Abstract
In this age of intensive industrialization and urbanization, mankind’s highest concern should be to analyze the effect of all metals accumulating in the environment, both those considered toxic and trace elements. With this aim in mind, a unique study was conducted to determine the potentially negative impact of Sn2+, Co2+, and Mo5+ in optimal and increased doses on soil biological properties. These metals were applied in the form of aqueous solutions of Sn2+ (SnCl2.2H2O), Co2+ (CoCl2 · 6H2O), and Mo5+ (MoCl5), each in the doses of 0, 25, 50, 100, 200, 400, and 800 mg kg−1 soil DM. The activity of dehydrogenases, urease, acid phosphatase, alkaline phosphatase, arylsulfatase, and catalase and the counts of twelve microorganism groups were determined on the 25th and 50th day of experiment duration. Moreover, to present the studied problem comprehensively, changes in the biochemical activity and yield of spring barley were shown using soil and plant resistance indices—RS. The study shows that Sn2+, Co2+, and Mo5+ disturb the state of soil homeostasis. Co2+ and Mo5+ proved the greatest soil biological activity inhibitors. The residence of these metals in soil, particularly Co2+, also generated a drastic decrease in the value of spring barley resistance. Only Sn2+ did not disrupt its yielding. The studied enzymes can be arranged as follows for their sensitivity to Sn2+, Co2+, Mo5+: Deh > Ure > Aryl > Pal > Pac > Cat. Dehydrogenases and urease may be reliable soil health indicators.
Keywords: Cobalt, Tin, Molybdenum, Soil enzymes, Microbiological activity
Introduction
In recent years, the attention of researchers has been focused mainly on heavy metals considered most harmful to the environment. These include, among others, Cd, Hg, Cr, Pb, and Cu (Wyszkowska et al. 2013; Zaborowska et al. 2015a). However, researchers have also increasingly focused on the contamination of soils with trace elements (Islam et al. 2015). This has its justification in that a common feature of all metals, regardless of their negative impact, is the fact that none of them biodegrade; they are characterized by a long biological half-life and show the potential to accumulate inside living organisms (Behbahaninia et al. 2009; Madrid et al. 2008). The side effects of their accumulation in the soil environment are therefore a problem which should be taken up and brought up for discussion, particularly in this age of dramatic industrialization and urbanization to meet the growing requirements of the human population.
Co2+ belongs to a group of transition metals. It most often assumes the +2, +3, and less often a +1 oxidation state. Only the 59Co isotope, representing 100 % of the isotopic composition of natural Co2+, is stable. It is a component of the following minerals: erythrite [Co3(AsO4)2.8H2O], glaucodot [(Co, Fe)AsS], and skutterudite (CoAs3) (Albanese et al. 2015; Shedd 2013). In Europe, the Mediterranean Basin countries are characterized by a higher Co2+ content than Northern Europe. This phenomenon is closely related to ophiolitic rocks (mafic and ultramafic) (Albanese et al. 2015). Contamination of soils with Co2+ is mainly caused by mining and smelting activity, fertilizer use, and sewage sludge spreading (Hamilton 2000). The production of liquid catalysts used in refineries is a source of environmental pollution with both Co2+ and Mo5+ (Lewis et al. 2012). In soil, this metal is closely correlated with Mn, Fe, and Al (Sterckeman et al. 2006). The bioavailability of Co2+ and, thus, its toxicity is also affected by the physicochemical properties of the soil environment such as structure, organic matter, pH, and complexing compounds (Luo 2010). Exposure to increased amounts of Co2+ in soil causes side effects both among soil microorganisms (Lock et al. 2006) and plants (Chatterjee and Chatterjee 2003). The response to an excess of Co2+ in a plant is heightened activity of superoxide dismutase (SOD), an enzyme responsible for O2 dismutation and an increase in iron sequestration and ferritin synthesis (Tewari et al. 2002). Co2+ plays a quite important role in the human body because it is the central atom of cobalamin, a coenzyme precursor, whose deficiency causes anemia (Paustenbach et al. 2013). Nevertheless, its genotoxic properties were also discovered, which should be taken into account in the overall Co2+ assessment (Kirkland et al. 2015).
The average Sn2+ content of the Earth’s crust is estimated at 2.5 mg kg−1. This metal is a component of cassiterite (SnO2), stannite (Cu2FeSnS4), teallite (PbSnS2), and montesite (PbSn4S5) (Pendias and Pendias 2001). In 2012, the world Sn2+ production amounted to 230 Mg (metric tons). The main leaders were China, Indonesia, Peru, and Bolivia (USDI 2013). Because Sn2+ is a component of nuclear waste, including 235U fission products, where the half-life of 126Sn is 105 years and of 121Sn 55.5 years (National Research Council 1983), the effects of soil Sn2+ mobility should be examined. Organotin compounds (OTC) which are part of fungicides, insecticides, bactericides, wood preservatives, and PVC stabilizers pose a potential threat to the environment (Hoch 2001). The inorganic tin forms are less toxic, but the effects of their soil presence are also less known (Marcic et al. 2006). Tendencies have been observed for the affinity of Sn2+ mobility to the soil size fraction and its organic matter content (Sterckeman et al. 2006). Due to its low soil solubility, Sn2+ translocation and uptake by plants is minimal, but increases with decreasing pH (Nakamaru and Uchida 2008).
