Abstract
Aim
To review the clinical feasibility of carbon ion radiotherapy (C-ion RT) for skull base tumors, especially for chordomas which are often seen in the skull base area.
Background
Skull base tumors treated by C-ion RT consist of primary chordomas and chondrosarcomas, and enormously extended head and neck cancer with a histology of adenoid cystic carcinomas, adenocarcinomas and malignant melanomas. These tumors are located on anatomically complex sites where they are close to important normal tissues and therefore demand better physical dose distribution to avoid unnecessary doses for surrounding normal tissues. These tumors are also known as radio-resistant tumors for low linear energy transfer (LET) radiotherapy and show favorable results after treatment by high LET carbon ion radiotherapy.
Materials and methods
Biological reports of C-ions for the chordoma cell line, clinical results of C-ion RT for skull base tumors, dose comparative studies between two representative facilities and tumor control probability (TCP) of chordomas by C-ion RT were reviewed.
Results
C-ion RT for skull base tumors, especially for chordomas, shows favorable results of tumor control and acceptable complications. The C-ion dose of 57.36 gray equivalent (GyE)/16 fractions/4 weeks will deliver 90% of local control for chordomas. The limiting doses for surrounding normal tissues are clearly revealed. The dose difference between institutes was assumed within 10%.
Conclusions
C-ion RT is recommended for skull base tumors because of high LET characteristics and clinical results.
Keywords: Carbon ion radiotherapy, Skull base tumor, Chordoma, TCP, High LET particle
1. Background
Skull base tumors are arising in complex anatomical regions and adjacent to the important normal tissues like the brain, cranial nerves, brainstem, eyeball and acoustic system. Also skull base tumors consist of various histological and clinical types including chordomas, chondrosarcomas, and other primary tumors; adenoid cystic carcinomas, adenocarcinomas, malignant melanomas and other metastatic and direct invasive tumors from neighboring anatomical sites. Surgical resection is recommended as the first therapeutic strategy, acquiring histological details with tumor removal.1 Due to the proximity of critical normal tissues, complete surgical resection can result in severe complications of surrounding normal tissues. In such a situation, highly conformed radiotherapy, like intensity modulated radiotherapy (IMRT), stereotactic radio-surgery (SRS) and charged particles will be recommended. Charged particles, like protons and carbon ions, have a Bragg peak and show better dose distribution than conventional X-rays, resulting in less doses to the surrounding normal tissues.2 For these tumors, high linear energy transfer (LET) radiotherapy is occasionally recommended because the biological characteristics have a high relative biological effectiveness (RBE). Carbon ions (C-ions) are charged particles the same as protons, and are characterized as high LET radiotherapy, different from low LET protons. The target tumors of high LET charged particles will be chordomas, chondrosarcomas, malignant meningiomas and non-squamous cell malignancies of the head and neck.
2. Aim
In this review article, the evidence of the high RBE feature of C-ions for skull base tumors, especially primary chordomas, which is the most popular histology in skull base tumors, was investigated in terms of biology, beam delivery, clinical results and dose comparative studies.
3. Materials and methods
3.1. Biology of high LET radiation for a chordoma cell line
A chordoma is a rare and slow growing malignancy arising from the remnants of the fetal notochord3 and there exist a few cell lines of chordomas established for biological study.4, 5 Also, the characteristics of the slow growth in chordomas make it difficult to investigate the behaviors in radiation exposure. Kato et al. investigated the nature of the U-CH1-N cell line which is a subpopulation of U-CH1 chordoma cells with a short doubling time.6 They reported the cell doubling time of U-CH1-N as 3 days which was a longer time than other cell lines, like HeLa (uterine cervix – 18 h) and U87-MG (malignant glioma – 24 h). Using this short-doubling-time cell line, they reported the RBE values of U-CH1-N at 10% survival as 1.30 in 13 keV/μm C-ion, 2.45 in 70 keV/μm C-ion and 3.86 in 200 keV/μm iron-ion.
Fujisawa et al. followed the comparative study of RBE between C-ion and proton therapy for chordomas using the U-CG1-N cell line.7 RBE values were calculated based on the D10 values. The RBE value of 70 MeV proton was 0.89, of 13–20 keV/μm C-ion was 0.85, of 20–30 keV/μm C-ion was 1.27, and >30 keV/μm C-ion was 1.69. C-ion killed cells depending on both the dose and the LET, while protons depended on the dose alone. They concluded the carbon ion radiotherapy may have an advantage for chordoma radiotherapy because of a higher cell-killing effect with high LET doses from biological observation in their studies.
