Abstract
Background
Carcinoma of unknown primary (CUP) of the pelvis is a challenging entity for the oncologist. The role of human papilloma virus (HPV)/p16 in carcinogenesis and prognosis is more established in the head and neck than in the pelvis. In the case of an HPV positive occult primary of the pelvis the radiation therapy target coverage is not well established.
Case reports
Case#1: A 69-year-old female with a left retroperitoneal and pelvic mass was treated with chemoradiation to a dose of 45 Gy in 25 fractions to elective lymph node regions and simultaneous boost to FDG-avid lymph nodes to 55 Gy in 25 fractions. A post-treatment PET-CT showed complete response of disease now 7 months post treatment. Case#2: A 58-year-old female with a large left retroperitoneal pelvic mass was treated post-operatively with chemoradiation to 45 Gy in 25 fractions with a pelvic boost to 54 Gy. She is clinically and radiographically with no evidence of disease at 4 years. Case#3: A 47-year-old female with left sided retroperitoneal pelvic mass that declined therapy. She ultimately died of progressive disease at 1 year after diagnosis.
Conclusion
Cisplatin based chemoradiation is effective for treating HPV/p16 + pelvic squamous cell cancers of unknown primary as long as the mass, regional lymph nodes and high risk pelvic primary sites are adequately covered.
Keywords: Carcinoma of unknown primary, HPV positive, Squamous cell carcinoma, Chemoradiation, p16 positive, Pelvis and retroperitoneum
Highlights
-
•
Adds 3 cases of pelvic squamous cell carcinoma of unknown primary
-
•
Similarities between pelvic and head and neck squamous cell of unknown primary
-
•
Radiation to highest risk sites of primary disease important
-
•
Prognosis not as bad as previously shown with aggressive chemoradiation
1. Introduction
Only 3–5% of all invasive cancers are classified as cancer of an unknown primary (CUP) (Fizazi et al., 2015, Greco and Hainsworth, 2009). Of these, approximately 5%–10% are squamous cell carcinomas (SCCs), many of which are human papillomavirus (HPV) positive and are most commonly located in the head and neck. HPV positive CUP SCCs in the retroperitoneum are exceedingly rare and as such there is not much is known about the etiology, pathogenesis, or ideal therapy (Clements et al., 2010, Oh et al., 2015, Chiec et al., 2014). These lesions likely represent lymphatic metastases from an occult primary and either surgical resection or definitive chemoradiation have been suggested to provide reasonable outcomes (Clements et al., 2010, Boneschi et al., 1999).
In the more common situation of SCC of unknown primary of the head and neck, HPV is a useful tool as it helps identify the mucosal at-risk primary site. Typically the definitive management for these cancers is with chemoradiation, as there is a strong link between HPV positivity and p16 overexpression with tumors that originate in the oropharynx (Fotopoulos and Pavlidis, 2015). In pelvic or retroperitoneal SCC of unknown primary HPV status may also be used as a guide to determine the at-risk mucosal sites. HPV is a common cause of genital, gynecologic and anal cancers with rates of 50% for vulvar cancers, 64–91% for vaginal cancers, 95% for cervical cancers and 90% for anal cancers (Parkin and Bray, 2006). However, HPV is rarely positive in endometrial or ovarian cancers (Ansari-Lari et al., 2004, Staebler et al., 2002, Lee et al., 2009, Idahl et al., 2010). These statistics in addition to patient risk factors, clinical presentation, and imaging findings may be used to guide treatment.
In this manuscript, we report on three cases of HPV positive pelvic and retroperitoneal SCC of unknown primary to build on the literature and to relate the approach to treatment to the more common situation found in CUP in the head and neck region.