Mo5+, as a trace element, is necessary in the environment in small amounts. The sources of excessive Mo5+ emission to the environment are mining; biosolids; fertilizers; the production of alloys, catalysts, and coal; and petroleum combustion (Mcgrath et al. 2010; Buekers et al. 2010). It occurs in the soil in the form of an oxyanion, in aluminosilicates and organic matter (Pyrzyńska 2007). In the soil environment, the Mo5+ amount is correlated with the presence of Fe, Al, and organic carbon content (Sterckemann et al. 2006), while with Cu2+, they are antagonists (Pyrzyńska 2007). In acidic soils, the dominant, soluble Mo5+ form is the anion MoO42−. In neutral and alkaline medium, Mo5+ forms mobile anionic complex compounds (Buekers et al. 2010). The biological role of Mo5+ is based on co-forming the pterin complex (a Mo cofactor), binding with enzymes participating in nitrogen (nitrate reductase) and sulfur (sulfite oxidase) metabolism, purine catabolism, and hormone biosynthesis. Over 50 enzymes of bacterial origin containing Mo5+ have been identified (Mendel and Bittner 2006). Nevertheless, both a deficiency and excessive exposure to this metal can cause abnormalities in the functioning of living organisms and, thus, ecosystems (Mcgrath et al. 2010), although according to Das et al. (2007), its direct effect on the metabolic processes of microorganisms is relatively low. An increased content of this element can possibly reduce nitrogen fixation.
Soil condition is closely related to microorganism activity and is considered a reliable indicator of the impact of environmental stress on soil (Epelde et al. 2008). Since the effects and range caused by contamination of soils with Sn2+, Co2+, and Mo5+ have not yet been widely studied, determining the biological activity of a soil environment subjected to the pressure of this group of metals seems a necessary step in a strategy to assess the scale of the problem related to Sn2+, Co2+, and Mo5+ accumulation or question the justifiability of the formulated hypothesis.
Materials and Methods
Experimental. Soil sampling and samples preparation
The study object was soil material from the Didactic and Experimental Center in Tomaszkowo. The area designated for research purposes, including protective belts, occupies around 4.5 ha. Soil with a granulometric composition of loamy sand (fraction of sand is 75 %, silt—17 %, clay—8 %) determined according to the US Department of Agriculture’s particle size distribution classification was collected from the arable-humus horizon of typical brown soils (Eutric Cambisol). The studied soil is characterized by the following properties: pH w 1 mol KCl dm3—6.3, Corg kg−1 dm of soil—6.4, hydrolytic acidity (HAC)—8.4 mmol(+) per kilogram of soil, sum of exchangeable bases (TEB) Ca2+, Mg2+, K+, and Na+—84 mmol(+) per kilogram of soil, cation exchange capacity (CEC)—92.40 mmol(+) per kilogram of soil, and base saturation (BS)—90.91 %. The next stage of the experiment was conducted in the greenhouse of the University of Warmia and Mazury in Olsztyn (NE Poland) based on a pot test, replicated five times.
The effect of the following variable factors was assessed: (1) the type of the heavy metals used: Sn2+ (SnCl2 · 2H2O), Co2+ (CoCl2 · 6H2O), and Mo5+ (MoCl5); (2) the degree of soil contamination with Sn2+, Co2+, and Mo5+ in milligrams per kilogram soil DM: 0, 25, 50, 100, 200, 400, and 800; and (3) test duration: 25 and 50 days. An analysis of the impact of the three metals on the yield of spring barley cv. Rabel was also performed.
Before conducting the experiment, the soil material was prepared by contaminating it with the individual heavy metals and adding NPKMg fertilizers. After mixing the soil in a polyethylene vessel and packing it in pots (3.5 dm3), in the amount of 3.2 kg per pot, the soil moisture in all the objects was brought to the level of 60 % of capillary water capacity. One level of fertilization with macro- and microelements was used and was expressed on an elemental basis in milligrams per kilogram soil: N—250 [CO(NH2)2], P—50 (KH2PO4), K—90 (KH2PO4), Mg—20 (MgSO4. 7H2O), Cu—5 (CuSO4 · 5H2O), Zn—5 (ZnCl2), Mo—5 (NaMoO4 · 2H2O), Mn—5 (MnCl2 · 4H2O), and oraz B—0.33 (H3BO3).
The vegetation of spring barley cv. Rabel was conducted for 50 days. Fifteen plants were left in each pot after seedling. The plant dry matter yield was determined after spring barley harvest at stage (according to the BBCH scale) 52—heading (20 % of inflorescence emerged).
Microbiological and biochemical analysis
In each soil sample, the activity of the enzymes dehydrogenases, catalase, urease, acid phosphatase, and alkaline phosphatase were determined in three replications, according to the methods given in Tables 1 and 2. Soil biochemical activity, except for catalase, was established using a Perkin-Elmer Lambda 25 spectrophotometer (MA, USA). The analyses of soil were performed after 25 and 50 days of the experiment.
Table 1.
Determined soil enzymes
| No. | Enzyme | Substrate | Unit | References |
|---|---|---|---|---|
| 1 | Dehydrogenases (EC 1.1) | 2,3,5-triphenyl tetrazolium chloride | Triphenyl formazan (μmol kg−1 dm of soil h−1) | Öhlinger (1996) |
| 2 | Catalase (EC 1.11.1.6) | H2O2—aqueous solution | O2 (mol kg−1 dm of soil h−1) | Alef and Nannpieri (1998) |
| 3 | Urease (EC 3.5.1.5) | Urea—aqueous solution | N-NH4 (mmol kg−1 dm of soil h−1) | |
| 4 | Acid phosphatase (EC 3.1.3.2) alkaline phosphatase (EC 3.1.3.1) | Disodium—4-nitrophenylphosphate hexahydrate | p-nitrophenol (mmol kg−1 dm of soil h−1) | |
| 5 | Arylsulphatase (EC 3.1.6.1) | Potassium-4-nitrophenyl-sulfate |
Table 2.
