Abstract
The scope of this study included the preparation of silver nanoforms with high antimicrobial efficacy, low cost, and ease of application. The term ‘silver nanoforms’ refers to silver located on the amorphous or crystalline titanium dioxide (TiO2). Silver nanoforms may be used as an alternative to antibiotics in killing bacteria. Pure and silver-incorporated titanium (used as a carrier) was prepared using the sol–gel-modified method. Physical and chemical properties of the samples were described, and the antibacterial activity was indicated using the following strains of bacteria: Staphylococcus aureus, Klebsiella pneumoniae (ESKAPE pathogens), and Escherichia coli. The results have shown that the antibacterial activity of silver nanoforms with amorphous TiO2 is much better than that in the samples based on anatase (crystalline TiO2). The sensitivity of the tested bacteria to silver nanoforms depends on physical and chemical properties of the nanoforms and individual characteristics of the bacteria. For the first time, significant participation of amorphous TiO2 in antibacterial compounds has been described through this study.
Introduction
Silver nanoforms are nanocompounds of silver located on the amorphous or crystalline titanium dioxide (TiO2). TiO2 is used as a carrier for biologically active silver (ions Ag+ and nanoparticles Ag0), causing (1) prevention of silver ions inactivation by environmental factors (such as NaCl), (2) prevention of silver nanoparticles aggregating with each other, (3) increase in the surface area (SBET) of silver nanoforms. The above points have one purpose: better (higher and faster) antibacterial activity of silver nanocompounds. TiO2 appears in the amorphous form and the following polymorphic crystalline ones: anatase, rutile, and brookite. Anatase is known for its highest photocatalytic properties, and for this reason, it has a potential for various biomedical and environmental applications [15, 20, 30]. Photocatalysis is a process during which, in a water environment, radicals are generated under the influence of UV light [4]. Radicals change the metabolism of bacteria cells, which causes their death. Up to now (October 2015), over 20,000 results in the PubMed have focused on the crystalline TiO2 (especially anatase) and its photocatalysis process. About 30 of them, concentrate on the comparison of their antibacterial efficacy, but only one refers to amorphous TiO2 as a carrier for silver and describes its potential antibacterial applications [7]. Research on bacterial resistance to antibacterial agents is an important trend in contemporary microbiology and the medical area. During the last six decades, resistance to antibiotics has increased, and when antibiotics were introduced in the 1950s, researchers expected that they would eradicate all bacterial diseases. In the early 1960s, resistance to penicillin among Gram-positive bacteria was noted, and the same phenomenon was soon observed in Gram-negative bacteria [24]. Multidrug resistance (MDR) in both Gram-positive and Gram-negative bacteria and extremely drug-resistant bacteria (XDR) is an important issue in the treatment of infectious diseases [22]. Antibiotic resistance control is a long-term international program which surveys MDR bacteria. Many international reports indicated that the resistance of bacteria to antibiotic drugs is still high [6]. Klebsiella pneumoniae as a nosocomial pathogen that also causes septicemia in newborns is increasingly multidrug-resistant [22]. MDR Staphylococcus aureus complicates the treatment of healthcare-associated infections. Also, carbapenems resistance in Gram-negative bacteria has been rapidly increasing [25]. Moreover, both K. pneumoniae and S. aureus belong to ESKAPE pathogens (together with Enterococcus faecium, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.). The ESKAPE group describes pathogens responsible for hospital infections, which are most difficult to treat. However, E. coli causing bacteremia infections and sepsis showed increasing resistance to fluoroquinolones [24]. The spread of resistance among pathogens is becoming a serious problem [14], and therefore searching for alternative drugs, such as those containing nanocomposites doped with silver, is very desirable. Silver ions and silver nanoparticles show a broad spectrum of antibacterial activity and indicate an oligodynamic effect [11, 18, 19]. Silver has been known for its antibacterial activity since ancient times, but the development of bionanotechnology has given us possibilities for using it as an active biomedical factor [16]. The toxicity of silver compounds depends on the bioavailability of silver, and silver ions rapidly bind to factors in the environment (such as Cl−) or aggregate with each other and eventually become unable to kill bacteria. The presence of a carrier (such as titanium dioxide) acts as a ligand for silver ions or nanoparticles and preservatives before decreasing the availability of silver [16]. Kedziora et al. [12] proposed the synthesis of TiO2/Ag compounds, where anatase indicated very good antibacterial efficacy. We modified the previous method, and in this work, other nanoforms of TiO2 were prepared—amorphous and crystalline (anatase) doped with silver—and their antibacterial activity was compared, with and without UV irradiation. This work proves excellent antibacterial activity of the nanoforms based on the amorphous TiO2—TiO2/Ag+ and TiO2/Ag0 (even without UV), much better than that based on the anatases—TiO2a/Ag+ and TiO2a/Ag0 (with UV irradiation). The amorphous nanoforms have a potential to be exploited in numerous biomedical, pharmaceutical, and industrial applications as an antimicrobial agent (e.g., for hard-to-heal wound infections as cream, ointment, bandages, disinfectants, etc., or as filters and preservatives in cosmetics). In order to maintain its excellent antibacterial activity, no additional factors such as UV irradiation are required.