2. Case reports
2.1. Case#1
69 year old female who presented with a right inguinal mass, left upper thigh numbness and pain for 3 months. She underwent excisional biopsy of the right inguinal mass, which was consistent with grade 3 A follicular lymphoma (CD 10, 20 and BCL 6 positive) and bone marrow biopsy was negative. This led to CT abdomen, which identified a left-sided retroperitoneal mass. CT-guided biopsy showed metastatic carcinoma with squamous cell differentiation. The lymphoma and squamous malignancy were not felt to be related and as the lymphoma was stage IA and low-grade she was recommended radiation therapy alone. Past medical history was significant only for a total abdominal hysterectomy and bilateral salpingo-oophorectomy performed for benign reasons approximately 20 years ago.
PET/CT was done and showed the left retroperitoneal mass (2.7 × 1.8 cm) as well as a left-sided pelvic mass (3.6 × 2.6 cm) with ureteral obstruction (Fig. 1a). Exam under anesthesia (EUA) with cystoscopy, anal colposcopy and directed biopsies of the anogenital region revealed no evidence of a primary. Otolaryngology work up was normal. Urology subsequently performed excisional biopsy of pelvic lymph nodes with ureterolysis to try to obtain more tissue and relieve pressure from the collecting system. The left pelvic mass showed metastatic squamous cell carcinoma strongly p16 positive consistent with high risk HPV involvement.
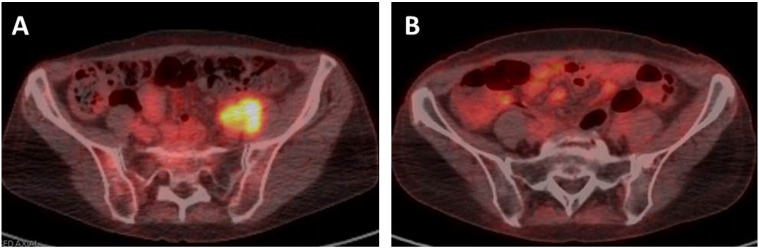
Fig. 1.
FDG PET/CT fused axial images showing (A) the left pelvic mass pre-treatment and (B) complete response to treatment with chemoradiation.
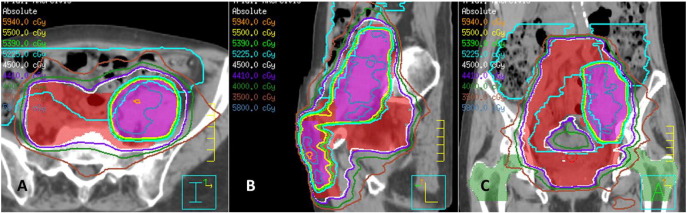
Recommendation was for chemoradiation and patient was treated with volumetric modulated arc therapy (VMAT) in order to encompass such a large volume of disease and the bilateral inguinal regions. She received 45 Gy in 25 fractions of 1.8 Gy to elective lymph node regions with a simultaneous integrated boost to FDG-avid lymph nodes to 55 Gy in 25 fractions (Fig. 2 a–c). The superior field border was extended to L2/3 to cover a full vertebral body above the grossly involved nodes. Inferiorly, although the punitive mucosal site was not identified, the vaginal cuff and upper 1/3 of the vaginal canal were included. She received weekly cisplatin at 40 mg/m2 concurrently.
Fig. 2.
VMAT treatment plan to 45Gy (red color wash) with an integrated boost to 55Gy (purple color wash) with (A) axial, (B) sagittal and (C) coronal representative images.
Three months post-treatment a PET/CT scan showed a complete response in the pelvic and retroperitoneal lymph nodes (Fig. 1b), but with interval development of a right middle lobe nodule with an SUV of 5. Patient underwent a right middle lobectomy and pathology showed necrotizing granulomas. She is alive and well with no evidence of disease 7 months post treatment.
2.2. Case#2
58 year old female who presented with left hip pain. A CT of the abdomen and pelvis showed a 6.7 × 7 cm retroperitoneal mass extending to the left pelvic side wall.