Activity enzymes in soil contaminated of Sn2+, Co2+, and Mo5+ (for average values of the research time) (kg1 DM soil h−1)
| Dose heavy metals (mg kg−1) | Deh | Ure | Pac | Pal | Cat | Aryl |
|---|---|---|---|---|---|---|
| μmol TFF | mmol N-NH4 | mmol PNP | mmol PNP | mol O2 | mmol PNP | |
| Sn2+ | ||||||
| 0 | 9.254 ± 0.063 | 1.050 ± 0.020 | 2.245 ± 0.066 | 2.195 ± 0.017 | 0.275 ± 0.008 | 0.236 ± 0.008 |
| 25 | 8.572 ± 0.070 | 0.954 ± 0.024 | 2.116 ± 0.060 | 2.161 ± 0.009 | 0.274 ± 0.013 | 0.215 ± 0.000 |
| 50 | 8.442 ± 0.083 | 0.911 ± 0.016 | 2.088 ± 0.017 | 2.118 ± 0.017 | 0.273 ± 0.008 | 0.204 ± 0.000 |
| 100 | 8.011 ± 0.044 | 0.923 ± 0.024 | 2.032 ± 0.024 | 2.095 ± 0.026 | 0.271 ± 0.010 | 0.204 ± 0.000 |
| 200 | 7.405 ± 0.125 | 0.882 ± 0.026 | 2.008 ± 0.023 | 1.970 ± 0.017 | 0.266 ± 0.013 | 0.174 ± 0.000 |
| 400 | 6.549 ± 0.081 | 0.800 ± 0.022 | 2.042 ± 0.032 | 1.912 ± 0.017 | 0.259 ± 0.012 | 0.177 ± 0.000 |
| 800 | 5.579 ± 0.072 | 0.648 ± 0.038 | 1.991 ± 0.000 | 1.803 ± 0.009 | 0.249 ± 0.010 | 0.166 ± 0.000 |
| Average | 7.687 | 0.881 | 2.074 | 2.036 | 0.267 | 0.194 |
| r | −0.938 | −0.872 | −0.584 | −0.850 | −0.854 | −0.868 |
| Co2+ | ||||||
| 0 | 8.551 ± 0.128 | 1.040 ± 0.037 | 2.263 ± 0.087 | 2.202 ± 0.009 | 0.276 ± 0.007 | 0.235 ± 0.000 |
| 25 | 6.714 ± 0.158 | 0.911 ± 0.019 | 2.061 ± 0.079 | 1.964 ± 0.032 | 0.266 ± 0.011 | 0.207 ± 0.000 |
| 50 | 4.836 ± 0.078 | 0.832 ± 0.033 | 2.032 ± 0.023 | 1.755 ± 0.000 | 0.251 ± 0.011 | 0.191 ± 0.000 |
| 100 | 2.971 ± 0.167 | 0.765 ± 0.019 | 1.941 ± 0.036 | 1.769 ± 0.018 | 0.239 ± 0.007 | 0.183 ± 0.000 |
| 200 | 2.102 ± 0.042 | 0.628 ± 0.021 | 1.921 ± 0.067 | 1.573 ± 0.033 | 0.200 ± 0.009 | 0.169 ± 0.000 |
| 400 | 2.138 ± 0.014 | 0.649 ± 0.014 | 1.799 ± 0.027 | 1.476 ± 0.027 | 0.200 ± 0.009 | 0.168 ± 0.000 |
| 800 | 1.940 ± 0.023 | 0.309 ± 0.019 | 1.760 ± 0.018 | 1.370 ± 0.027 | 0.194 ± 0.011 | 0.155 ± 0.000 |
| Average | 4.179 | 0.733 | 1.968 | 1.730 | 0.232 | 0.187 |
| r | −0.677 | −0.902 | −0.752 | −0.791 | −0.797 | −0.735 |
| Mo5+ | ||||||
| 0 | 8.720 ± 0.187 | 1.016 ± 0.023 | 2.218 ± 0.065 | 2.208 ± 0.026 | 0.272 ± 0.008 | 0.252 ± 0.008 |
| 25 | 7.708 ± 0.059 | 0.885 ± 0.009 | 2.126 ± 0.025 | 2.189 ± 0.039 | 0.243 ± 0.009 | 0.239 ± 0.000 |
| 50 | 6.599 ± 0.034 | 0.640 ± 0.025 | 2.025 ± 0.040 | 2.090 ± 0.018 | 0.241 ± 0.008 | 0.206 ± 0.000 |
| 100 | 6.170 ± 0.082 | 0.654 ± 0.012 | 1.894 ± 0.024 | 2.078 ± 0.018 | 0.226 ± 0.012 | 0.170 ± 0.000 |
| 200 | 5.075 ± 0.054 | 0.605 ± 0.012 | 1.778 ± 0.015 | 2.004 ± 0.018 | 0.223 ± 0.013 | 0.167 ± 0.000 |
| 400 | 3.467 ± 0.084 | 0.527 ± 0.016 | 1.540 ± 0.039 | 1.707 ± 0.009 | 0.211 ± 0.009 | 0.141 ± 0.000 |
| 800 | 1.681 ± 0.081 | 0.152 ± 0.010 | 1.463 ± 0.033 | 1.386 ± 0.028 | 0.195 ± 0.010 | 0.087 ± 0.000 |
| Average | 5.631 | 0.640 | 1.863 | 1.952 | 0.230 | 0.180 |
| r | −0.929 | −0.888 | −0.892 | −0.988 | −0.811 | −0.915 |
| LSD0.05 | a—0.654 b—0.043 a, b—0.113 |
a—0.016 b—0.010 a, b—0.028 |
a—0.032 b—0.021 a, b—0.055 |
a—0.015 b—0.010 a, b—0.026 |
a—0.007 b—0.005 a, b—0.013 |
a—0.161 b—0.105 a, b—0.279 |
a dose of heavy metal, b kind of heavy metal, Deh dehydrogenases, Ure urease, Pac acid phosphatase, Pal alkaline phosphatase, Cat catalase, Aryl arylsuphatase
On days 25 and 50 of the experiment, the soil samples were tested for the number of twelve microorganism groups: cellulolytic bacteria, ammonification bacteria, nitrogen-immobilizing bacteria, Arthrobacter sp., Azotobacter sp., and Pseudomonas sp. on a medium described by Wyszkowska et. al. (2008), Actinobacteria—on the medium developed by Küster and Williams with the addition of nystatin and actidione (Parkinson et al. 1971), fungi—on Martin medium (1950), and copiotrophic bacteria, copiotrophic spore-forming bacteria, oligotrophic bacteria, and oligotrophic spore-forming bacteria on Onta and Hattori (1983) medium. The number of microorganisms was determined with a colony counter.
