Abstract
Surgery has evolved greatly over the last decades thanks to the more sophisticated and conservative surgical approaches and also thanks to the progress of diagnostic imaging. An added value is represented by the increased experience of the professionals and the close multidisciplinarity of the procedures including neurosurgeons, otolaryngologists and maxillo-facial surgeons. One of the most recent developments is the endoscopic surgery allowing for more conservative and cosmetically satisfactory outcomes.
Radiation therapy has greatly changed over the last decades thanks to the technology advances related both to the availability of new imaging modalities and techniques of radiation delivery. Delivery of radiation evolved from three-dimensional conformal techniques to stereotactic and intensity-modulated radiation therapy. Particle therapy has the potential to further improve in the near future thanks to the progress of technology. Proton therapy allows for optimization of dose deposition in the target with lesser dose in the healthy tissues and ion therapy, currently using carbon ions, has been more recently introduced with the advantage of more effective treatments in case of less radio-sensitive tumours thanks to a higher biological effectiveness.
A relevant concept that can significantly improve the results is that of interaction and integration of different disciplines not only within the surgical field. The cooperation between surgeons of various disciplines, radiation oncologists and medical oncologists together with professionals from other disciplines, such as pathology and radiology is nowadays required in an effort to customize and optimize the treatment in each single patient.
Keywords: Skull base, Surgery, Radiotherapy, History
1. Background
The base of skull is an anatomical district characterized by various tissues that separate the intracranial content from the facial structures. A peculiar aspect of skull base tumours is the extreme heterogeneity of the lesions that may arise from or involve this specific anatomic site. Many are benign and may require a single treatment modality but others are malignant and can aggressively evolve with frequent recurrences and sometimes also distant metastases. Another particular aspect is the proximity of the skull base to many structures deputed to relevant physiologic functions that may not allow wide surgical resections in many cases. For this reason, malignant and also benign lesions cannot always be radically removed with a consequent high risk of local recurrence.
Surgery and radiotherapy have been the two main modalities employed in the treatment of these tumours and their clinical and technical progresses substantially have contributed to the improvement of the prognosis of patients affected by skull base malignancies.
The present article describes the main steps in the development of surgery and radiotherapy over the last decades.
2. Surgery
The term “cranial base surgery” appeared in the surgical literature around the end of the 1960s and soon became familiar to practitioners and made them aware of a new principle of exposing some areas of the brain, neck and face.1 As in every surgical procedure, there is an access route, a target area, and a surgical corridor obtained by removing, displacing, or bypassing anatomical structures; in cranial base surgery the approach route involves the bone of the skull base and the adjacent soft tissues.
The skeleton of the skull base is a bony diaphragm separating the brain on one side from the neck and face on the other. Skull base surgery is used to deal with lesions within the base and extending into the cranial cavity and/or neck or face as well as with lesions originating close to the base, but not necessarily extending into the base. The route used for the procedure thus involves an area of the bone of the base and contiguous soft tissues forming part of the neck, face and brain, but, conversely, it can involve a conventional mode of access – craniotomy, or upper neck or face approach that is enlarged to include the skull base as well.
The method was gradually developed, improving with experience and especially gaining from the imaging radiology that facilitated the diagnosis of the nature and extent of the disease to be treated, and enabled a more accurate preoperative planning of the procedure. The skull base ceased to be an unsurpassable barrier to the neurosurgeon on one hand, and to the otolaryngologist and maxillofacial surgeon on the other. It became a site approached by means of multidisciplinary combinations of techniques borrowed from all these specialties, mostly with the aid of a microscope, and involving the brain and dura, nerves and vessels, sensory organs, bone and face. At the same time, the procedure had to prepare grounds for the subsequent functional and aesthetic reconstructions. In short, we are talking about primary lesions of the skull base that may or may not extend into the cranial cavity, face or neck, or about lesions of the brain, neck or face lying in the vicinity of the skull base but not necessarily extending within it. The route used for the surgical procedure involves the skull base and the contiguous cranium, brain, neck and face.
The translabyrinthine approach was the first typical example of such surgery.2, 3 Fig. 1 is an axial CT showing the bone removal needed to access to the cerebello-pontine angle. Initially intended as a procedure for removing an acoustic neuroma, the translabyrinthine approach helped to shape the principle that bone could be removed to expose lesions not only within the skull base (the internal auditory canal in the example shown), but also in the cranial cavity adjacent to the base, i.e. the cerebello-pontine angle.