Materials and Methods
Production of Pure Amorphous and Crystalline TiO2 and TiO2 Doped with Silver (TiO2/Ag+, TiO2a/Ag+, TiO2/Ag0 and TiO2a/Ag0) Nanocompounds
All used reagents were described in a study by Kedziora et al. [12]. Titanium n-butoxide [Ti(O-Bu)], hydrofluoric acid, and ammonium hydroxide were added as described by Kedziora et al. [12]. The duration of stirring was established experimentally. In the previous study [12], diamminesilver(I) [Ag(NH3)2]+ was added during the above procedure to form nanosilver grain. In this work, we (1) finished the gel synthesis (by washing with methyl alcohol and water, drying at 80 °C, and transforming to anatase, if necessary); and then (2) treated the powder of amorphous or crystalline TiO2 with diamminesilver(I) and a reducing factor (glucose) during impregnation and reduction. After step 2, we received nanocompounds of amorphous and crystalline: TiO2—TiO2/Ag+, TiO2a/Ag+ (as an impregnation effect) and TiO2/Ag0 and TiO2a/Ag0 (as a reduction effect). To prepare the anatase, amorphous TiO2 was calcinated under the same conditions as described previously [12]. The content of silver (Ag+ or Ag0) was measured by dissolving the composite in acetic acid or nitric acid accordingly, and then measured using atomic absorption spectroscopy (AAS, Perkin Elmer 1100). Results of nanoforms characteristics are presented in Table 1.
Table 1.
Physicochemical description of prepared samples
| Sample | Kind of TiO2 | Size of TiO2 (nm) | Size of silver nanoparticles (nm) | Content of silver (wt%) |
|---|---|---|---|---|
| TiO2 | Amorphous | ≤100 | n/a | n/a |
| TiO2/Ag0 | Amorphous | ≤100 | 20 | 10 |
| TiO2/Ag+ | Amorphous | ≤100 | n/a | 10 |
| TiO2a | Anatase | ≤40 (aggregate) | n/a | n/a |
| TiO2a/Ag0 | Anatase | ≤40 (aggregate) | ≤10 | 1 |
| TiO2a/Ag+ | Anatase | ≤40 (aggregate) | n/a | 1 |
n/a not applicable
Characteristics of Pure TiO2 and TiO2 Doped with Silver (TiO2/Ag+, TiO2a/Ag+, TiO2/Ag0 and TiO2a/Ag0) Nanocomposites
The description of morphology is one of the basic features of materials in the nanoscale. The samples were characterized as follows: size and shape—with transmission electron microscopy (TEM, Philips CM20 Super Twin); crystalline structure—with X-ray diffraction (XRD, Stöe); porosity—with nitrogen adsorptions isotherms (Autosorb 1-C, Quantachrome Instruments); and content of silver—with atomic absorption spectroscopy (AAS, Perkin-Elmer). Diffraction patterns (degrees and intensity of peaks measured with XRD) were compared with the referenced patterns from the database of diffraction patterns: International Centre for Diffraction Data (PDF2 Database ICDD). Porosity measured with N2 adsorption and desorption provides information about the pore size and the corresponding surface area. Porosity size is of fundamental importance in developing the surface area—a basic feature in nanotechnology. The smaller the pore sizes observed the larger the surface area described. A shape of the isotherm confirms a pore size. To measure the silver content in the nanocompounds with AAS, the solubility of silver ions in citric acid and that of silver nanoparticles in the nitric acid were used. The results obtained with the above techniques make the interpretation of antibacterial activity of silver nanomaterials easier. The photocatalytic activity of nanocompounds was measured using a model reaction of estimated decomposition of methyl blue (at room temperature, RT) [28].
Bacteria Strains
In all, 10 bacteria strains were used for antimicrobial study—three reference strains from American Type Culture Collection: Staphylococcus aureus ATCC 6538 (Gram-positive bacteria), Escherichia coli ATCC 11229 (Gram-negative bacteria), and Klebsiella pneumoniae ATCC 4352 (Gram-negative bacteria); and seven of the same genera strains, isolated from infected wounds purchased from the DiaLab Medical Laboratory. The antibiogram is presented in Table 2. Clinical isolates of E. coli 475, 555, 574, and K. pneumoniae 626 strains are resistant to penicillin, penicillin with inhibitor, and cefalosporins, the second generation drugs frequently used as the first applied antibiotics. S. aureus strains (173, 187) are methicillin resistant (MRSA), resistant to chinolon, trimethoprim–sulfamethoxazole, macrolides, and lincosamides. S. aureus 298 is a methicillin-sensitive (MSSA) strain. The antibiogram was done according to Kirby-Bauer disk diffusion-susceptibility test protocol [5].