Calculations and statistical analysis
The activity of soil enzymes and spring barley yield were used to determine soil and plants resistance (RS) to Sn2+, Co2+, and Mo5+ contamination. Calculations were made with a formula proposed by Orwin and Wardle (2004):
where: D0—difference between control soil (C0) and contaminated soil after 25 days of incubation (t0). The values of RS remain in the range of 0 to 1, where 1 indicates strong soil resistance, i.e., negligible effects of external factors. The lower the resistance, the stronger the impact of a given factor on the soil environment.
The results were processed statistically using Statistica 10.0 software (StatSoft, Inc. 2012). Homogeneous groups were calculated by Tukey’s test, at p = 0.01. Pearson’s simple correlation coefficients between increasing Sn2+, Co2+, and Mo5+ doses and the activity of individual enzymes were also determined. The effect of the time of soil residence of the tested metals on its biochemical activity was illustrated using principal component analysis (PCA). The reaction of microorganisms to soil contamination with Sn2+, Co2+, and Mo5+ was analyzed using a clustering method (Ward’s method dendrogram). The percentage of variation for all analyzed variables (η2) was determined with the analysis of variance (ANOVA).
Results and discussion
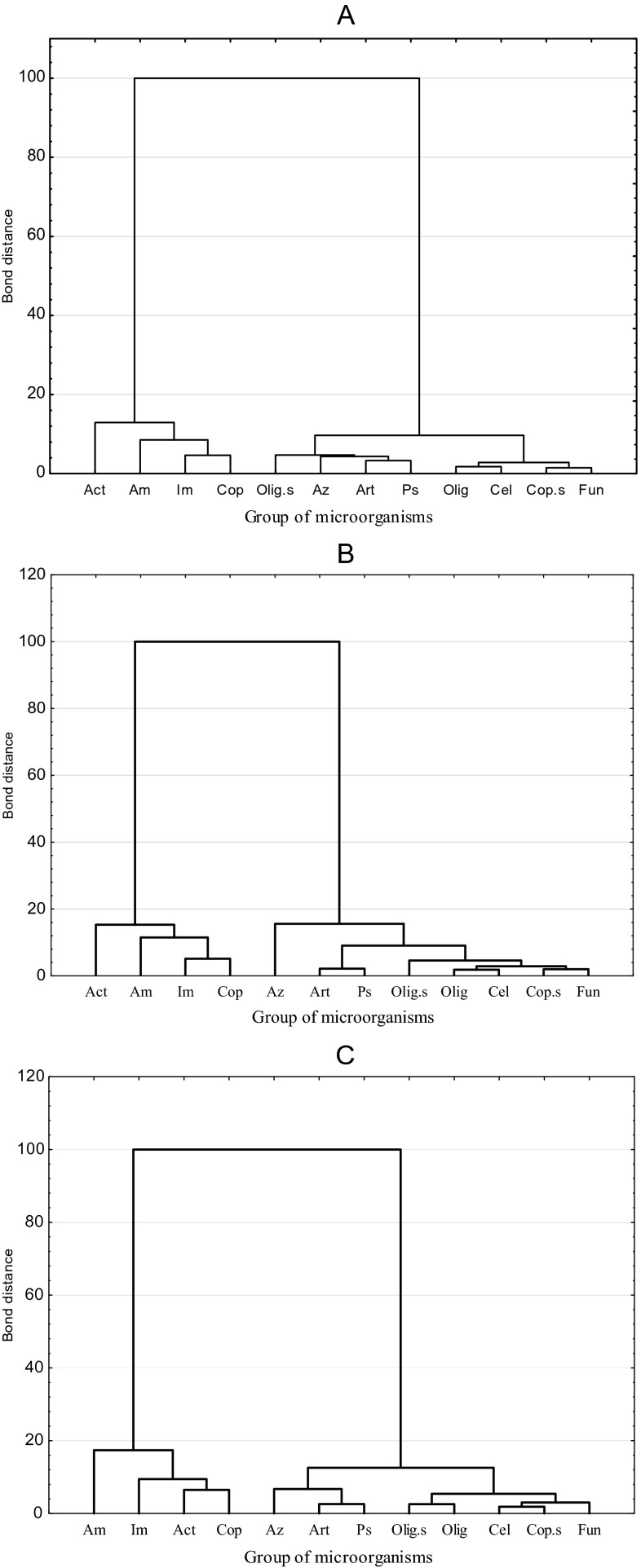
A significant inhibitory impact of all the used metals on soil biological properties was noted in the conducted study. This thesis can be substantiated by high values of the η2 coefficient. The major factor modifying the condition of the soil subjected to the pressure of Sn2+, Co2+, and Mo5+ to the highest degree was the dose (54.25 %) and, to a lower degree, the xenobiotic type (11.7 %). The reaction of microorganisms to individual metals was similar (Fig. 1a–c). This was illustrated in cluster analysis diagrams by Ward’s method. Two separate clusters consisting of several subclusters with homogeneous variances were formed by the following: ammonifying, nitrogen-immobilizing, copiotrophic bacteria and Actinobacteria (the first cluster) and Arthrobacter sp., Azotobacter sp., Pseudomonas sp., oligotrophic bacteria, oligotrophic spore-forming bacteria, cellulolytic bacteria, fungi, and copiotrophic spore-forming bacteria (the second cluster). The obtained tendencies are puzzling because Pseudomonas sp., due to its ability to produce exopolysaccharide (EPS) responsible for biosorption or bioaccumulation, acquires features of resistance to heavy metals (Kilic and Donmez 2008). On the other hand, genus Azotobacter bacteria, which were located within the same cluster, are considered some of most sensitive to this group of xenobiotics (Borowik et al. 2014). Wang et al. (2010) suggest that gram-positive bacteria are more susceptible to contamination with heavy metals than gram-negative bacteria. In their toxicity ranking, Co2+ took the place: Cr > Pb > As > Co > Zn > Cd > Cu. Root exudates accumulating in the barley rhizosphere, including: glucose, glutamic acid, citric acid, and oxalic acid (Renella et al. 2006), which are not neutral for microbiological activity and diversity, could also prove a factor moderating the reactions of individual microorganism groups.