Fig. 1.
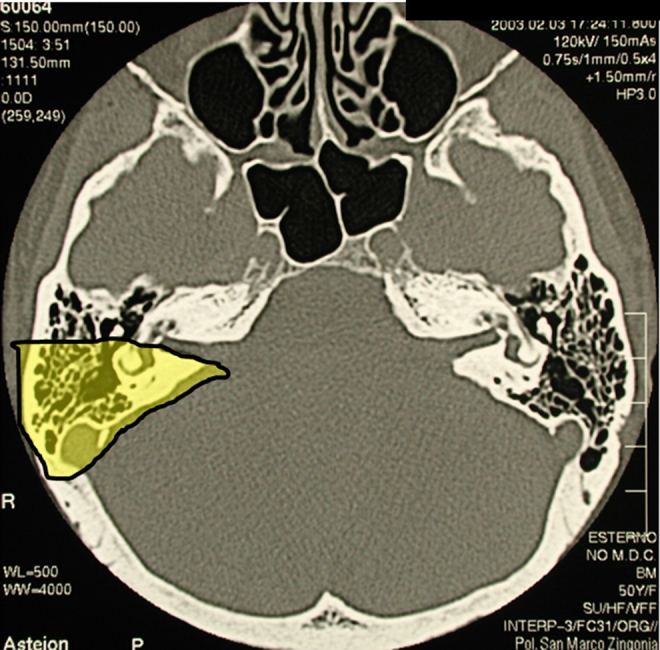
Axial CT bone window of the skull base showing extent of bone removal using the translabyrinthine approach to the cerebello-pontine angle (yellow area).
A second historical contribution came from the system of infratemporal approaches which prompted the concept that a surgical corridor could be obtained by removing, displacing or bypassing anatomical structures to expose a lesion arising in the skull base and growing into the neck, face or cranium.4 In the infratemporal approach A, bone removal extends from the petrous dura to the lower temporal bone and tympanum to expose a surgical field from the dura (and c.p.a.) to the neck. The facial nerve running through the middle of the field is displaced supero-anteriorly to clear the way to the jugular foramen. Similar principles apply to the infratemporal approaches B and C. The purpose of this system of approaches is to expose a wide area of the skull base, from the jugular foramen to the nasopharynx.
The idea of entering via the skull base and expanding the field into the adjacent cranium and neck, as well as the conventional craniotomy enlarged to involve the skull base, became well established. It was applied to a number of procedures involving the techniques used in different specialties, by a single surgeon or by teams of two or more surgeons and gave rise to a surgical subspecialty. The fundamental features of this subspecialty today are: a preoperative diagnosis of the pathology, a precise knowledge of its extension, a planned approach to expose the lesion and performance of the reconstruction. The so-called tailored approach to individual lesion entails choosing one from a number of tried and tested procedures and removal and reconstruction steps.
A modern endoscope is a tube with lenses at both ends that provide a picture of the surgical field to the eye or a TV screen. A straight or angled instrument is brought right into the field, closer than a microscope, and it has given rise to a new subspecialty as well.5 Anterior approaches to the skull base rely on natural apertures (the nose and mouth) allowing for a much wider exposure than when entering via the paranasal sinuses, to reach the fronto-nasal dura, sella and clivus. The dura can be entered to reach the brain's frontal lobe, parasellar area and pre-pontine cistern. This transdural route allows a safe dura repair. Advances are still being made in endoscopy of the skull base, its present limits being the internal carotid arteries and the brain.
Procedures that cross the skull base were known in both neurosurgery and otolaryngology, e.g. for the removal of paragangliomas growing from the skull base into the endocranium, or for transnasal hypophysectomy, the cranio-facial and transfrontal approaches to wounds and tumours of the anterior cranial fossa, or the fronto-temporal or suboccipital approaches. It was in the 1980s that microscopic neurosurgery reached a widespread excellence following the work by Malis and Yasargil and focused on the principles and procedures of skull base surgery. Among several procedures available, worth mentioning is the subtemporal–infratemporal approach, which runs along the trigeminal nerve from its ganglion to the foramen ovale and the parapharyngeal space.6 The procedure for reaching the cerebello-pontine angle extends from the subtemporal fossa posteriorly through the petrous apex.7 More anteriorly, the procedures include the pterional approach enlarged to include the orbito-zigomatic ridge and extending both to the middle cerebral fossa and the upper neck, the transfrontal route enlarged to include the fronto-orbital ridge and the transfrontal-ethmoidal approach.8, 9, 10 As for the posterior edge of the skull base, the available procedures include the approaches to the foramen magnum and the cranio-cervical junction.11 Experience suggested that some approaches, such as the transapex and retrolabyrinthine approaches to the cerebello-pontine angle, could be dismissed in favour of the retrosigmoid approach, although the anterior and lateral approaches enlarged to include the skull base bone remain in use. The evolution seen in the surgical procedures has also been promoted by the expertise gained with a surgical microscope which makes good use of the extra space provided by a transbasal enlargement.
