Abstract
Objective
To report our experience using ipilimumab, a monoclonal antibody targeting CTLA-4, combined with radiation therapy in women diagnosed with mucosal melanoma of the lower genital tract.
Methods
We retrospectively identified all patients who received ipilimumab with concurrent radiation treatment of mucosal melanoma of the lower genital tract at Memorial Sloan Kettering Cancer Center from 2012 to 2015. Various clinicopathologic data and treatment response were abstracted and analyzed.
Results
Four patients were identified. Median age was 61.5 years (range 44–68); 3 were diagnosed with vaginal melanoma, 1 with cervical melanoma. All would have required extensive surgical procedures to remove entirety of disease. Median size of lesions was 4.7 cm (range, 3.3–5.3); all were Ballantyne stage I. Median number of doses of upfront ipilimumab was 4 (range, 3–4). Two patients suffered CTCAE grade 3 adverse events (colitis, rash). All received external beam radiation: 3 to 3000 cGy, 1 to 6020 cGy. Post-radiation surgical resection was performed in 3 patients (75%); 1 (33%) of 3 patients achieved complete pathologic response. Complete local radiographic response was observed in all patients after completion of initial therapy and surgery. Two developed recurrence at 9 and 10 months post-diagnosis (mediastinum, lung); 2 remain disease-free at 20 and 38 months.
Conclusions
Mucosal melanoma of the lower genital tract is rare, and data-driven treatment strategies limited. Immunotherapy has demonstrated durable efficacy in the treatment of cutaneous melanomas. Our small case series shows a favorable response to combined ipilimumab and radiation therapy. Larger studies are needed to validate these promising results.
Keywords: Gynecologic mucosal melanoma, Vaginal melanoma, Cervical melanoma, Ipilimumab, Immunotherapy, Radiation therapy
Highlights
-
•
Mucosal melanoma of the lower genital tract is rare, and data-driven treatment strategies limited.
-
•
Our small case series shows a favorable response to combined ipilimumab and radiation therapy.
-
•
Larger studies are needed to validate these promising results.
1. Introduction
Mucosal melanoma accounts for approximately 1.4% of all melanomas diagnosed in the United States (Mihajlovic et al., 2012). The subset of mucosal melanoma localized to the lower genital tract (LGT) constitutes a small percentage of these rare tumors. The Surveillance Epidemiology and End Results database noted only 644 cases of vulvar melanoma from 1973 to 2003 (Sugiyama et al., 2007). Thirty-seven cases of newly diagnosed vaginal melanoma were reported at MD Anderson Cancer Center over a similar time frame (1980–2009) (Frumovitz et al., 2010). Cervical melanoma is the rarest of these tumors, comprising 3–9% of all diagnosed mucosal melanomas of the LGT (Pusceddu et al., 2012, Myriokefalitaki et al., 2013). Survival for patients with this rare malignancy remains poor. A recent study from our institution reported 5-year overall survival (OS) rates of 60% for patients with vulvar melanoma and 20% for those with vaginal melanoma, in a cohort of 118 patients (Leitao, 2014).
Given the rarity of mucosal melanoma of the LGT, much of the data regarding treatment and care has been extrapolated from larger studies that include cutaneous and mucosal melanomas of varied origin. The mainstay of treatment for these tumors is primary surgical resection, with the goal of achieving negative margins (Garbe et al., 2010). However, this goal is often difficult to achieve in melanomas of the LGT due to close approximation of tumor to vital anatomic structures such as the bladder and rectum. Attempting to obtain negative margins through an exenterative type of procedure is not recommended in this setting, as many studies have demonstrated that radical surgery confers no survival benefit (Leitao, 2014, DeMatos et al., 1998a, Brand et al., 1989). Exploration of preoperative treatment with chemotherapy and/or radiation to circumvent the need for extensive surgical resection has been limited (Leitao et al., 2014). Standard chemotherapeutics such as dacarbazine, which are FDA-approved for use in advanced cutaneous melanoma, show limited activity in the metastatic setting, and trials of neoadjuvant chemotherapy for patients with resectable melanoma indicate that they are no more likely to respond than those with stage IV disease (Shah et al., 2010). Radiation treatment has customarily been used in the palliative setting for women with advanced, symptomatic disease (Huguenin et al., 1998).