Fig. 1.
Similarity of microbial reaction to contamination of soil with a Sn2+, b Co2+, and c Mo5+. Am ammonifying bacteria, Im nitrogen-immobilizing bacteria, Act Actinobacteria, Cop copiotrophic bacteria, Cop.s spore-forming copiotrophic bacteria, Az Azotobacter sp., Art. Arthrobacter sp., Ps. Pseudomonas sp., Olig oligotrophic bacteria, Olig.s spore-forming oligotrophic bacteria, Cel cellulolytic bacteria, Fun fungi
Based on an analysis of changes in soil biochemical properties, it can be clearly stated that regardless of the type of applied metal, dehydrogenases, and urease are the most sensitive enzymes (Table 1). In the objects with 800 mg Sn2+, Co2+, and Mo5+ per kilogram soil, their activity decreased, respectively, by: 39.71 and 38.28 %, 77.37 and 70.28 %, and 80.72 and 85.04 % compared to control samples. Taking into account the sensitivity of individual enzymes to the tested metals, they can be arranged as follows: for Sn2+, Deh > Ure > Aryl > Pal > Pac > Cat; for Co2+, Deh > Ure > Pal > Aryl > Cat > Pac; and for Mo5+, Ure > Deh > Aryl > Pac > Cat > Pal. Each metal showed varied toxicity. Based on own research, Co2+ can be considered the strongest inhibitor of biochemical properties. Under its pressure, enzymatic activity was inhibited, on average, by 25.24 %. Mo5+ ranked second (24.00 %) and Sn2+ third (11.42 %).
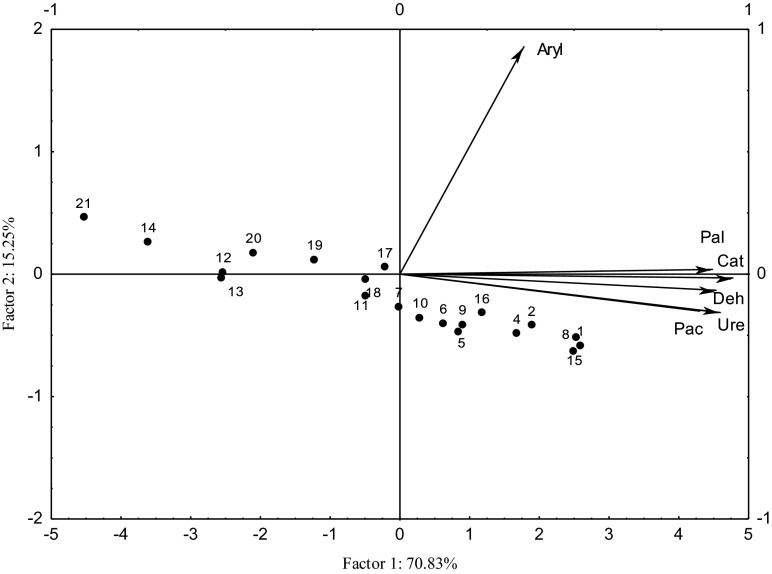
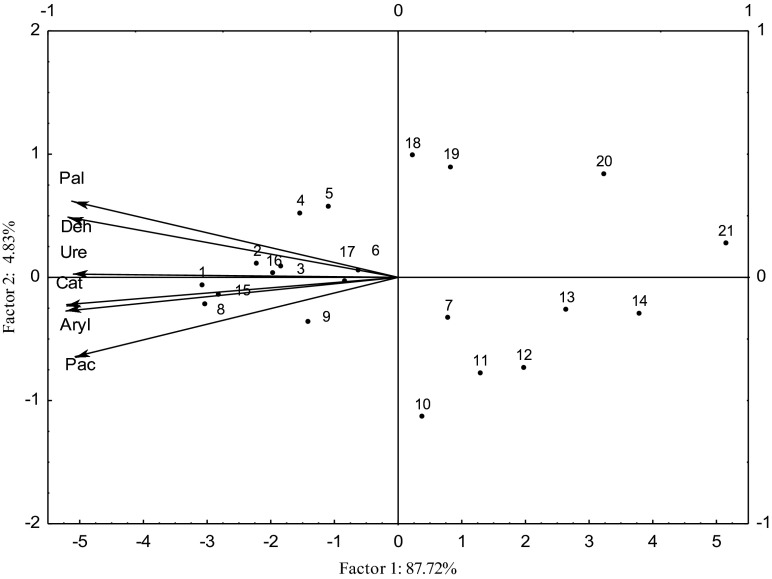
A PCA analysis including the time of soil Sn2+, Co2+, and Mo5+ residence revealed detailed relationships important in the study (Figs. 2 and 3). Both after 25 and 50 days of the experiment, the distribution of vectors around the axis representing the first principal component describing 70.83 and 87.72 % of the total data variance, respectively, indicates that regardless of heavy metal type, the activity of all the enzymes was positively correlated with this variable. Arylsulfatase activity was of the greatest importance only in the objects incubated for 25 days, for the second principal component defining 15.25 %. The distances between cases and the values of their coordinates indicate a negative effect of both Co2+ and Mo5+ in doses above 200 mg metal per kilogram soil DM (Fig. 2). After 50 days of study duration, a higher toxic impact of Co2+ was manifested—from the dose of 100 mg Co2+ per kilogram soil DM, and for Mo5+ after the application of 400 and 800 mg Mo5+ per kilogram soil DM (Fig. 3). Soil contamination with Sn2+ did not significantly disturb its biochemical properties in either of these objects.