The field of neurosurgery has changed remarkably since the introduction of the endoscope. The transnasal and the transbuccal routes enable lesions in the anterior cranial fossa, sella and parasellar areas to be exposed, as well as the pterygoid, petrous and occipital bones.12 The creation of an approach to the median cisterns through the clivus is an important achievement, too. The original contribution of endoscopic brain surgery is a short, direct access through natural apertures with no need for any brain retraction. Overlaps remain between the microscopic and endoscopic avenues. Experience will enable their respective indications to be integrated with the great contribution expected from imaging and tumour biology. What remains to be universally achieved is for the same surgeon to master both the microscope and the endoscope so that the power of these different means and methods can be combined to enable the best possible targeted conservative surgery.
3. Radiotherapy
The evolution of radiation treatment for skull base tumours imitates the evolution of radiation therapy for other tumour types. The very first reports published in the literature around the half of the 20th century describe radiation treatments for pituitary adenoma in 1946, tumours of the glomus jugulare in 1953 and craniopharyngioma in 1955.13, 14, 15 In the following years, treatments for meningioma in 1963, chordoma in 1967 and chondrosarcoma in 1969 were reported.16, 17, 18 These series were treated with very simple and old techniques by using orthovoltage and cobalt radiations often with a palliative intent.
The subsequent evolution was related not only to the technological progress of radiotherapy machines but also to the implementation of new imaging modalities. The advent of CT-scan in the 1970s changed dramatically the way to deliver radiotherapy due to the ability to detect target and non-target structures and to identify the density of the tissues to X-rays and consequently to obtain a reliable calculation of dose deposited in the tumour and in the rest of the body. Similarly, the advent of MRI in the early 1980s improved substantially the ability to precisely define the tumour extension with respect to the surrounding healthy tissues and to better identify radiation-sensitive structures, such as various components of the central nervous system and the cranial nerves including the optic pathways and other structures.
The first report on the use of stereotactic radiation technique was for radiotherapy of acoustic neuroma in 1979.19 Stereotactic radiotherapy evolved rapidly for the treatment of acoustic neuroma, craniopharyngioma and pituitary adenoma.20 This irradiation modality in a single shot used an invasive head frame fixed to the patient's skull and a gamma-knife machine with cobalt sources or a conventional linear accelerator rotating around the isocentre.
A few years later a technique with a relocatable head frame was adopted by Gill et al., allowing for stereotactic fractionated radiotherapy with conventional linear accelerator.21 The main advantage of multiple fractionation with respect to a single fraction was represented by the radiobiological advantage with decreased risk of radiation induced damage to the tissues susceptible to late side effects, i.e. the central nervous system and the optic pathway.
Over the same years, radiation therapy evolved from two-dimensional to three-dimensional techniques with precise dose calculation in the three spatial dimensions. The implementation of sophisticated beam delivery devices, such as the multileaf collimators, contributed to precisely deliver volumetric treatments. At the beginning of the 21st century, the intensity-modulated radiation therapy (IMRT) technique was implemented with further reduction of the high-middle dose volumes outside the target. In the last years, technology evolved further and innovative linear accelerators were introduced in clinical practice, i.e. tomotherapy and cyberknife that led to an optimization of the dose distribution with a very precise control of the target before and during treatment (Image Guided Radiation Therapy – IGRT).
In parallel to X-rays radiation therapy, since the 1970s, particle therapy appeared in the scenario of radiation oncology. The rationale of using charged particles is mainly related to the advantageous physical dose deposition in the Bragg peak region that can be appropriately spread out to cover the tumour volume with substantial sparing of proximal and distal tissues compared to photons. Moreover, ion beams have a narrower lateral penumbra compared to protons and are able to increase the biological efficacy thanks to their higher linear energy transfer (LET).