More recently, the role of immunotherapy in cutaneous melanoma has been explored, with favorable results (Larkin et al., 2015, Robert et al., 2011, Hodi et al., 2010). A 2010 phase 3 study investigating the use of ipilimumab–a monoclonal antibody that blocks cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)–in patients with previously treated metastatic melanoma demonstrated a nearly 4-month OS advantage as compared to a peptide vaccine alone (Hodi et al., 2010). Recent literature has also pointed to a potential modulation of the immunotherapeutic effect of CTLA-4 blockade with concomitant radiation (Postow et al., 2012, Twyman-Saint Victor et al., 2015). In this case series, we report on our experience using combined ipilimumab and radiation in the treatment of women diagnosed with mucosal melanoma of the LGT.
2. Methods
After Institutional Review Board approval, we retrospectively identified all patients at Memorial Sloan Kettering Cancer Center who received ipilimumab with concurrent radiation for treatment of mucosal melanoma of the LGT between 2012 and 2015. Review was based on data collected from outpatient, operative, and radiation oncology notes. Demographic data collected included age, race, body mass index (BMI), documented comorbidity, and genetic mutational status. Initial date of diagnosis and pathologic tumor features were noted. Use of ipilimumab and number of doses received, as well as radiation treatment and dosage, were captured. Retrospective toxicity grading was as per the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (NCI 2009).
Treatment response was graded as per RECIST guidelines version 1.1, and retrospectively reviewed (Eisenhauer et al., 2009). A complete response (CR) was defined as disappearance of all target lesions, with any pathological lymph nodes demonstrating a reduction in short axis to < 10 mm. Partial response (PR) was characterized by a minimum 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for disease progression. Definition of response as CR, PR, or SD was establishable only if no new lesions arose during treatment. Recurrence of disease was based on pathologic or radiographic evidence. OS was measured from date of initial diagnosis until date of death or until most recent known status in patients who were still alive at the time of data collection.
3. Results
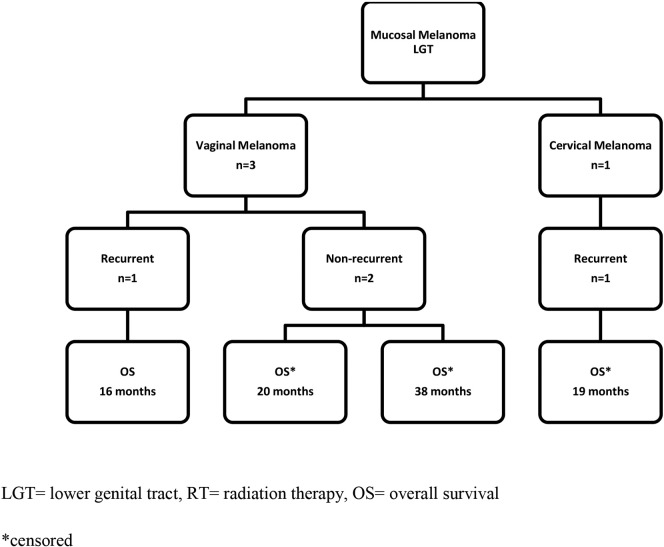
Four patients with mucosal melanoma of the LGT treated with concurrent ipilimumab and radiation were identified (Table 1). Three patients were diagnosed with vaginal melanomas, and 1 patient was diagnosed with a cervical melanoma (Fig. 1).
Table 1.
Clinicodemographic summary of patients with melanoma of the lower genital tract.