Fig. 2.
Enzyme activity in soil contaminated with Sn2+, Co2+, and Mo5+ after 25 days of experiments—PCA method. Vectors represent the analyzed variables. Deh dehydrogenases, Ure urease, Pal alkaline phosphatase, Pac acid phosphatase, Cat catalase. Dose Sn2+, Co2+, and Mo5+ mg kg−1 DM soil: 0 (cases 1, 8, 15), 25 (2, 9, 16), 50 (3, 10, 17), 100 (4, 11, 18), 200 (5, 12, 19), 400 (6, 13, 20), and 800 (7, 14, 21). Cases 1–7 with Sn2+, 8–14 with Co2+, and 15–21 with Mo5+
Fig. 3.
Enzyme activity in soil contaminated with Sn2+, Co2+, Mo5+ after 50 days of experiments—PCA method. Vectors represent the analyzed variables. Deh dehydrogenases, Ure urease, Pal alkaline phosphatase, Pac acid phosphatase, Cat catalase. Dose Sn2+, Co2+, and Mo5+ mg kg−1 DM soil: 0 (cases 1, 8, 15), 25 (2, 9, 16), 50 (3, 10, 17), 100 (4, 11, 18), 200 (5, 12, 19), 400 (6, 13, 20), and 800 (7, 14, 21). Cases 1–7 with Sn2+, 8–14 with Co2+, and 15–21 with Mo5+
The problem of soil stability, besides microbiological and biochemical indicators, defines its quality in a broader spectrum (Griffiths and Phillipot 2013). Therefore, the soil resistance index (RS) dealing with this phenomenon was calculated in the study (Table 3). After the application of 25 mg Sn2+, Co2+, and Mo5+ per kilogram soil DM, the lowest resistance values were noted in the case of Sn2+ and Mo5+ for urease and Co2+ for dehydrogenases. The RS index also highlighted the interrelationship between deepening soil homeostasis disturbance and increasing the inhibition power of the tested heavy metals. The soil balance was disturbed most severely by Co2+, followed by Mo5+ and Sn2+, reducing the resistance of all the enzymes, on average, by 32.35, 30.48, and 11.47 % compared to the samples with the lowest dose of the metals.
Table 3.
Indicators of enzymes resistance (RS) to soil contamination with Sn2+, Co2+, and Mo5+ after 50 days of the research
| Dose heavy metals (mg kg−1) | Deh | Ure | Pac | Pal | Cat | Aryl |
|---|---|---|---|---|---|---|
| Sn2+ | ||||||
| 25 | 0.863ab | 0.763a | 0.905abc | 0.952ab | 0.958ab | 0.772b |
| 50 | 0.823b | 0.752a | 0.860abcd | 0.853de | 0.992a | 0.780b |
| 100 | 0.811b | 0.742a | 0.756cde | 0.865cd | 0.992a | 0.784b |
| 200 | 0.717c | 0.762a | 0.792abcd | 0.874cd | 0.877bc | 0.631c |
| 400 | 0.511f | 0.758a | 0.798abcd | 0.770f | 0.832c | 0.651c |
| 800 | 0.406g | 0.355e | 0.783bcd | 0.624g | 0.827c | 0.503d |
| average | 0.688 | 0.689 | 0.816 | 0.823 | 0.913 | 0.687 |
| r | −0.964* | −0.883* | −0.478 | −0.959* | −0.833* | −0.933* |
| Co2+ | ||||||
| 25 | 0.654d | 0.753a | 0.888abc | 0.800f | 0.915abc | 0.781b |
| 50 | 0.353g | 0.500d | 0.850abcd | 0.556h | 0.840c | 0.610c |
| 100 | 0.193h | 0.536cd | 0.774bcd | 0.576gh | 0.706d | 0.507d |
| 200 | 0.114i | 0.526cd | 0.743cde | 0.552h | 0.593ef | 0.496d |
| 400 | 0.111i | 0.469d | 0.593ef | 0.499i | 0.601ef | 0.491d |
| 800 | 0.111i | 0.191f | 0.588ef | 0.435j | 0.554f | 0.385e |
| average | 0.256 | 0.496 | 0.739 | 0.570 | 0.702 | 0.545 |
| r | −0.618 | −0.884* | −0.895* | −0.727* | −0.781* | −0.775* |
| Mo5+ | ||||||
| 25 | 0.893a | 0.659ab | 0.961a | 0.989a | 0.838c | 0.920a |
| 50 | 0.597de | 0.565bcd | 0.937ab | 0.924b | 0.833c | 0.619c |
| 100 | 0.591e | 0.617bc | 0.760cde | 0.913bc | 0.680de | 0.504d |
| 200 | 0.487f | 0.570bcd | 0.705def | 0.813ef | 0.660de | 0.484d |
| 400 | 0.202h | 0.323e | 0.543f | 0.589gh | 0.556f | 0.370e |
| 800 | 0.150hi | 0.032g | 0.548f | 0.394j | 0.534f | 0.199f |
| average | 0.486 | 0.461 | 0.742 | 0.770 | 0.683 | 0.516 |
| r | −0.870* | −0.984* | −0.839* | −0.983* | −0.849* | −0.842* |
The same superscripted letters in the columns indicate homogeneous groups; n = 17
r correlation coefficient, Deh dehydrogenases, Ure urease, Pac acid phosphatase, Pal alkaline phosphatase, Cat catalase, Aryl arylsuphatase
*significant for p = 0.01