3.2. Beam delivery and treatment planning system
A present, there are two representative beam delivery systems for C-ion RT.
One is a passive beam delivery system at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, and is based on the relationships between RBE and LET of human salivary gland tumor (HSG) cells for flattening the spread out Bragg peak (SOBP) and on the clinical experience with fast neutrons for determining the clinical RBE.8 Accelerated beams are broadened laterally by wobbler magnets and a scatterer; and they are longitudinally spread out by a ridge filter. A collimator and a compensator are used to clip the irradiation fields and to conform the distal shape of the irradiation field to those of the planned target volume of a patient, respectively. SOBPs were designed for the carbon beam to have uniform biological responses throughout the SOBP. The physical dose distributions of the beam agreed very well with the predicted depth dose distributions; also, the biological responses were satisfactorily flat in the SOBP. RBE values of carbon beams were determined based on experimental results of cell responses, on values expected with the linear-quadratic model, and on experiences with neutron therapy. They use fixed RBE values independent of dose levels, and the RBE system depends only on LET of the C-ion radiation fields. The clinical dose is obtained by multiplying a ratio between the biologic dose at the neutron-equivalent point and the clinically observed RBE value, 3.0 to the entire biologic dose distributions.
Another system is a dynamic beam delivery at Gesellschaft fuer Schwerionenforschung (GSI) in Darmstadt, Germany.9 The RBEs are determined for each single beam through the local effect model (LEM) which assumes that equal local doses should lead to equal local effects, independent of the radiation quality. The first version, LEM I, is based on a radial dose distribution of each charged particle crossing into a cell nucleus, as well as on the radiosensitivity and repair capacity of the tissue. They reported that the original version of the LEM I underestimated the ratio of RBE in the Bragg peak region as compared with the RBE in the entrance. The next extension of the LEM II still showed the same tendency. Implementation of the modified track structure LEM III almost completely compensates for these systematic deviations, and predictions of RBE by LEM III for high and low energetic C-ions show good agreement for a wide panel of different cell lines, as well as for the tolerance of the rat spinal cord.
3.3. Clinical results of carbon ion radiotherapy
Since December 1997, C-ion radiotherapy (RT) has been performed at the GSI by means of the intensity-controlled raster scan technique for patients with chordomas and low-grade chondrosarcomas of the skull base. The median total dose was 60 gray equivalent (GyE) (57–70 GyE) (weekly fractionation of 7 fractions with a fraction dose of 3.0 GyE) delivered in 20 fractions within 3 weeks. Schulz-Ertner et al. reported the preliminary results of C-ion RT in 96 patients with chordomas of the skull base in 2007.10 The actuarial 3- and 5-year local control rates were 80.6% and 70.0%, respectively, and the overall 3- and 5-year overall survival (OS) rates were 91.8% and 88.5% with acceptable toxicity. Delivery of target doses exceeding 60 cobalt-gray equivalent (CGE) in 12 patients improved local control significantly (p = 0.029, log-rank) with a 5-year local control probability increasing from 63% to 100%. They tried to demonstrate the dose-tumor control probability (TCP) curves by means of a logistic model (P(D) = eb0+b1D/(1 + eb0+b1D), where P is the probability of tumor control, D is the dose (Gy) applied to the skull base chordoma, and b0 and b1 are constants requiring estimation for TCP) together with photon and proton studies. The resulting parameters were b0 = 5.7072, b1 = 0.087 and D50 (dose for 50% tumor control) = 65.6 Gy converting the carbon dose to the effective dose of 2 Gy per fraction using α/β = 2 Gy. Even if there were some uncertainties in the data used for curve fitting, the reported dose–response relationship represented that the high dose irradiation would increase the possibility of chordoma control. Uhl et al. updated long-term results of C-ion RT for 155 patients with skull base chordomas treated at the GSI.11 The median follow-up after treatment was 72 months (range, 12–165 months). The resulting 3-year, 5-year, and 10-year LC rates were 82%, 72%, and 54%, respectively, whereas the 3-year, 5-year, and 10-year OS rates were 95%, 85%, and 75%, respectively. No higher late toxicity could be detected after carbon ion treatment.
Mizoe et al. reported the results of dose escalation Phase I/II study of C-ion RT for 33 patients with skull base chordoma at NIRS in Chiba, Japan.12 All the patients were treated by 16 fractions for 4 weeks with total doses of 48.0, 52.8, 57.6, and 60.8 Gy equivalents (GyE). The local control rate was 85.1% (standard error [SE], 8%) at 5 years and 63.8% (SE, 19%) at 10 years. The OS rate of the 33 patients was 87.7% (SE, 7%) at 5 years and 67% (SE, 14%) at 10 years. With multiportal irradiation and possibly repeated surgical removals, normal tissues showed a mild reaction, and no severe morbidity of important organs occurred. As a result of the dose escalation study of C-ion RT for skull base tumors, a dose fractionation of 60.8 GyE/16 fractions for 4 weeks was decided as a recommended dose because of acceptable normal tissue reactions and good local tumor control.