| Patient A | Patient B | Patient C | Patient D | |
|---|---|---|---|---|
| Primary site | Vagina | Vagina | Vagina | Cervix |
| Age | 44 | 68 | 61 | 62 |
| BMI | 20.4 | 23.7 | 24.4 | 26 |
| Pathology IHC staining | Melan-A, MITF, HMB-45 | MITF, HMB-45 | Melan-A, MITF, HMB-45, tyrosinase | Melan-A, HMB-45, S100 |
| Ipilimumab doses | 4 | 4 | 4 | 3 |
| Radiation therapy | EBRT 3000 cGy | EBRT 6020 cGy | EBRT 3000 cGy | EBRT 3000 cGy |
| Post-Ipi/RT imaging | SD | PR | CR | SD |
| Surgical resection | Vaginectomy | WLE | n/a | Hysterectomy, BSO, vaginectomy |
| Post-op imaging | CR | CR | CR | CR |
| Recurrence | No | Yes | No | Yes |
| Vital status | NED | DOD | NED | AWD |
BMI = body mass index, IHC = immunohistochemistry, Melan-A = melanoma antigen, MITF = microphthalmia-associated transcription factor, HMB-45 = human melanoma black 45, EBRT = external beam radiation therapy, Ipi = ipilimumab, SD = stable disease, PR = partial response, CR = complete response, WLE = wide local excision, BSO = bilateral salpingo-oophorectomy, NED = no evidence of disease, DOD = dead of disease, AWD = alive with disease.
All patients had a documented local CR postoperatively.
Fig. 1.
Survival outcomes in patients with melanoma of the LGT treated with ipilimumab and radiation.
3.1. Patient A
Patient A is a 44-year-old Caucasian female who initially presented with vaginal discharge. She was found to have a 4.7 cm, nearly completely circumferential vaginal tumor encompassing the right-upper and mid vagina, precluding primary surgical resection. No distant disease was noted on imaging at time of initial diagnosis. Biopsy confirmed a Ballantyne stage I vaginal melanoma. Genetic testing was negative for mutations in BRAF, NRAS, or c-KIT. Patient A received treatment with 4 doses of ipilimumab at 3 mg/kg, delivered intravenously every 3 weeks. The treatment course was complicated by a CTCAE grade 3 generalized maculo-papular rash, which responded to outpatient therapy with topical and oral steroids; and a CTACE grade 1 diarrhea. Concurrent treatment with external beam radiation (EBRT) was given to a dose of 3000 cGy in 5 fractions. Post-treatment imaging after completion of EBRT demonstrated SD. The patient underwent surgical resection with a partial vaginectomy 33 days after completion of EBRT. Final pathology revealed no evidence of disease in the surgical specimen, and postoperative imaging showed no evidence of residual disease. Patient A subsequently received maintenance ipilimumab every 12 weeks for 1 year at an outside institution. No recurrence of disease was noted at 38 months of follow-up.
3.2. Patient B
Patient B is a 68-year-old Asian female who initially presented with vaginal bleeding. She was found to have a 3.3 cm multifocal vaginal lesion. Pathology was consistent with a Ballantyne stage I vaginal melanoma. No distant disease was noted on imaging at time of initial diagnosis. Genetic testing revealed no mutations in BRAF, NRAS, or c-KIT. Patient B received 4 doses of ipilimumab with no complications, followed by EBRT to 6020 cGy in 28 fractions. Imaging after completion of initial treatment demonstrated a partial radiographic response. Fifty-seven days post-radiation, surgical resection with wide local excision was performed. Final pathology revealed presence of disease, with positive surgical margins. The patient received adjuvant therapy with combination cisplatin and dacarbazine for 5 cycles. Follow-up imaging at 3 months post-surgery demonstrated a complete local radiographic response. Recurrence of disease with lung metastases was noted at 10 months following initial diagnosis, and 6 months following completion of treatment with ipilimumab and radiation. Patient B died of disease 16 months after initial diagnosis.
3.3. Patient C
Patient C is a 61-year-old Black female who initially presented with vaginal bleeding. She was found to have an unresectable stage I vaginal melanoma encompassing the entire vaginal canal. No evidence of distant disease was identified at that time. No genetic testing was performed. Four doses of ipilimumab at 3 mg/kg were given, without complication. The patient subsequently received EBRT to 3000 cGy in 5 fractions. Post-treatment imaging demonstrated a complete radiographic response to initial therapy. No surgical intervention was performed and no additional treatment given. Patient C showed no evidence of disease recurrence at 20 months following initial diagnosis.