Many researchers share the view that a decrease in the enzymatic activity of soils is a manifestation of abiotic stress caused by accumulated heavy metals in excessive amounts (Kucharski et al. 2011; Wyszkowska et al. 2013; Zaborowska et al. 2015b; Xian et al. 2015). Dehydrogenases are considered the most sensitive parameters used to assess the effects of soil environment contamination (Gil-Sotres et al. 2005). In the toxicity series (ED50) for dehydrogenases, Co2+ was placed as follows: Hg (2 mg) > Cu (35 mg) > Cr6+ (71 mg) > Cr3+ (75 mg) > Cd2+ (90 mg) > Ni2+ (100 mg) > Zn2+ (115 mg) > As3+ (168 mg) > Co2+ (582 mg) > Pb2+ (652 mg kg−1) (Welp 1999). Although catalase belongs to the same class of oxidoreductases, it reacted extremely differently to the pressure caused by Sn2+, Co2+, and Mo5. Wyszkowska et al. (2009) also observed that this enzyme succumbed to the pressure of heavy metals, but not as negatively as dehydrogenases. Similar to the authors’ own research, arylsulfatase sensitivity to this group of xenobiotics was similar to acid phosphatase and alkaline phosphatase (Wyszkowska et al. 2010). It is supposed that low arylsulfatase activity values were not necessarily generated only by high doses of the metals. Knauff et al. (2003) claim that this could be caused by the release of H+ protons from barley roots, which reduces its activity, decreasing soil pH. Tabatabai (1977) stresses that Sn2+, Co2+, and Mo6+ all have an inhibitory effect on urease activity. The author proposed the following series of divalent ions as inhibitors of this enzyme: Ag2+ > Hg2+ > Cu2+ > Cd2+ > Zn2+ > Sn2+ > Mn2+. An attempt to explain the relationship between the time of soil residence of tested metals and decreasing values of microbiological indicators was made by Moreno et al. (2003). They claim that this may be related to the narrowing of the pool of substrates available to microorganisms.
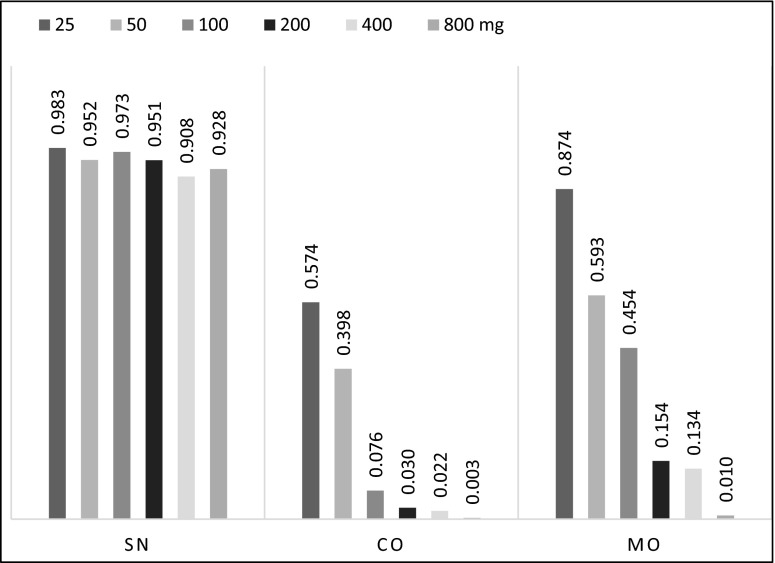
The xenobiotics introduced into the soil, except for Sn2+, also contributed to a drastic growth inhibition of the cultivated plant (Fig. 4). Characteristic symptoms of the disturbances in the biological mechanisms of spring barley, as the effects of stress related to soil contamination with the discussed metals, were also root system deformation and leaf chlorosis. Only Sn2+, regardless of the metal dose, generated high spring barley RS values, oscillating between 0.908 and 0.983. Unquestionably, Co2+ proved most toxic which, even in the amount of 100 mg kg−1 soil DM, decreased this plant’s resistance sixfold compared to the objects with 25 mg Co2+ per kilogram soil DM, reducing its value almost to zero (0.076). An inhibitory effect was also observed for Mo5+, particularly in its higher doses, above 200 mg Mo5+ per kilogram soil DM. The response to Mo5+ toxicity was also observed by McGrath et al. (2010), who found that Mo5+ uptake by plants was closely correlated with soil pH and the antidote consisted of the competition between S2+ and Mo5+. In the presence of S2+ in soil, the absorption of this metal by a plant and, as a consequence, its inhibitory effect decreases. The results of own research correspond to those obtained by Li et al. (2009). Co2+ in an amount from 53.6 mg to 91 mg kg−1 soil DM, depending on soil type, caused 50 % spring barley growth inhibition. Mico et al. (2008) indicate that after the application of 45 mg Co2+ per kilogram soil DM the barley yield disappears. This metal mainly accumulates in plant roots (Lotfy and Mostafa 2014).
Fig. 4.
Index of resistance (RS) of spring barley depending on Sn2+, Co2+, and Mo5+ (mg kg−1 DM soil) pollution
Conclusions
The study results indicate that trace elements present in excess in soil are able to disturb its homeostasis. They verify both soil microbiological diversity and biochemical activity. After optimal amounts are exceeded, inhibition of its biological activity and, as a consequence, reduced plant yields can be expected.