Schulz-Ertner et al. reported the effectiveness and toxicity of carbon ion radiotherapy in chondrosarcomas of the skull base.13 Between November 1998 and September 2005, 54 patients with low-grade and intermediate-grade chondrosarcomas of the skull base were treated with C-ion RT using the raster scan technique at the GSI. All patients had gross residual tumors after surgery. The median total dose was 60 CGE (weekly fractionation 7 × 3.0 CGE). The median follow-up was 33 months (range, 3–84 months). Only 2 patients developed local recurrences. The actuarial local control rates were 96.2% and 89.8% at 3 and 4 years; OS was 98.2% at 5 years. They concluded that C-ion RT is an effective treatment for low- and intermediate-grade chondrosarcomas of the skull base offering high local control rates with low toxicity. Uhl et al. reported the follow up results of 79 patients with chondrosarcoma of the skull base added to previous report treated between 1998 and 2008.14 The follow-up period after irradiation was 91 months (range, 3–175 months). The 3-year, 5-year, and 10-year local control rates were 95.9%, 88%, and 88%, respectively; the corresponding OS rates were 96.1%, 96.1%, and 78.9%, respectively. They concluded that carbon ion therapy is a safe and effective treatment in patients with chondrosarcoma of the skull base.
3.4. Tolerance doses of surrounding normal tissues
Hasegawa et al. reported the tolerance dose for retention of visual acuity in C-ion RT.15 Of 163 patients with H&N and skull base tumor treated between 1994 and 2000, 54 optic nerves of 30 patients were included in the irradiation volume. All patients had a follow-up period of more than 4 years. The median prescribed total dose was 56.0 gray equivalents (GyE) at 3.0–4.0 GyE per fraction per day (range, 48–64 GyE; 16–18 fractions; 4–6 weeks). Of 54 optic nerves (ONs), 35 ONs had been irradiated under 57 GyE (maximum dose [Dmax]) resulting in no visual loss. Conversely, 11 of the 19 ONs (58%) irradiated with more than 57 GyE (Dmax) suffered a decrease of visual acuity. In all of these 19 cases, the ONs had been involved in the tumor before carbon ion radiotherapy. In the multivariate analysis, a dose of 20% of the volume of the ON (D20) was significantly associated with visual loss. They concluded that the occurrence of visual loss seemed to be correlated with a delivery of more than 60 GyE to the 20% of the ON volume.
Koto et al. reported the risk factors of radiation induced brain injury, which consisted of damage to the temporal lobes, frontal lobes, and cerebellum, after C-ion radiotherapy for skull base tumors.16 Between April 1997 and January 2009, 47 patients with skull base tumors were irradiated by C-ion RT with a total dose of 48.0–60.8 GyE/16 fractions/4 weeks. Of these patients, 39 who were followed up with magnetic resonance imaging (MRI) for more than 24 months were analyzed. The median follow-up period was 67 months. They reported the occurrence rate of grade 2 or higher brain injury at 5 years was 34.3% for the patients with V50 larger than 4.6 ml, and 15.6% for the patients with V50 smaller than 4.6 ml (p = 0.0192). They concluded that the occurrence of grade 2 or higher brain injury correlated with the irradiated volume (4.6 ml) of V50 (p = 0.004, hazard ratio = 1.229, 95% CI = 1.069–1.412).
3.5. Dose comparative study between two representative facilities
Uzawa et al. reported the comparative study of the biological effectiveness of 290 MeV/amu carbon-ion beams in Chiba, Japan, and in Darmstadt, Germany. The overall difference of RBE between the two facilities was 0–5% for gut crypt survival and 3–7% for HSG cell killing.17 They concluded the carbon-ion beams at the NIRS, Japan, and the GSI, Germany, are biologically identical after single and daily fractionated irradiation.