Table 2.
Antibiogram of bacterial isolates
| Bacterial isolates | Am | Amc | Cxm | Ctx | Caz | Atm | An | Cip | SXT | Imp | Mem | TZP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli 475a | S | R | R | S | S | S | S | S | S | S | S | S | |||
| E. coli 555a | S | R | R | S | S | S | S | S | S | S | S | S | |||
| E. coli 574a | S | R | R | S | S | S | S | S | S | S | S | S | |||
| K. pneumoniae 626a | S | R | R | S | S | S | S | S | S | S | S | S |
| P | Amc | Cxm | Ctx | Caz | Ge | An | Cip | Sxt | Imp | Mem | E | Cc | Va | Fox | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus 173a | R | R | R | R | R | S | S | R | R | R | R | R | R | S | R |
| S. auerus 298a | R | S | S | S | S | S | S | S | R | S | S | S | S | S | S |
| S. auerus 187a | R | R | R | R | R | R | R | R | R | R | R | R | R | S | R |
S sensitive, R resistant, Am ampicillin, AMC amoxicillin-clavulanic acid, CXM cefuroxime, CTX cefotaxime, CAz ceftazidime, AN amikacin, Ge gentamicin, ATM aztreonamum, CIP ciprofloxacin, Imp imipenem, Mem meropenem, Tzp piperacillin–tazobactam, SXT trimethoprim–sulfamethoxazole, P penicillin, E erytromycin, CC clindamycin, VA vancomycin, FOX cefoxitin
aBacterial isolates, numbered by Medical Laboratory DiaLab
Antibacterial Effect of Nanocomposites: TiO2/Ag+, TiO2a/Ag+, TiO2/Ag0 and TiO2a/Ag0
The antibacterial effect of amorphous (TiO2/Ag+, TiO2/Ag0) and crystalline (TiO2a/Ag+, TiO2a/Ag0) nanocompound was determined by minimal inhibitory concentration (MIC) values according to the reference methods of Clinical and Laboratory Standards Institute (CLSI) for the determination of MICs of aerobic bacteria by broth microdilution [8, 27] with minor modification. The stock of the antimicrobial agent’s dilution was prepared in microplates, and the final inoculum was 106 CFU/ml per spot. Nanoforms-free spots with a medium, pure TiO2 and TiO2a and cultures were used as growth controls. To study the bacterial inactivation effect of titanium nanocomposites after UV irradiation, the tested samples were irradiated with UV light (Philips, 30 W/cm2) for 30 min.
Results and Discussion
Synthesis and Characterization of Silvered TiO2
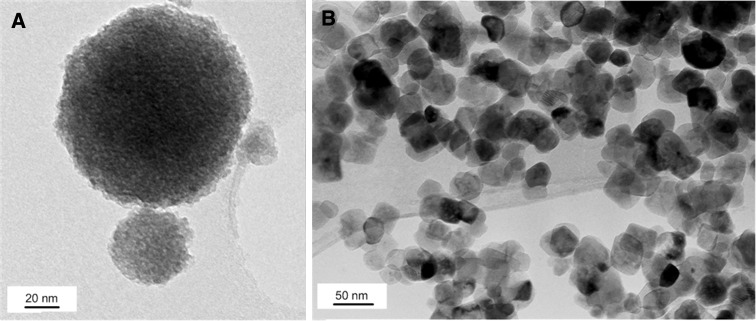
Sol–gel synthesis is one of the most popular methods used for nanocompounds production [2, 3, 23] and its modification leads to obtaining various products with differences in physicochemical and biological properties. In comparison with the study by Kedziora et al. [12], we showed that a source of silver [diamminesilver(I)] may be added during the synthesis of powder forming silver grain and to the ready gel (this work). The way of synthesizing has an impact on the surface area and antibacterial efficacy. In Fig. 1, the TEM images of the prepared basic samples are shown. Figure 1a presents amorphous TiO2, and anatase (TiO2a) is presented in Fig. 1b. The size of amorphous TiO2 is less than or equal to 100 nm. Calcination of amorphous TiO2 caused a decrease in grain size (from 100 to 40 nm), but silver grain embodied to TiO2 structure [12] decreased the surface area (SBET). The specific surface area of the powder decreased from 424.0 ± 8.0 m2/g (for amorphous TiO2) to 52.8 ± 0.2 m2/g (for crystalline TiO2a) (Table 3). High reduction of surface area is probably related to the aggregation of TiO2 during annealing.
Fig. 1.
TEM images of amorphous TiO2 (a) and crystalline TiO2 (b)
Table 3.