Interestingly, the very first tumour types treated by charged particles were the tumours of the base of skull since they were very often located closely to radio-sensitive critical structures, such as the brainstem, the optic pathway and the brain parenchyma, and required high-radiation doses. As a matter of fact, the largest series of skull base tumours treated by radiotherapy were reported by centres using proton therapy.
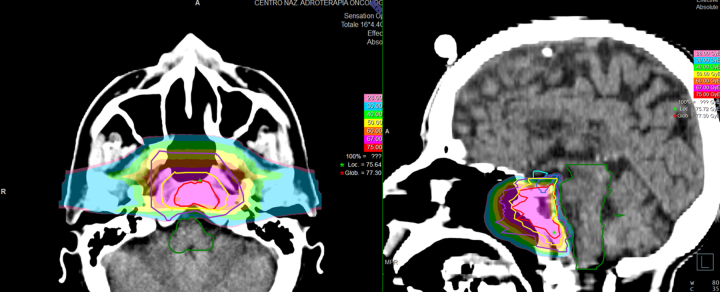
One of the first clinical series was reported from Massachusetts General Hospital (MGH) using proton-beam radiation therapy for the treatment of chordoma and chondrosarcoma of the base of skull and cervical spine.22 In 1982, the authors reported the results of treatment of 10 patients, six with chordoma, three with chondrosarcoma, and one with a neurofibrosarcoma. Local control was achieved for all but one patient with marginal failure after a follow-up period ranging from 2 months to 6 years. High doses of radiation, up to 76 Cobalt Gray Equivalents (CGE), were delivered without significant morbidity. In the following years, a number of retrospective and prospective clinical trials were reported by institutions from the USA, Europe and Japan equipped with proton facilities showing that very favourable clinical results could be achieved with limited toxicity in the treatment of skull base tumours.23, 24, 25, 26, 27 Most of the experiences focused on skull base chordoma and chondrosarcoma. At MGH, 375 chordomas and 246 chondrosarcomas of the base of skull were treated by protons obtaining 10-year local control rates of 54% and 98%, respectively.23 At Loma Linda University Medical Center (LLUMC), 58 patients were treated to a total dose of 65–79 GyE with 5-year local control and overall survival rates of 59% and 79% for chordoma and of 75% and 100% for chondrosarcoma, respectively.24 At the Centre de Protontherapie (CPO) in Orsay in France, 100 cases of chordoma of the skull base and cervical spine were treated to a median total dose of 67 GyE obtaining 4-year local control and overall survival rates of 54% and 81% respectively.25 At Paul Scherrer Institut in Villigen, Switzerland, 47 patients affected by skull base chordoma were treated to a mean total dose of 74 GyE reporting a local control rate of 81% at 5 years.26 In Japan, the results on 16 patients treated at Tsukuba were published with local control of 100% at 3 years for chordoma and 86% for chondrosarcoma and an overall survival rate of 100%.27 At the Centre for Oncological Hadrontherapy (CNAO) in Italy, the preliminary results on the first patients treated with protons were recently reported with very low-toxicity rates (Fig. 2).28
Fig. 2.
Chordoma treated with carbon ion radiotherapy at CNAO (Pavia, Italy) after endoscopic surgery. Carbon ions were delivered with active scanning and Intensity-Modulated Particle Therapy (IMPT) to a total dose of 70.4 Gy (RBE), 4.4 Gy (RBE)/fraction, in 16 daily fractions, 4 days a week.
One of the last developments of radiation therapy is related to the use of high-LET radiation, such as carbon ions. The two main pioneering institutions that experienced this treatment modality are the National Institute of Radiological Sciences (NIRS) in Japan and the Heidelberg Ion Therapy Centre (HIT) in Germany. Thanks to a very precise spatial dose distribution and a high-biological efficacy (RBE), the results obtained in clinical series were very favourable even in the case of radioresistant tumours, such as chordoma and chondrosarcoma of the cranial base.29, 30 This treatment modality has been considered to date as experimental due to the difficulty and uncertainty in predicting the real map of biological effects and the relative shortness of follow-up of clinical series. At present, the number of centres able to treat by carbon ions is increasing worldwide and the physical, radiobiological and clinical research activities are expected to progress fast and make available solid results in terms of long-term local control, survival and side effects.