Fossati et al. reported the comparison results of C-ion treatment planning systems (TPSs) between NIRS and GSI.18 In this study, carbon ion plans were optimized on target volumes of cubic and spherical shapes, for RBE weighted dose prescription levels ranging from 3.6 to 4.4 GyE by 0.2 GyE steps. Plans were calculated for target sizes from 4 to 12 cm in steps of 2 cm defining three beam geometries which were single beam, opposed beam and orthogonal beam configurations. Physical dose distributions of NIRS-based and LEM-based treatment plans were compared. LEM-based prescription doses that minimize differences in physical dose distributions between the two systems were found. These doses were compared with the mean RBE-weighted dose obtained with a Monte Carlo code (FLUKA) interfaced with LEM I. In the investigated dose range, LEM-based RBE weighted prescription doses should be higher than NIRS reported prescription doses. The optimal dose depends on a target size, shape and position, number of beams and dose level. The opposed beam configuration resulted in the smallest average prescription dose difference (0.45 ± 0.09 GyE). The second approach of recalculating the RBE-weighted dose of NIRS with a Monte Carlo code interfaced with LEM resulted in no significant difference with the results obtained from the planning study.
4. Results
TCP of carbon ion radiotherapy for skull base chordomas
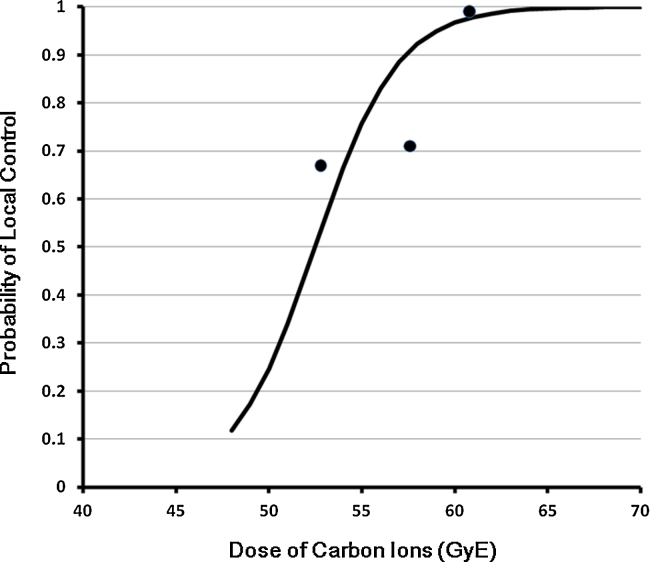
From the clinical data of Mizoe et al.,12 tumor control probability (TCP) of chordomas treated by carbon ions was estimated. This curve was calculated from the results of a single institutional and prospective dose escalation study. The objective patients were 16 cases treated by a phase I/II dose escalation study and 14 cases treated by a phase II study. One case treated by 48.0 GyE/16 fractions/4 weeks in the phase I/II study was excluded from analysis because it was the only one treated by 48.0 GyE. The local control rate on each dose points were 67% (2/3) at 52.8 GyE, 71% (5/7) at 57.6 GyE and 100% (19/19) at 60.8 GyE. The formula used was the following integral logistic model:
where P is the probability of local control, D is the dose (GyE) applied to the skull base chordoma, and a and b are constants requiring estimation for TCP. For the calculation of the constants, the least squares method was applied. Constant a and constant b were estimated at −23.6473 and 0.4506, respectively (Fig. 1). From the calculated equation, the 50% local control dose was 52.48 GyE and the 90% dose was 57.36 GyE.
Fig. 1.
Dose and tumor control probability (TCP) curve estimated from NIRS results. The 50% local control dose was 52.48 GyE and the 90% dose was 57.36 GyE.
5. Discussion
The chordoma cell line showed a high RBE nature for C-ion RT. The RBE was 1.69 in more than 30 keV/μm C-ion7 and 2.45 in 70 keV/μm of C-ion.6 The RBE of chordoma cells showed dose dependency for C-ion irradiation.8 Clinical reports also showed high RBE results for chordoma,12, 14 chondrosarcoma14 and other skull base tumors. These results should confirm the clinical position of C-ion RT in the management of skull base tumors.
The high RBE nature of C-ions is not only seen in tumor control but also in normal tissue reactions. Many clinical results showed acceptable morbidity of C-ion RT, and some literature reported the dose-complication relationships of the optic nerves15 and the brain.16 These data and other reports19, 20 of C-ion RT showed the clinical feasibility of C-ion RT for skull base tumors because of the acceptable morbidity of surrounding normal tissues.
Ares et al. reported the 5-year local control rates as 81% for chordomas and 94% for chondrosarcomas in their spot-scanning proton RT.21 They observed 4 patients (6%) with a high grade late toxicity. Deraniyagala et al. reported the 2-year local control rates of proton therapy as 86% for chordomas.22 They observed Grade 2 toxicity in 18% of the patients in the form of unilateral hearing loss. No grade 2 or higher optic or brainstem toxicities were observed. These results of proton RT for chordomas and chondrosarcomas showed clinically acceptable results. Future results of proton RT with dose escalation and for different histology tumors other than chordomas and chondrosarcomas will be needed.