Surface area (S BET) and pore size get with nitrogen adsorptions
| Sample | S BET (m2/g) | Pore size (nm) |
|---|---|---|
| TiO2 | 424.0 ± 8.0 | 4 |
| TiO2a | 52.8 ± 0.2 | 22 |
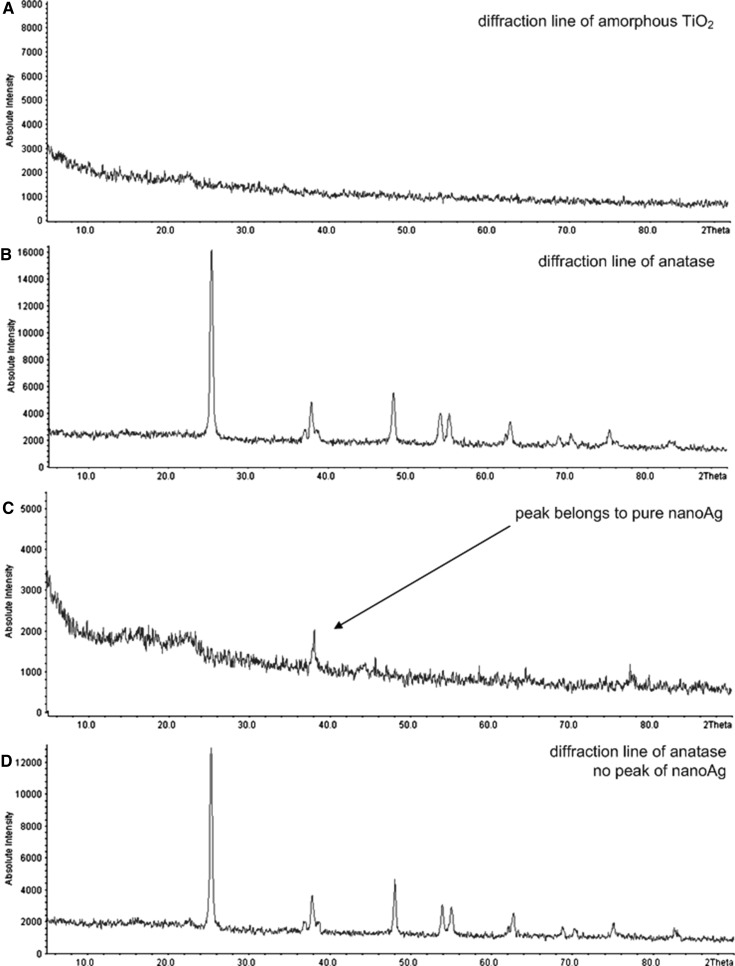
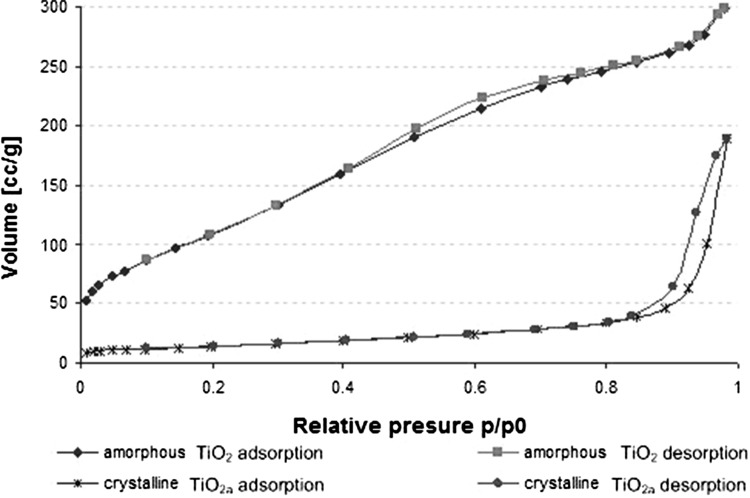
In a study by Kedziora et al. [12], adding diamminesilver(I) to the sol of TiO2 was proposed. It caused a decrease in the size of silver nanoparticles to less than 5 nm [12]. Calcination of amorphous titanium (TiO2) did not change the silver crystallite size. X-ray diffraction lines of amorphous and crystalline TiO2 are shown in Fig. 2. All of these are own results, and the obtained data were compared with the ICDD database. We confirmed that amorphous TiO2 powder calcinated at 400 °C is already well crystallized to anatase (Fig. 2b, d). All reflections in the diffraction patterns shown in Fig. 2b were identified as belonging to anatase (according to ICDD Database No. 99-200-3744, 38.78 2Theta). In the pattern observed in Fig. 2a, c, the amorphous nature of TiO2 was also proved [lack of clear peaks and shoulder peak around 23° (2θ)]. In Fig. 2c, there is a visible peak showing the presence of pure nano Ag [peak around 38° (2θ), with silver content estimated at 10 wt% with AAS, Table 1] embedded in amorphous titanium samples (according to ICDD Database No. 99-101-3086, 38.25 2Theta). In Fig. 2d (diffraction lines of TiO2a/Ag0), peaks of anatase can be observed. However, no visible silver peak in Fig. 2d probably results from (1) a low content of silver (estimated at 1 wt% for TiO2a/Ag0, see Table 1, proved with AAS) or (2) interference of the anatase and nanosilver peaks. N2 adsorption–desorption isotherms of the amorphous and crystalline TiO2 are illustrated in Fig. 3. The isotherms of adsorption and desorption do not cover each other, which confirms that the prepared materials (amorphous TiO2 and crystalline TiO2a) are porous. From the shape of hysteresis, it can be concluded that the material is mesoporous (the pore diameter of titanium dioxide is between 2 and 50 nm) [21]. Photocatalytic properties of the prepared anatase were experimentally confirmed by measuring the decomposition of methyl blue. Average time of decomposition of methyl blue by crystalline anatase (TiO2a) was estimated at 90 min.