3.4. Patient D
Patient D is a 62-year-old Asian female who initially presented with vaginal bleeding. She was found to have a 5.3 cm cervical mass adherent to the rectum, involving the vagina and anus. The lesion was consistent with a Ballantyne stage I primary cervical melanoma. No distant disease was identified on imaging at time of initial diagnosis. Genetic testing revealed no evidence of BRAF, NRAS, or c-KIT mutations. Patient D received only 3 doses of ipilimumab, due to dose-limiting CTCAE grade 3 diarrhea. She initially required treatment with oral steroids, and was subsequently hospitalized and treated with infliximab for severe steroid-refractory colitis. The patient was also noted to have an infusion reaction during treatment, characterized by chest and back pain and shortness of breath, with a negative cardiac work-up. She received post-ipilimumab EBRT to 3000 cGy in 5 fractions. Imaging after completion of EBRT demonstrated SD. The patient underwent hysterectomy with bilateral salpingo-oophorectomy and upper vaginectomy 97 days after completing treatment. Final pathology revealed presence of disease, with negative surgical margins. A complete radiographic response was noted on post-operative imaging 3 weeks after surgery. On surveillance imaging 6 months after completing initial treatment with ipilimumab/radiation and 9 months following initial diagnosis, the patient was found to have a metastatic mediastinal lymph node. Patient D remains alive with disease 19 months after initial diagnosis and has been treated with pembrolizumab for recurrent disease, with SD noted.
4. Discussion
Mucosal melanoma of the LGT is rare, and data-driven treatment strategies remain limited. In this study, we present 4 cases of mucosal melanoma of the vagina and cervix that, at time of diagnosis, would have required an extensive surgical procedure to remove the entirety of disease; however, all 4 patients received initial treatment with a combination of concurrent ipilimumab and radiation. Ipilimumab was dosed at 3 mg/kg IV every 3 weeks as per the FDA approval for advanced melanoma. The clinicodemographic features of the patients in our study are in keeping with known characteristics of patients with melanoma of the LGT, including common presentation and age at initial diagnosis. All 4 patients received a minimum of 3 doses of ipilimumab with concurrent EBRT. CTCAE grade 3 adverse effects of colitis and rash were noted in 2 patients, although treatment was held in only 1 patient due to severity of symptoms. Three patients with persistent disease after ipilimumab/radiation underwent less extensive surgical resection. All patients demonstrated local CR after completing initial treatment. Two patients have remained without disease recurrence since that time.
Immunotherapy has demonstrated efficacy in the treatment of cutaneous melanomas, with durable effects. The initial results of a phase 3 study of metastatic melanoma, comparing the addition of ipilimumab to a glycoprotein peptide vaccine (gp100), showed an improvement in median OS of 10 months, as compared to 6.4 months in patients receiving gp100 alone (Hodi et al., 2010). A 2011 study of previously untreated patients with metastatic disease also demonstrated a significantly prolonged OS in patients receiving ipilimumab with dacarbazine (DTIC), a standard alkylating chemotherapeutic drug, in contrast to those receiving dacarbazine alone (Robert et al., 2011). Notably, patients in this study undergoing combination therapy also achieved durable results, with survival rates at 3 years of 20.8% for the ipilimumab-containing regimen and 12.2% for the DTIC-alone regimen. In a more recent analysis of OS in ipilimumab-treated patients with advanced melanoma, pooled data from 10 prospective trials demonstrated a median OS of 11.4 months, with a plateau in the survival curve around year 3, and survival rates ranging from 20 to 26% (Schadendorf et al., 2015). Additional immunotherapeutic regimens have shown further promise, including the addition of PD-1 checkpoint inhibitors nivolumab to an ipilimumab-containing regimen and pembrolizumab for patients with ipilimumab-refractory melanoma (Larkin et al., 2015, Ribas et al., 2015).