Her gynecologic history is significant for a total abdominal hysterectomy and bilateral salpingo-oopherectomy 15 years ago for endometriosis, however, she reports multiple colposcopies for cervical intraepithelial neoplasia III.
She had a PET/CT as well as EUA with directed biopsies without identification of a primary site of disease. At an outside hospital, she underwent an exploratory laparotomy for pelvic mass resection and was found to have disease adherent to the bladder, vaginal and left pelvic side wall. Pathology showed p16 positive poorly differentiated SCC.
She received post-operative cisplatin-based chemoradiation to 45 Gy in 25 fractions to the whole pelvis using a 3D four field technique which covered the vaginal cuff and upper 1/3 of the vagina. This was followed by a boost of 9Gy using a 3D three field oblique technique for a total dose of 54 Gy. The pelvic field borders extended from L4/5 down to the obturator foramen and posteriorly included the sacrum. The reason for using 3D conformal with a sequential boost vs. IMRT is unknown as she received treatment at an outside hospital. Unfortunately, during this process she was hospitalized repeatedly for small bowel obstructions.
Now, 4 years later, Patient 2 began to develop severe abdominal pain. She was noted to have hematuria and was treated for multiple UTIs. She underwent cystoscopy with biopsy and was found to have stromal fibrosis consistent with late radiation changes. She is currently clinically and radiographically with no evidence of disease.
2.3. Case#3
47 year old G11P9 female who initially presented with several weeks of left leg pain. She had a CT scan of the chest, abdomen and pelvis which showed a left pelvic side wall mass in the location of an expected left ovary causing compression of the left common iliac vein with thrombosis in the left external iliac and femoral veins. She was taken to the OR at the outside facility where a biopsy was obtained of the pelvic sidewall mass. Pathology from the small specimen that was obtained was reviewed here and showed a high grade malignant neoplasm with extensive necrosis.
She underwent PET/CT which showed the left sided retroperitoneal mass measuring 7.4 × 6.5 × 6.9 cm with central necrosis with an SUV of 15.1, a more superior lesion resembling a periaortic lymph node measuring 2.5 × 3.6 × 1.7 cm with an SUV of 12.1 (Fig. 3). EUA with cervical pap test, high resolution anoscopy and directed biopsies revealed no primary lesion.
Fig. 3.

Maximal intensity projection image at presentation showing the large FDG avid mass in the left pelvis.
To obtain more tissue, ultrasound guided biopsy of the left pelvic mass with FNA showed p16 positive moderately differentiated squamous cell carcinoma.
She was recommended chemoradiation upfront vs. induction chemotherapy followed by chemoradiation vs. palliative radiotherapy for pain control, but ultimately opted not to receive any treatment. She passed away of progressive disease 1 year after her diagnosis.
3. Discussion
Presentation with pelvic or retroperitoneal HPV/p16 positive squamous cell carcinoma of unknown primary is rare. These 3 cases add to the 7 previously reported cases in the literature to date (Table 1) (Clements et al., 2010, Oh et al., 2015, Chiec et al., 2014). In each case, the women were worked up in a manner analogous to that of patients presenting with neck nodes with an unknown primary of the head and neck. Each patient had a thorough evaluation to determine the primary site of disease including EUA with biopsies and PET/CT, but no primary was identified. In head and neck cancer presenting with unknown primary, the primary is felt to be a small/submucosal tumor, to have had spontaneous regression or yet to manifest. HPV or p16 status is relied on as a tool to identify potential mucosal at-risk sites and to improve targeting of the chemoradiation. With pelvic or retroperitoneal SCC of unknown primary, HPV/p16 status can be similarly useful. In the cases presented, the anal mucosa was not covered as a high risk mucosal site, as it was considered lower risk in these cases based on history. In a patient with risk factors for anal cancer, however, this may need to be included as a target.
Table 1.