Fig. 2.
XRD patterns of: a amorphous TiO2, b crystalline TiO2a, c amorphous TiO2/Ag0 and d crystalline TiO2a/Ag0
Fig. 3.
N2 adsorption and desorption by amorphous TiO2 and crystalline TiO2a
The knowledge of the nanocarrier morphology (size, shape, porosity, surface area, and crystal structures) is a fundamental issue and has an important impact on the following aspects: (1) silver content in the nanocompounds, (2) antibacterial efficacy of silver nanocompounds, (3) interaction of bacteria cells with silver nanocompounds, (4) antibacterial mechanism of action, and (5) mechanism of potential bacterial resistance to silver nanomaterials. Furthermore, this knowledge is fundamental for further research concerning the influence of physicochemical properties of the compounds on the answer of bacteria cells.
Antibacterial Activity
Results of the antibacterial efficacy of silver nanocomposites are summarized in Tables 4 and 5. We checked antimicrobial activity of temporary forms—TiO2 impregnated with silver ions Ag+ (TiO2/Ag+ and TiO2a/Ag+) and permanent forms—TiO2 doped with silver nanoparticles Ag0 (TiO2/Ag0 and TiO2a/Ag0) against Gram-positive and Gram-negative bacteria. Pure TiO2 and TiO2a did not show antibacterial activity in the applied concentration (1024 µg/ml). The best efficacy was indicated in the sample based on amorphous titanium dioxide used as a carrier for silver ions (TiO2/Ag+) where MIC was equal for ions with and without UV irradiation and reached 0.05 µg/ml. Excellent efficacy is indicated in the silver nanoparticles (Ag0) also located on the amorphous TiO2 (TiO2/Ag0). Also, MIC of TiO2/Ag0 comes to 0.4 µg/ml (the same values for UV irradiation and without it). Bionanotechnologists seem to overlook the amorphous TiO2, but we proved its excellent properties as a carrier for antibacterial agents such as silver (both silver ions and nanoparticles). It is very attractive for medical and biological applications—shows a very good antibacterial efficacy even without UV treatment. Annealing to the anatase structure may be limited and stopped after the amorphous TiO2 phase. Silver nanoforms based on the crystalline TiO2a indicated lower antibacterial activity than those based on an amorphous one even after UV treatment. MIC values for TiO2a/Ag+ reached the level of 0.8–3.2 µg/ml without UV irradiation and 0.4–1.6 µg/ml for TiO2a/Ag0 after UV treatment. However, for TiO2a/Ag0 MIC values were established as 25.6–51.2 µg/ml without UV and 12.8–25.6 µg/ml after UV irradiation. It is worth underlining that the tested clinical isolates of E. coli (475, 555, 574) and K. pneumoniae 626 are resistant to penicillin and cefalosporins, and two of the tested S. aureus are MRSA bacteria strains (S. aureus 173, and 187, Table 2). When compared with the bacterial sensitivity and resistance to antibiotics, the obtained MIC values for silver nanoforms result from a different antibacterial mechanism of action. Yanez et al. [29] confirmed a good reduction of bacteria by TiO2 nanotubes doped with polyethylene composites to over 40–99 % after UV light and about 28–43 % after white light irradiation. Results of our study confirm that the major reason for high antibacterial activity of silver nanoforms is the surface area, higher in amorphous than crystalline titanium dioxide. The increased SBET of anatase is due to lower bacterial susceptibility. Li et al. [15] also showed that a diameter of TiO2 is one of the most important factors in the antibacterial efficacy. In contrast to our study, Li et al. [15] indicated that anatase has higher antibacterial activity than amorphous titania. In studies by Jasiorski et al. [10] and Wiglusz et al. [26], very good efficacy of silver nanoparticles deposited on other amorphous compounds such as silica surface [10] and hydroxyapatites [26] was proved. Amorphous TiO2 has a potential to become a perfect carrier for antibacterial metals such as silver. In comparison with the previous research [12] the MIC values for the TiO2 and TiO2a impregnated with silver ions (TiO2/Ag+, TiO2a/Ag+) on S. aureus, E. coli, and K. pneumoniae were significantly lower than the MIC values obtained for Ag nanoparticles (Tables 4, 5), so antibacterial sensitivity depends on both the kinds of carrier and silver oxidation. Silver ions react quickly but, unlike silver nanoparticles, are rapidly inactivated by environment factors, e.g., Cl−. Gao et al. [7] also proposed the production of amorphous TiAg2O which revealed very effective antibacterial properties, cytocompatibility, and long lasting effectivity. Moreover, Zhao et al. [31] proved the long-term antibacterial ability of silver nanoparticles and biointegration. In contrast to Keleher et al. [13], we demonstrated less sensibility of Gram-negative bacteria to silver nanoparticles immobilized on the anatase (TiO2a/Ag0) than in the case of Gram-positive strains. However, Jamuna-Thevi et al. [9] and Mirzajani et al. [17] proved good activity of silver nanoparticles embedded on the anatase against S. aureus. They also proved that the minimum antibacterial inhibitory concentration of Ag0 was less than the maximum cytotoxic concentration. Abdal-hay et al. [1] suggested that coating the Ti with different polymer layers causes more efficacy against bacteria.