Although typically confined to case series and single-institution studies, data regarding the treatment and survival outcomes of patients with mucosal melanoma of the LGT illustrate a difficult-to-control disease with poor outcomes in all stages (Brand et al., 1989, Leitao et al., 2014, DeMatos et al., 1998b, Harting and Kim, 2004, Kingston et al., 2004, Bennani et al., 2013). Historically, 5-year survival rates for patients with vaginal melanoma have been calculated to be as low as 5–10% (Harting and Kim, 2004). In a 1989 case series from UCLA, the OS for 7 cases of primary vaginal melanoma was 13% (Brand et al., 1989). More recent data demonstrate 5-year survival rates of 20% for patients with vaginal melanoma (Frumovitz et al., 2010, Leitao, 2014). In a literature review of 78 cases of cervical melanoma, 5-year survival rates in patients with stage I and stage II disease were 18.8% and 11.1%, respectively (Pusceddu et al., 2012). Over a period of 25 years at Duke University Medical Center, a total of 30 patients with vulvar mucosal melanoma, 9 patients with vaginal mucosal melanoma, and 4 patients with cervical mucosal melanoma were diagnosed (DeMatos et al., 1998a). Although two-thirds of these patients were found to have localized disease at time of initial diagnosis, and all but 1 patient underwent resection with curative intent, the majority of treatment failures were local. Outcomes for patients with advanced disease are even more dismal; in a 2004 study from UT Houston and MD Anderson Cancer Center, only 4 of 11 patients with advanced vulvovaginal melanoma demonstrated a PR to biochemotherapy, and none showed a CR (Harting and Kim, 2004). Clearly, novel therapeutics and treatment strategies for this rare malignancy are needed.
Although limited by small numbers, our case series shows that select patients can receive concurrent neoadjuvant radiation and checkpoint inhibition to reduce the morbidity of a planned surgical resection—which, historically, has a low chance of systemic cure. Our findings are particularly interesting in light of the known relatively radio-resistant nature of melanoma, as well as previous studies reporting high rates of adverse events in patients undergoing radiation treatment combined with other immunotherapies (Barker et al., 2013). However, recent studies showing the efficacy of ipilimumab used concurrently with radiation in patients with metastatic melanoma highlight the potential synergy of this combination (Postow et al., 2012, Bot et al., 2012, Patel et al., 2015, Kiess et al., 2015, Silk et al., 2013). In a 2013 retrospective review of 29 patients at Memorial Sloan Kettering Cancer Center with unresectable, advanced melanoma, who underwent non-brain radiation with induction or maintenance ipilimumab, the combination of these two therapies did not appear to compromise local effect or survival benefits (Barker et al., 2013). A recently published phase 1 study from the University of Pennsylvania of 22 patients with metastatic melanoma also demonstrated promising results, with an overall response rate of 36% in patients receiving combined therapy (Twyman-Saint Victor et al., 2015).
Participation in clinical trials, when available, should be strongly encouraged for patients with gynecologic mucosal melanoma of the LGT. Providers should advocate for inclusion of gynecologic patients in large prospective trials of melanoma patients, given the overall rarity of this tumor type and the potential utility of immunotherapy and novel treatments in this patient population. The importance of a multidisciplinary approach with involvement of a melanoma specialist is paramount, and provides the best opportunity for disease control and cure.
Conflict of interest statement
Dr. Carvajal declares a financial relationship with AstraZeneca (grant, consulting), outside the submitted work; and financial relationships with Novartis, Merck, Iconic Therapeutics, Genentech, Janssen, and Aura Biosciences (all for consulting), outside the submitted work.
None of the other authors declare any potential conflicts of interest.
No other disclosures are made by any of the authors.
Footnotes
This study was funded in part through the NIH/NCI Support GrantP30 CA008748.
