation and alternating polarity respectively
Discussion
Prevalence of ANSD estimated from the total population tested for hearing loss was 0.42 % (N = 11) in the present study. This is similar to the report by Davis and Hirsh [2] which is 0.5 % of his clinical population. The prevalence of ANSD is found to be 5.06 % in children with sensory neural hearing loss in this study conducted at Rajiv Gandhi Government General Hospital and Madras Medical College, Chennai, Tamil Nadu. Reports of the prevalence rates in the literature are extremely variable probably due to the difference in the age range considered, testing methods used and population being considered for the study.
In developing countries like India very few reports regarding the prevalence of ANSD have been documented. Kumar and Jayaram [6] have reported low prevalence rate of 1 in 183 in population with sensory neural hearing loss which include children, adults and geriatrics at Mysore. Recently Mittal et al. [9] has reported the prevalence of 5.3 % among 487 children below 12 years of age. In 183 patients with sensory neural hearing loss cases 26 has been identified as ANSD. Their study showed an increased prevalence rate of 14.2 % at New Delhi. Unlike the available literature, this study shows the prevalence of ANSD as 5.06 % among sensory neural hearing loss children which could be due to the variation across the region.
In comparison with the prevalence rates estimated in Western population [8, 14] the prevalence of ANSD is slightly lower in our study which could be due to the type of child population being studied which differs in age. The prevalence rate of 5.06 % in the present study correlates well with the reports by Rodríguez Domínguez et al. [13] which is around 5.26 % in children with sensory neural hearing loss.
The audiological characteristics of the identified children showed varied degree of hearing thresholds from normal hearing sensitivity in 18 % of population with ANSD and mild to profound degree of hearing impairment in 82 % of the population with ANSD. This correlates with the results of Starr et al. [15] which shows in 80 % of their clients with ANSD, the primary complaint reported was hearing loss. All of them identified in the present study had bilateral ANSD (100 %) with ‘A’ type tympanogram with absent reflexes. This report approximates with the results of the study by Berlin et al. [1] which shows 92.69 % bilateral ANSD and absent of middle ear muscle reflexes in 89.19 % of their clients. DPOAEs or TEOAEs were observed in 54 % and CM was observed in 46 % of population with ANSD which shows preserved cochlear function. The presence of OAE among 54 % of the population being tested contrasts with the study by Sininger and Oba [16] which shows 80 % clear OAEs in patients with ANSD. This decreased percentage of OAEs in our study could be due to the fact that in older ANSD patient’s absence of OAE may have been present at some time before our baseline testing. ABR were significantly abnormal for the children tested which shows severe auditory nerve dysfunction in children with ANSD. Our finding with ABR (absent in 82 % and presence of wave V only in 18 %) correlates with the results of Sininger and Oba [16] which shows absent ABR in 70 %, and presence of wave V only in 19 % of their population with ANSD. Audiological characteristics in ANSD in this study correlated with the various similar characteristic studies reported in the literature [1, 15]. From these audiological findings, we suggest use of test battery approach for the assessment of hearing in all the children. We emphasize the use of objective tests like tympanometry, stapedial reflex test, OAE and ABR testing as a routine test procedure for identification of ANSD even up to the age of 12 years.
Conclusion
Our data shows that the prevalence of ANSD among hearing impaired children is not rare accounting to 5.06 % of sensory neural hearing loss population. Identification and management of children with ANSD is still challenging. New born Universal Hearing Screening, school screening programs with follow up assessment protocols would help us in early identification of children with ANSD. A team approach is necessary for the management of Children with ANSD to improve their abilities in speech, language and communication which would in turn help them in efficient learning and education to become self sufficient.
References
- 1.Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, et al. Multi site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder) Int J Audiol. 2010;49:30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- 2.Davis H, Hirsh S. A slow brainstem response for low frequency audiometry. Audiology. 1979;18:445–461. doi: 10.3109/00206097909072636. [DOI] [PubMed] [Google Scholar]
- 3.Foerst A, Beutner D, Lang-Roth R, Huttenbrink KB, von Wedel H, et al. Prevalence of auditory neuropathy/synaptopathy in a population of children with profound hearing loss. Int J Pediatr Otorhinolaryngol. 2006;70:1415–1422. doi: 10.1016/j.ijporl.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Kraus N. Auditory neuropathy: an historical and current perspective. In: Sininger YS, Starr A, editors. Auditory neuropathy: a new perspective on hearing disorders. Samdiago: Singular-Thomson learning; 2001. pp. 1–14. [Google Scholar]
- 5.Kraus N, Bradlow MA, Cunningham CJ, King CD, Koch DB, Nicol TG, et al. Consequences of neural synchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar AU, Jayaram M. Prevalence and audiological characteristics in individuals with auditory neuropathy/auditory dys-synchrony. Int J Audiol. 2006;45:360–366. doi: 10.1080/14992020600624893. [DOI] [PubMed] [Google Scholar]
- 7.Lee JSM, McPherson B, Yuen KCP, Wong LLN. Screening for auditory neuropathy in a school for hearing impaired children. Int J Pediatr Otorhinolaryngol. 2001;61:39–46. doi: 10.1016/S0165-5876(01)00543-2. [DOI] [PubMed] [Google Scholar]
- 8.Maris M, Venstermans C, Boudewyns AN. Auditory neuropathy/dys-synchrony as a cause of failed neonatal hearing screening. Int J Pediatr Otorhinolaryngol. 2011;75:973–975. doi: 10.1016/j.ijporl.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Mittal R, Ramesh AV, Panwar SS, Nilkanthan A, Nair S, Mehra PR. Auditory neuropathy spectrum disorder: its prevalence and audiological characteristics in an Indian tertiary care hospital. Int J Pediatr Otorhinolaryngol. 2012;76(9):1351–1354. doi: 10.1016/j.ijporl.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Rance G, Beer DE, Cone-wesson B, Shephard RK, Dowell RC, King AM, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20:238–252. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rance G, Barker EJ, Sarant JZ, Ching TY. Receptive language and speech production in children with auditory neuropathy/dys-synchrony type hearing loss. Ear Hear. 2007;28:694–702. doi: 10.1097/AUD.0b013e31812f71de. [DOI] [PubMed] [Google Scholar]
- 12.Rea PA, Gibson WPR. Evidence for surviving outer hair cell function in congenitally deaf ears. Laryngoscope. 2003;113:230–234. doi: 10.1097/00005537-200311000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez Domínguez FJ, Cubillana Herrero JD, Cañizares Gallardo N, Pérez Aguilera R. Prevalence of auditory neuropathy: prospective study in a tertiary-care center. Acta Otorinolaryngol. 2007;58(6):239–245. doi: 10.1016/S0001-6519(07)74920-8. [DOI] [PubMed] [Google Scholar]
- 14.Sanyelbhaa Talaat H, Kabel AH, Samy H, Elbadry M. Prevalence of auditory neuropathy among infants and young children with severe to profound hearing loss. Int J Pediatr Otorhinolaryngol. 2009;73(7):937–939. doi: 10.1016/j.ijporl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Starr A, Picton TW, Sininger Y, Hood L, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 16.Sininger YS, Oba S. Patients with auditory neuropathy: who are they and what can they hear? In: In Sininger YS, Starr A, editors. Auditory neuropathy: a new perspective on hearing disorders. Samdiago: Singular-Thomson Learning; 2001. pp. 15–35. [Google Scholar]