6. Conclusions
It is clear that the C-ion RT should be favorable in radiotherapy for skull base tumors because of high LET effects for the tumors and conformed dose distribution for the normal tissues resulting in a high tumor control rate and acceptable normal tissue morbidity.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Di Maio S., Temkin N., Ramanathan D., Sekhar L.N. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg. 2011;115:1094–1105. doi: 10.3171/2011.7.JNS11355. [DOI] [PubMed] [Google Scholar]
- 2.Jereczek-Fossa B.A., Krengli M., Orecchia R. Particle beam radiotherapy for head and neck tumors: radiobiological basis and clinical experience. Head Neck. 2006;28:750–760. doi: 10.1002/hed.20448. [DOI] [PubMed] [Google Scholar]
- 3.Salisbury J.R. The pathology of the human notochord. J Pathol. 1993;171:253–255. doi: 10.1002/path.1711710404. [DOI] [PubMed] [Google Scholar]
- 4.Bruederlein S., Sommer J.B., Meltzer P. Molecular characterization of putative chordoma cell lines. Sarcoma. 2010 doi: 10.1155/2010/630129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheil-Bertram S., Kappler R., von Baer A. Molecular profiling of chordoma. Int J Oncol. 2014;44:1041–1055. doi: 10.3892/ijo.2014.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato T.A., Tsuda A., Uesaka M. In vitro characterization of cells derived from chordoma cell line U-CH1 following treatment with X-rays, heavy ions and chemotherapeutic drugs. Radiat Oncol. 2011;6:116. doi: 10.1186/1748-717X-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa H., Genik P.C., Kitamura H., Fujimori M., Uesaka M., Kato T.A. Comparison of human chordoma cell-kill for 290 MeV/n carbon ions versus 70 MeV protons in vitro. Radiat Oncol. 2013;8:91. doi: 10.1186/1748-717X-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai T., Endo M., Minohara S. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer M., Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45:3319–3330. doi: 10.1088/0031-9155/45/11/314. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Ertner D., Karger C.P., Feuerhake A. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68:449–457. doi: 10.1016/j.ijrobp.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 11.Uhl M., Mattke M., Welzel T. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014 doi: 10.1002/cncr.28877. [June 19] [DOI] [PubMed] [Google Scholar]
- 12.Mizoe J., Hasegawa A., Takagi R., Bessho H., Onda T., Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base. 2009;19:219–224. doi: 10.1055/s-0028-1114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz-Ertner D., Nikoghosyan A., Hof H. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. 2007;67:171–177. doi: 10.1016/j.ijrobp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Uhl M., Mattke M., Welzel T. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy. First report of long-term results. Cancer. 2014;120:1579–1585. doi: 10.1002/cncr.28606. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa A., Mizoe J.E., Mizota A., Tsujii H. Outcomes of visual acuity in carbon ion radiotherapy: analysis of dose-volume histograms and prognostic factors. Int J Radiat Oncol Biol Phys. 2006;64:396–401. doi: 10.1016/j.ijrobp.2005.07.298. [DOI] [PubMed] [Google Scholar]
- 16.Koto M., Hasegawa A., Takagi R. Risk factors for brain injury after carbon ion radiotherapy for skull base tumors. Radiother Oncol. 2014;111:25–29. doi: 10.1016/j.radonc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Uzawa A., Ando K., Koike S. Comparison of biological effectiveness of carbon-ion beams in Japan and Germany. Int J Radiat Oncol Biol Phys. 2009;73:1545–1551. doi: 10.1016/j.ijrobp.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Fossati P., Molinelli S., Matsufuji N. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57:7543–7554. doi: 10.1088/0031-9155/57/22/7543. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S., Kawase T., Yoshida K., Hasegawa A., Mizoe J. Skull base chordomas: efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir. 2009;151:759–769. doi: 10.1007/s00701-009-0383-5. [DOI] [PubMed] [Google Scholar]
- 20.Mizoe J.E., Hasegawa A., Jingu K. Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol. 2012;103:32–37. doi: 10.1016/j.radonc.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Ares C., Hug E.B., Lomax A.J. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–1118. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Deraniyagala R.L., Yeung D., Mendenhall W.M. Proton therapy for skull base chordomas: an outcome study from the university of Florida proton therapy institute. J Neurol Surg B: Skull Base. 2014;75:53–57. doi: 10.1055/s-0033-1354579. [DOI] [PMC free article] [PubMed] [Google Scholar]