Presentation and histology of the 10 cases of pelvic HPV/p16 + SCC of unknown primary.
| Pt | Presentation | Location of mass | Histology | IHC |
|---|---|---|---|---|
| 1 | 56 year old female with left pelvic mass found incidentally on ultrasound for routine health evaluation | Left retroperitoneal parametrium | Squamous | HPV 18 + |
| 2 | 34 year old female with right sided DVT | Right psoas | Squamous | HPV −/p16 + |
| 3 | 27 year old female with left sided DVT | Left psoas | Squamous | P16 + |
| 4 | 43 year old female unknown presentation | Left psoas | Poorly differentiated | HPV +/p16 + |
| 5 | 44 year old female with left sided DVT | Left psoas | Poorly differentiated | HPV +/p16 + |
| 6 | 52 year old female with right sided DVT | Right psoas, liver and lung mets | Poorly differentiated | HPV +/p16 + |
| 7 | 54 year old female unknown presentation | Right pelvic lymph node | Squamous | HPV +/p16 + |
| 8 | 69 year old female with a right inguinal mass, left upper thigh numbness and pain for 3 months | Left retroperitoneum | Squamous | HPV +/p16 + |
| 9 | 58 year old female with left hip pain | Left retroperitoneum | Poorly differentiated | P16 + |
| 10 | 47 year old female with several weeks of left leg pain | Left retroperitoneum | Squamous | P16 + |
Pt 1: Ref. Oh et al., 2015.
Pts 2–7: Ref. Clements et al., 2010.
Pts 8–10: current series.
IHC: Immunohistochemistry.
Retroperitoneal adenopathy at diagnosis is inherently considered metastatic disease, however, from these series, there is growing evidence to suggest that with aggressive management with cisplatin-based chemoradiation the outcomes may be comparable to locally advanced cervical or anal cancers. In our series, the 2 women who received chemoradiation therapy are alive and without evidence of disease (one at 7 months and the other at 4 years). Similarly, in the series from MDACC, women treated with chemoradiation were alive with or without disease for an average of 22 months (range 6–48 months) (Table 2) (Clements et al., 2010). Larger series of a more diverse group of patients with unknown primaries of the abdomen and pelvis have also shown survival benefits using definitive radiotherapy (Kelly et al., 2012).
Table 2.
Treatment and outcomes of the 10 cases of pelvic HPV/p16 + SCC of unknown primary.
| Pt | Treatment | Radiation details | Clinical outcomes |
|---|---|---|---|
| 1 | Complete resection and left pelvic lymph node dissection followed by chemotherapy with cisplatin/5-FU and planned RT | Not described | Not reported |
| 2 | Chemoradiation with cisplatin | 40 Gy/10 fx to the pelvis followed by 10 Gy/4 fx for persistent pain | Stable disease for 1 year with progression in cervix, alive with disease at 23 months |
| 3 | Taxol or carboplatin with progression followed by definitive chemoradiation | 45 Gy to the pelvis followed by IMRT boost to 63 Gy to gross disease | Died of disease at 1 year |
| 4 | Palliative radiation with multiple chemotherapy regimens at relapse | 45 Gy/25 fx to the pelvis | Progression in primary tumor and regional nodes at 1 year |
| 5 | Taxol or carboplatin followed by definitive chemoradiation | 45 Gy to the pelvis followed by IMRT boost to 63 Gy to gross disease | NED at 6 months |
| 6 | Palliative radiation with multiple chemotherapy regimens at relapse | 10 Gy × 1 to the pelvis repeated at 6 weeks | Died of disease at 8 months |
| 7 | Resection followed by chemoradiation with carboplatin | Initial RT not none, but received 66 Gy at the time of recurrence | NED at 48 months |
| 8 | Resection with residual gross disease followed by chemoradiation with weekly cisplatin | IMRT to 55 Gy/25 fx to gross disease and 45 Gy/25 fx to at-risk nodal regions | NED at 7 months |
| 9 | Resection with residual gross disease followed by chemoradiation with weekly cisplatin | 45 Gy/25 fx to the pelvis with conformal RT followed a boost to 54 Gy to gross disease | NED at 4 years |
| 10 | Refused therapy | None | Died of disease at 1 year |
In this series, each woman presented with hip pain and was found to have large volume disease in the pelvis and retroperitoneum. Interesting, there is variation in the literature of presentation, including more occult presentations such as incidental finding on transvaginal ultrasound and DVT (Table 1) (Clements et al., 2010, Oh et al., 2015). Although CUP in the head and neck is uncommon, it can be diagnosed at an earlier stage as the cervical neck nodes are visible and noticeable to a patient prior to causing other symptoms. It is a possibility that CUP in the pelvis and retroperitoneum is actually more common than is reported, but is not found until disease becomes symptomatic.