Table 4.
MIC (µg/ml) of amorphous TiO2 doped with silver (TiO2/Ag+ and TiO2/Ag0) with and without UV irradiation
| Bacteria strains | MIC [µg/ml] TiO2/Ag+ | MIC [µg/ml] TiO2/Ag0 | ||
|---|---|---|---|---|
| Without UV | With UV | Without UV | WITH UV | |
| S. aureus ATCC 6538 | 0.05 | 0.05 | 0.4 | 0.4 |
| S. aureus 173a | 0.05 | 0.05 | 0.4 | 0.4 |
| S. aureus 187a | 0.05 | 0.05 | 0.4 | 0.4 |
| S. aureus 298a | 0.05 | 0.05 | 0.4 | 0.4 |
| E. coli ATCC 11229 | 0.05 | 0.05 | 0.4 | 0.4 |
| E. coli 475a | 0.05 | 0.05 | 0.4 | 0.4 |
| E. coli 555a | 0.05 | 0.05 | 0.4 | 0.4 |
| E. coli 574a | 0.05 | 0.05 | 0.4 | 0.4 |
| K. pneumoniae ATCC 4352 | 0.05 | 0.05 | 0.4 | 0.4 |
| K. pneumoniae 626a | 0.05 | 0.05 | 0.4 | 0.4 |
aBacterial isolates
Table 5.
MIC (µg/ml) of anatase doped with silver (TiO2a/Ag+ and TiO2a/Ag0) with and without UV irradiation
| Bacteria strains | MIC (µg/ml) TiO2a/Ag+ | MIC (µg/ml) TiO2a/Ag0 | ||
|---|---|---|---|---|
| Without UV | With UV | Without UV | With UV | |
| S. aureus ATCC 6538 | 0.8 | 0.4 | 25.6 | 12.8 |
| S. aureus 173a | 1.6 | 0.8 | 25.6 | 12.8 |
| S. aureus 187a | 1.6 | 0.8 | 25.6 | 12.8 |
| S. aureus 298a | 1.6 | 0.8 | 25.6 | 12.8 |
| E. coli ATCC 11229 | 3.2 | 1.6 | 25.6 | 12.8 |
| E. coli 475a | 3.2 | 1.6 | 51.2 | 25.6 |
| E. coli 555a | 1.6 | 0.8 | 51.2 | 25.6 |
| E. coli 574a | 3.2 | 1.6 | 25.6 | 12.8 |
| K. pneumoniae ATCC 4352 | 3.2 | 1.6 | 51.2 | 25.6 |
| K. pneumoniae 626a | 3.2 | 1.6 | 51.2 | 25.6 |
aBacterial isolates
In summary, we propose that silver nanoforms based on the amorphous TiO2 have a potential to be used in the inhibition of growth or treatment of an infection caused by MDR E. coli and K. pneumoniae strains or MRSA S. aureus.
Conclusions
Various modifications of the sol–gel technology make it possible to synthesize different silver nanoforms with great possibilities to be used in the therapy of infections. Titanium dioxide with its physical and chemical properties may be used as a carrier of silver ions and nanoparticles. Amorphous TiO2 is an ideal carrier for biologically active silver, both ions and nanoparticles, even without UV irradiation. The surface area of the carrier has an impact on the antibacterial efficacy. We have proven an alternative way to antibiotics for killing bacteria and have proposed amorphous TiO2 as a carrier for biologically and medically active silver.