References
- Barker C.A., Postow M.A., Khan S.A., Beal K., Parhar P.K., Yamada Y. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol. Res. 2013;1:92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennani A., el Fatemi H., Erraghay S., Mobakir H., Ameurtess H., Souuaf I. The primary melanoma of the female genital tract: report of three cases and review of literature. Pan Afr. Med. J. 2013;16:58. doi: 10.11604/pamj.2013.16.58.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot I., Blank C.U., Brandsma D. Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J. Neurol. 2012;259:1976–1978. doi: 10.1007/s00415-012-6488-4. [DOI] [PubMed] [Google Scholar]
- Brand E., Fu Y.S., Lagasse L.D., Berek J.S. Vulvovaginal melanoma: report of seven cases and literature review. Gynecol. Oncol. 1989;33:54–60. doi: 10.1016/0090-8258(89)90603-3. [DOI] [PubMed] [Google Scholar]
- DeMatos P., Tyler D., Seigler H.F. Mucosal melanoma of the female genitalia: a clinicopathologic study of forty-three cases at Duke University Medical Center. Surgery. 1998;124:38–48. [PubMed] [Google Scholar]
- DeMatos P., Tyler D.S., Seigler H.F. Malignant melanoma of the mucous membranes: a review of 119 cases. Ann. Surg. Oncol. 1998;5:733–742. doi: 10.1007/BF02303485. [DOI] [PubMed] [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Frumovitz M., Etchepareborda M., Sun C.C., Soliman P.T., Eifel P.J., Levenback C.F. Primary malignant melanoma of the vagina. Obstet. Gynecol. 2010;116:1358–1365. doi: 10.1097/AOG.0b013e3181fb8045. [DOI] [PubMed] [Google Scholar]
- Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Spatz A. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur. J. Cancer. 2010;46:270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Harting M.S., Kim K.B. Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res. 2004;14:517–520. doi: 10.1097/00008390-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Maanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenin P.U., Kieser S., Glanzmann C., Capaul R., Lutolf U.M. Radiotherapy for metastatic carcinomas of the kidney or melanomas: an analysis using palliative end points. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:401–405. doi: 10.1016/s0360-3016(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Kiess A.P., Wolchok J.D., Barker C.A., Postow M.A., Tabar V., Huse J.T. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015;92:368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston N.J., Jones R.W., Baranyai J. Recurrent primary vulvovaginal malignant melanoma arising in melanoma in situ–the natural history of lesions followed for 23 years. Int. J. Gynecol. Cancer. 2004;14:628–632. doi: 10.1111/j.1048-891X.2004.14414.x. [DOI] [PubMed] [Google Scholar]
- Larkin J., Hodi F.S., Wolchok J.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- Leitao M.M., Jr. 2014. Management of Vulvar and Vaginal Melanomas: Current and Future Strategies; pp. e277–e281. (Am Soc Clin Oncol Educ Book). [DOI] [PubMed] [Google Scholar]
- Leitao M.M., Jr., Cheng X., Hamilton A.L., Siddiqui N.A., Jurgenliemk-Schulz I., Mahner S. Gynecologic Cancer InterGroup (GCIG) consensus review for vulvovaginal melanomas. Int. J. Gynecol. Cancer. 2014;24:S117–S122. doi: 10.1097/IGC.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Mihajlovic M., Vlajkovic S., Jovanovic P., Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int. J. Clin. Exp. Pathol. 2012;5:739–753. [PMC free article] [PubMed] [Google Scholar]
- Myriokefalitaki E., Babbel B., Smith M., Ahmed A.S. Primary malignant melanoma of uterine cervix FIGO IIa1: A CASE report with 40 months ongoing survival and literature review. Gynecol. Oncol. Case Rep. 2013;5:52–54. doi: 10.1016/j.gynor.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.R., Lawson D.H., Kudchadkar R.R., Carthon B.C., Oliver D.E., Okwan-Duodu D. Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. Neuro-Oncology. 2015;17:1312–1321. doi: 10.1093/neuonc/nov093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu S., Bajetta E., Carcangiu M.L., Formisano B., Ducceschi M., Buzzoni R. A literature overview of primary cervical malignant melanoma: an exceedingly rare cancer. Crit. Rev. Oncol. Hematol. 2012;81:185–195. doi: 10.1016/j.critrevonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O'Day S., Weber J., Garbe C. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah G.D., Socci N.D., Gold J.S., Wolchok J.D., Carvajal R.D., Panageas K.S. Phase II trial of neoadjuvant temozolomide in resectable melanoma patients. Ann. Oncol. 2010;21:1718–1722. doi: 10.1093/annonc/mdp593. [DOI] [PubMed] [Google Scholar]
- Silk A.W., Bassetti M.F., West B.T., Tsien C.I., Lao C.D. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2:899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama V.E., Chan J.K., Shin J.Y., Berek J.S., Osann K., Kapp D.S. Vulvar melanoma: a multivariable analysis of 644 patients. Obstet. Gynecol. 2007;110:296–301. doi: 10.1097/01.AOG.0000271209.67461.91. [DOI] [PubMed] [Google Scholar]
- Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]