This series is inherently limited by a small number of cases and retrospective nature. Although we are not able to determine whether HPV/p16 caused these squamous cell cancers, it is a helpful tool to develop a treatment paradigm with reasonable outcomes in these patients.
The authors have no financial or other conflict of interests to report.
References
- Ansari-Lari M.A. Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am. J. Surg. Pathol. 2004;28(2):160–167. doi: 10.1097/00000478-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Boneschi M. Primary retroperitoneal tumors. Treatment modality and prognostic factors. Minerva Chir. 1999;54(11):763–768. [PubMed] [Google Scholar]
- Chiec L. Male pelvic squamous cell carcinoma of unknown primary origin. Case Rep. Oncol. Med. 2014;2014:953698. doi: 10.1155/2014/953698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A. The presence of human papillomavirus or p16 in six cases of retroperitoneal carcinoma. Obstet. Gynecol. 2010;116(5):1042–1046. doi: 10.1097/AOG.0b013e3181f88ddf. [DOI] [PubMed] [Google Scholar]
- Fizazi K. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26(Suppl. 5):v133–v138. doi: 10.1093/annonc/mdv305. [DOI] [PubMed] [Google Scholar]
- Fotopoulos G., Pavlidis N. The role of human papilloma virus and p16 in occult primary of the head and neck: a comprehensive review of the literature. Oral Oncol. 2015;51(2):119–123. doi: 10.1016/j.oraloncology.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Greco F.A., Hainsworth J.D. Introduction: unknown primary cancer. Semin. Oncol. 2009;36(1):6–7. doi: 10.1053/j.seminoncol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Idahl A. Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, human papillomavirus, and polyomavirus are not detectable in human tissue with epithelial ovarian cancer, borderline tumor, or benign conditions. Am. J. Obstet. Gynecol. 2010;202(1) doi: 10.1016/j.ajog.2009.07.042. (p. 71 e1–6) [DOI] [PubMed] [Google Scholar]
- Kelly P. Role of definitive radiation therapy in carcinoma of unknown primary in the abdomen and pelvis. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(5):2012–2017. doi: 10.1016/j.ijrobp.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Lee H.B. A case of advanced gynecologic pelvic tumors showing the diagnostic utility of HPV analysis. J. Gynecol. Oncol. 2009;20(4):251–253. doi: 10.3802/jgo.2009.20.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H.J. HPV-Related Retroperitoneal Squamous Cell Carcinoma of Unknown Primary: A Case Report. Cancer Res. Treat. 2015;47(4):954–957. doi: 10.4143/crt.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D.M., Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl. 3) doi: 10.1016/j.vaccine.2006.05.111. (p. S3/11–25) [DOI] [PubMed] [Google Scholar]
- Staebler A. Hormone receptor immunohistochemistry and human papillomavirus in situ hybridization are useful for distinguishing endocervical and endometrial adenocarcinomas. Am. J. Surg. Pathol. 2002;26(8):998–1006. doi: 10.1097/00000478-200208000-00004. [DOI] [PubMed] [Google Scholar]