Acknowledgments
This work was supported by the project “Academy of Development as the key to strengthening human resources of the Polish economy” co-financed by the European Union under the European Social Fund (beneficiary A. Kedziora, 08/12MDAR/2014). The authors thank Professor Leszek Kepinski for TEM images, Jerzy Kowalczyk PhD for research support, and DiaLab Medical Laboratory for bacteria strains.
Abbreviations
- [Ag(NH3)2]+
Diamminesilver(I)
- AAS
Atomic absorption spectroscopy
- ATCC
American Type Culture Collection
- CFU/ml
Colony-forming units per ml
- CLSI
Clinical and Laboratory Standard Institute
- ESKAPE
Group of six pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.)
- ICDD
International Centre for Diffraction Data (PDF2 Database ICDD)
- MDR
Multidrug resistance
- MIC
Minimal inhibitory concentration
- MRSA
Methicillin-resistant S. aureus
- MSSA
Methicillin-sensitive S. aureus
- RT
Room temperature
- SBET
Surface area
- TEM
Transmission electron microscopy
- Ti(O-Bu)
Titanium n-butoxide
- TiO2
Amorphous titanium dioxide
- TiO2/Ag+
Amorphous titanium dioxide doped with silver ions
- TiO2/Ag0
Amorphous titanium dioxide with silver nanoparticles
- TiO2a
Crystalline titanium dioxide (anatase)
- TiO2a/Ag+
Crystalline titanium dioxide (anatase) doped with silver ions
- TiO2a/Ag0
Crystalline titanium dioxide (anatase) with silver nanoparticles
- UV
Ultraviolet light
- XDR
Extremely drug resistance
- XRD
X-ray diffraction
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Abdal-hay A, Hwang MG, Lim JK. In vitro bioactivity of titanium implants coated with biocomponent hybrid biodegradable polymers. J Sol Gel Sci Technol. 2012;64:756–764. doi: 10.1007/s10971-012-2912-6. [DOI] [Google Scholar]
- 2.Amin SA, Pazouki M, Hosseinnina A. Synthesis of TiO2–Ag nanocomposites with sol–gel method and investigation of its antibacterial activity against E. coli. Powder Technol. 2009;196:241–245. doi: 10.1016/j.powtec.2009.07.021. [DOI] [Google Scholar]
- 3.Barudin NHA, Sreekantan S, Ong MT, Lai CW. Synthesis, characterization and comparative study of Nano-Ag–TiO2 against Gram-positive and Gram-negative bacteria under fluorescent light. Food Control. 2014;46:480–487. doi: 10.1016/j.foodcont.2014.05.046. [DOI] [Google Scholar]
- 4.Cao H, Qiao Y, Liu X, Lu T, Cui T, Meng F, Chu PK. Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta Biomater. 2013;9:5100–5110. doi: 10.1016/j.actbio.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 5.European Committee on Antimicrobial Susceptibility Testing (2015) Breakpoints tables for interpretation of MICs and zones diameters, version 5.0. http://www.eucast.org
- 6.Fournier S, Buisson-Burn C, Jarllier V. Twenty years of antimicrobial resistance control programme in a regional multi hospital institution, with focus on emerging bacteria (VRE and CPE) Antimicrob Resist Infect Control. 2012;1:1–4. doi: 10.1186/2047-2994-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao A, Hang R, Huang X, Zhao L, Zhang X, Wang L, Tang B, Ma S, Chu P. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35:4223–4235. doi: 10.1016/j.biomaterials.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Hryniewicz W, Sulikowska A, Szczypa K, Skoczynska A, Luczak-Kadłubowska A, Gniatkowski M. Rekomendacje doboru testów do oznaczania wrażliwości bakterii na antybiotyki i chemioterapeutyki. Warszawa: Polish National Institute of Public Health; 2012. [Google Scholar]
- 9.Jamuna-Thevi K, Bakar SA, Ibrahim S, Shahab N, To MRM. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum. 2011;86:235–241. doi: 10.1016/j.vacuum.2011.06.011. [DOI] [Google Scholar]
- 10.Jasiorski M, Leszkiewicz A, Brzezinski S, Bugla-Ploskonska G, Malinowska G, Borak B, Karbownik I, Baszczuk A, Strek W, Doroszkiewicz W. Textile with silver silica spheres: its antimicrobial activity against Escherichia coli and Staphylococcus aureus. J Solgel Sci Technol. 2009;51:330–334. doi: 10.1007/s10971-009-1902-9. [DOI] [Google Scholar]
- 11.Kedziora A, Gorzelanczyk K, Bugla-Ploskonska G. Positive and negative aspects of silver nanoparticles usage. Biol Int. 2013;53:67–76. [Google Scholar]
- 12.Kedziora A, Strek W, Kepinski L, Bugla-Ploskonska G, Doroszkiewicz W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J Solgel Sci Technol. 2012;62:79–86. doi: 10.1007/s10971-012-2688-8. [DOI] [Google Scholar]