Nowadays, radiotherapy has demonstrated to be able to become an effective complimentary treatment to surgery for several tumour types but it could represent an even radical approach alternative to surgery not only in the case of inoperable patients or unresectable disease but also when treatment outcome is comparable to surgery in terms of local control but with less severe side effects.
4. Discussion and conclusions
Surgery has evolved greatly over the last decades thanks to the more sophisticated techniques, the progress of diagnostic imaging, the increased experience of the professionals and the close multidisciplinarity of the procedures including neurosurgeons, otolaryngologists and maxillo-facial surgeons. Transfacial and intracranial approaches have became less risky in terms of intraoperative and postoperative complications and tumours located in sites considered as inaccessible or very risky, such as the cavernous sinuses, can be safely removed. One of the most recent developments was the endoscopic surgery that allowed, when feasible, more conservative and cosmetically satisfactory outcomes. Neuronavigation was recently also implemented in skull base surgery allowing for more precise and conservative approaches.
Radiation therapy has greatly changed over the last decades thanks to the technology advances related both to the availability of new imaging modalities and techniques of radiation delivery. Delivery of radiation evolved from two- and three-dimensional conformal techniques to stereotactic and intensity-modulated radiation therapy. Particle therapy, first employed for treating skull base tumours in the early 1970s, has the potential to further improve in the near future thanks to the progresses of technology. Proton therapy has recently moved from passive scattering to active scanning system allowing for intensity-modulated proton therapy (IMPT) and, consequently, for optimization of dose deposition in the target with less dose in the healthy tissues. Ion therapy, currently with carbon ions, has been more recently introduced with the advantage of more effective treatments in the case of less radio-sensitive tumours thanks to a higher biological effectiveness.
Medical oncology can also contribute to the management of malignant tumours arising from the base of skull or originating from other structures and invading the skull base. Thanks to chemotherapy and molecular targeted drugs, patients can be offered more effective first or second line treatments, sometimes in combination with radiation therapy.
Another aspect that has significantly changed over time is the concept of interaction and integration of the different disciplines. The cooperation between surgeons allowed for more complex and useful approaches. However, surgery, although performed through very complex and sophisticated procedures, cannot have always the potential to achieve oncologically radical resections especially when the tumour removal is done by “piecemeal” resection. In these cases, radiation therapy should actually be discussed upfront even before the choice of the surgical approach. In other cases, surgery and radiation can represent two alternative treatment modalities and the selection of one of them should be discussed also with the patient in relation to the chances of success and the risk of side effects.
The multidisciplinary team should include also professionals from other disciplines, first of all pathologists and radiologists, who can contribute to clarify pathological, ultrastructural and biomolecular characteristics of the lesions and describe the real extension of the tumour and its relationship with the healthy surrounding structures.
All these aspects justify the foundation of scientific skull base societies at national and international levels that promote progress and disseminate the knowledge about the biological and clinical aspects of this peculiar anatomic district.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Hamberger C., Wersall J., editors. Disorders of the skull base region. Nobel symposium; Stockholm; 1969. [Google Scholar]
- 2.House W.F. Transtemporal bone microsurgical removal of acoustic neuromas. Arch Otolaryngol. 1964;80:735–738. [PubMed] [Google Scholar]
- 3.House W.F. Monograph 2. Acoustic neuroma. Arch Otolaryngol. 1968;88:575–715. [PubMed] [Google Scholar]
- 4.Fisch U. Infratemporal fossa approach to tumors of the temporal bone and base of the skull. J Laryngol Otol. 1978;92:949–967. doi: 10.1017/s0022215100086382. [DOI] [PubMed] [Google Scholar]
- 5.Wigand M.E. Thieme; Stuttgart/New York: 1989. Endoskopische Chirurgie der Nasennebenhoelen und der forderen Schaedelbasis. [Google Scholar]
- 6.Sekhar L.N., Schramm V.L., Jr., Jones N.F. Subtemporal–preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67:488–499. doi: 10.3171/jns.1987.67.4.0488. [DOI] [PubMed] [Google Scholar]
- 7.Kawase T., Shiobara R., Toya S. Anterior transpetrosal–transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869–876. [PubMed] [Google Scholar]
- 8.Samii M., Draf W. Springer-Verlag; Berlin/Heidelberg: 1989. Surgery of the skull base. An interdisciplinary approach. [Google Scholar]
- 9.Sekhar L. Surgical management of tumors involving the cavernous sinus. In: Sekhar L., Schramm V.L. Jr., editors. Tumors of the cranial base. Futura Publishing Company; New York: 1987. pp. 393–419. [Google Scholar]
- 10.Raveh J., Laedrach K., Speiser M. The subcranial approach for fronto-orbital and antero-posterior skull base tumors. Arch Otolaryngol Head Neck Surg. 1993;119:385–393. doi: 10.1001/archotol.1993.01880160029006. [DOI] [PubMed] [Google Scholar]
- 11.George B., Dematons C., Cophignon J. Lateral approach to the anterior portion of the foramen magnum. Surg Neurol. 1988;29:484–490. doi: 10.1016/0090-3019(88)90145-0. [DOI] [PubMed] [Google Scholar]
- 12.Kassam A.B., Gardner P., Snydermann C., Mintz A., Carrau R. Expanded endonasal fully endoscopic completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa and infratemporal fossa. Neurosurg Focus. 2005;19:E6. [PubMed] [Google Scholar]
- 13.Buschke F. Radiotherapy of pituitary adenomas. West J Surg Obstet Gynecol. 1950;58:271–278. [PubMed] [Google Scholar]
- 14.Alexander E., Jr., Adams S. Tumor of the glomus jugulare: follow-up study two years after roentgen therapy. J Neurosurg. 1953;10:672–674. doi: 10.3171/jns.1953.10.6.0672. [DOI] [PubMed] [Google Scholar]
- 15.Dutta S., Bhattacharyya A. Craniopharyngioma. J Indian Med Assoc. 1955;16:325–326. [PubMed] [Google Scholar]
- 16.Turnball I.M., Tom M.I. Pigmented meningioma. J Neurosurg. 1963;20:76–80. doi: 10.3171/jns.1963.20.1.0076. [DOI] [PubMed] [Google Scholar]
- 17.Wright D. Nasopharyngeal and cervical chordoma – some aspects of their development and treatment. J Laryngol Otol. 1967;81:1337–1355. doi: 10.1017/s0022215100068341. [DOI] [PubMed] [Google Scholar]
- 18.Minagi H., Newton T.H. Cartilaginous tumors of the base of skull. Am J Roentgenol Radium Ther Nucl Med. 1969;195:308–313. doi: 10.2214/ajr.105.2.308. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch A., Noren G., Anderson H. Audiologic findings after stereotactic radiosurgery in nine cases of acoustic neuroma. Acta Otolaryngol. 1979;88:155–160. doi: 10.3109/00016487909137155. [DOI] [PubMed] [Google Scholar]
- 20.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797–803. doi: 10.1136/jnnp.46.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill S.S., Thomas D.G.T., Warrington A.P., Brada M. Relocatable frame for stereotactic external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1991;20:599–603. doi: 10.1016/0360-3016(91)90076-g. [DOI] [PubMed] [Google Scholar]
- 22.Suit H.D., Goitein M., Munzenrider J. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56:377–385. doi: 10.3171/jns.1982.56.3.0377. [DOI] [PubMed] [Google Scholar]
- 23.Munzenrider J.E., Liebsch N.J. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175:57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 24.Hug E.B., Loredo L.N., Slater J.D. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–439. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 25.Noel G., Feuvret L., Calugaru V. Chordomas of the spine and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44:700–708. doi: 10.1080/02841860500326257. [DOI] [PubMed] [Google Scholar]
- 26.Ares C., Hug E.B., Lomax A.J. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–1118. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 27.Fuji H., Nakasu, Ishida Y. Feasibility of proton beam therapy for chordoma and chondrosarcoma of the skull base. Skull Base. 2011;21:201–206. doi: 10.1055/s-0031-1275636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orecchia R., Vitolo V., Fiore M.R. Proton beam radiotherapy: report of the first ten patients treated at the “Centro Nazionale di Adroterapia Oncologica (CNAO)” for skull base and spinal tumours. Radiol Med. 2014;119:277–282. doi: 10.1007/s11547-013-0345-0. [DOI] [PubMed] [Google Scholar]
- 29.Mizoe J., Hasegawa A., Takagi R., Bessho H., Onda T., Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base. 2009;19:219–224. doi: 10.1055/s-0028-1114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhl M., Mattke M., Welzel T. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014;120:3410–3417. doi: 10.1002/cncr.28877. [DOI] [PubMed] [Google Scholar]