- 13.Keleher J, Bashant J, Heldt N, Johnson L, Li Y. Photo-catalytic preparation of silver-coated TiO2 particles for antibacterial applications. World J Microbiol Biotechnol. 2002;18:133–139. doi: 10.1023/A:1014455310342. [DOI] [Google Scholar]
- 14.Li B, Yi Y, Wang Q, Woo PCY, Tan L, Jing H, Gao GF, Liu CH. Analysis of drug resistance determinants in Klebsiella pneumoniae isolates from a Tertiary-Car Hospital in Beijing. PLoS ONE. 2012 doi: 10.1371/journal.pone.0042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Cui B, Feng B, Wang J, Lu X, Weng J. Antibacterial activity of TiO2 nanotubes: influence of crystal phase, morphology and Ag deposition. Appl Surf Sci. 2013;284:179–183. doi: 10.1016/j.apsusc.2013.07.076. [DOI] [Google Scholar]
- 16.Mijnendonckx K, Leys N, Mahillon J, Silver S, Houdt R. Antimicrobial silver: uses, toxicity and potential for resistance. Biometals. 2013;26:609–621. doi: 10.1007/s10534-013-9645-z. [DOI] [PubMed] [Google Scholar]
- 17.Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res Microbiol. 2011;162:542–549. doi: 10.1016/j.resmic.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Prabakar K, Sivalingam P, Mohamed Rabeek SI, Muthuselvam M, Devarajan AA, Karthick R, Suresh MM, Wembonyama JP. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial Gram-negative pathogens. Colloids Surf B Biointerfaces. 2013;104:282–288. doi: 10.1016/j.colsurfb.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, Khmel IA. Antibacterial effects of silver nanoparticles on Gram-negative bacteria: influence on the growth and biofilm formation, mechanisms of action. Colloids Surf B Biointerfaces. 2013;102:300–306. doi: 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Rosario AV, Christinelli WA, Barreto RN, Pereira EC. Investigation of photocatalytic activity of metal-doped TiO2 nanoparticles prepared by Pechini method. J Solgel Sci Technol. 2012;64:734–742. doi: 10.1007/s10971-012-2910-8. [DOI] [Google Scholar]
- 21.Sarbak Z. Adsorpcja i adsorbenty. Teoria i zastosowanie. Poznan: Wydawnictwo Naukowe UAM; 2000. [Google Scholar]
- 22.Veleba M, Higgins PG, Gonzales G, Seifert H, Schneiders T. Characterization of RarA, a Novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56:4450–4458. doi: 10.1128/AAC.00456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viana MM, Mohallem NDS, Miquita DR, Balzuweit K, Silva-Pinto E. Preparation of amorphous and crystalline Ag/TiO2 nanocomposite thin films. Appl Surf Sci. 2012;265:130–136. doi: 10.1016/j.apsusc.2012.10.151. [DOI] [Google Scholar]
- 24.Virella G. Microbiology and infectious diseases. Philadelphia: Wiliam & Wilkins; 1997. [Google Scholar]
- 25.Wang L, Gu H, Lu X. A rapid low-cost real time PCR for the detection of Klebsiella pneumoniae carbapenemase genes. Ann Clin Microbiol Antimicrob. 2012;91:361–366. doi: 10.1186/1476-0711-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiglusz RJ, Kedziora A, Lukowiak A, Doroszkiewicz W, Strek W. Hydroxyapatites and europium(III) doped hydroxyapatites as a carrier of silver nanoparticles and their antimicrobial activity. J Biomed Nanotechnol. 2012;8:1–8. doi: 10.1166/jbn.2012.1424. [DOI] [PubMed] [Google Scholar]
- 27.Wikler MA, Cockerill FR, Craig WA, Dudley MN, Eliopoulos GM, Hecht DW (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically approved standard—seventh edition M7-A5 NCCLS, 26
- 28.Wyrwa J, Rekas M. Fotokatalityczna degradacja błękitu metylenowego przy użyciu nanokrystalicznego TiO2. Ceram Mater. 2011;63(3):524–527. [Google Scholar]
- 29.Yanez D, Guerrero S, Lieberwirth I, Ulloa MT, Gomez T, Rabagliati FM, Zapata PA. Photocatalytic inhibition of bacteria by TiO2 nanotubes-doped polyethylene composites. Appl Catal A. 2015;489:255–261. doi: 10.1016/j.apcata.2014.10.051. [DOI] [Google Scholar]
- 30.Yin HB, Wada Y, Kitamura T, Kambe S, Murasawa S, Mori H, Sakata T, Yanagida S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J Mater Chem. 2001;11:1694–1703. doi: 10.1039/b008974p. [DOI] [Google Scholar]
- 31.Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni W, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32:5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]