Co2+ proved the most toxic. It generated not only very low enzyme activity and resistance values but also contributed to a drastic spring barley yield reduction. Mo5+, although it is an element participating in nitrogen and sulfur metabolism and is part of bacterial enzymes in increased amounts (similar to Co2+), became an important inhibitor of the activity of dehydrogenases and urease. Soil reaction to an excess of Sn2+ was also negative, but the scale of the problem was not as alarming. The grown plant reacted exceptionally positively to its increased soil doses. Spring barley resistance did not undergo significant changes.
To conclude, this experiment is an important link in a series of studies on the awareness of the quality of the environment in which we function. It reveals the existence of side effects to the contamination of soils with trace elements caused by the growing push towards an increased standard of living and consumerism.
Acknowledgments
The research was supported by the Polish Ministry of Science and Higher Education.
References
- Albanese S, Sadeghi M, Lima A, Cicchella D, Dinelli E, Valera P, Falconi M, Demetriades A, Vivo B. GEMAS: cobalt, Cr, Cu and Ni distribution in agricultural and grazing land soil of Europe. Journal of Geochemical Exploration. 2015;154:81–93. doi: 10.1016/j.gexplo.2015.01.004. [DOI] [Google Scholar]
- Alef, K., Nannipieri, P. (1998) Methods in applied soil microbiology and biochemistry. Eds., London, Academic Press Harcourt Brace & Company p. 576.
- Behbahaninia A, Mirbagheri SA, Khorasani N, Nouri J, Javid AH. Heavy metal contamination of municipal effluent in soil and plants. Journal of Food, Agriculture and Environment. 2009;7(3 - 4):851–856. [Google Scholar]
- Borowik A, Wyszkowska J, Kucharski J, Baćmaga M, Tomkiel M. Pressure exerted by zinc on the nitrification process. Journal of Elementology. 2014;19(2):327–338. [Google Scholar]
- Buekers J, Mertens J, Smolders E. Toxicity of the molybdate anion in soil is partially explained by the effects of the accompanying cation or by soil pH. Environmental Toxicology and Chemistry. 2010;29:1274–1278. doi: 10.1002/etc.162. [DOI] [PubMed] [Google Scholar]
- Chatterjee J, Chatterjee C. Management of phytotoxicity of cobalt in tomato by chemical measures. Plant Science. 2003;164:793–801. doi: 10.1016/S0168-9452(03)00066-9. [DOI] [Google Scholar]
- Das AK, Chakraborty R, Cervera ML, Guardia M. A review on molybdenum determination in solid geological samples. Talanta. 2007;71:987–1000. doi: 10.1016/j.talanta.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Epelde L, Becerril JM, Hernández-Allica J, Barrutia O, Garbisu C. Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Applied Soil Ecology. 2008;39:299–310. doi: 10.1016/j.apsoil.2008.01.005. [DOI] [Google Scholar]
- Gil-Sotres F, Trasar – Cepeda C, Leiros MC, Seoane S. Different approaches to evaluate soil quality using biochemical properties. Soil Biology & Biochemistry. 2005;37:877–887. doi: 10.1016/j.soilbio.2004.10.003. [DOI] [Google Scholar]
- Griffiths BS, Phillipot L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiology Reviews. 2013;37:112–129. doi: 10.1111/j.1574-6976.2012.00343.x. [DOI] [PubMed] [Google Scholar]
- Hamilton EI. Environmental variables in a holistic evaluation of land contaminated by historic mine wastes: a study of multi-element mine wastes in West Devon, England using arsenic as an element of potential concern to human health. Science of the Total Environment. 2000;249:171–221. doi: 10.1016/S0048-9697(99)00519-7. [DOI] [PubMed] [Google Scholar]
- Hoch M. Organotin compounds in the environment—an overview. Applied Geochemistry. 2001;16:719–743. doi: 10.1016/S0883-2927(00)00067-6. [DOI] [Google Scholar]
- Islam S, Ahmed K, Al-Mamun H. Distribution of trace elements in different soils and risk assessment: a case study for the urbanized area in Bangladesh. Journal of Geochemical Exploration. 2015;158:212–222. doi: 10.1016/j.gexplo.2015.07.017. [DOI] [Google Scholar]
- Kilic NK, Donmez G. Environmental conditions affecting exopolysaccharide production by Pseudomonas aeruginosa, Micrococcus sp. and Ochrobactrum sp. Journal of Hazardous Materials. 2008;154:1019–1024. doi: 10.1016/j.jhazmat.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Brock T, Haddouk H, Hargeaves V, Lloyd M, Mc Garry S, Proudlock R, Sarlang S, Sewald K, Sire G, Sokołowski A, Ziemann C. New investigations into the genotoxicity of cobalt compounds and their impact on overall assessment of genotoxic risk. Regulatory Toxicology and Pharmacology. 2015;73:311–338. doi: 10.1016/j.yrtph.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Knauff U, Schulz M, Scherer HW. Arylsulfatase activity in the rhizosphere and roots of different crop species. European Journal of Agronomy. 2003;19:215–223. doi: 10.1016/S1161-0301(02)00035-7. [DOI] [Google Scholar]
- Kucharski J, Wieczorek K, Wyszkowska J. Changes in the enzymatic activity in sandy loam soil exposed to zinc pressure. Journal of Elementology. 2011;16(4):577–589. [Google Scholar]
- Lewis RC, Gaffney SH, Le MH, Unice KM, Paustenbach DJ. Airborne concentrations of metals and total dust during solid catalyst loading and unloading operations at a petroleum refinery. International Journal of Hygiene and Environmental Health. 2012;215:514–521. doi: 10.1016/j.ijheh.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Li HF, Gray C, Mico C, Zhao FJ, McGrath SP. Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere. 2009;75(7):979–986. doi: 10.1016/j.chemosphere.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Lock K, Schamphelaere KAC, Becaus S, Criel P, Van Eeckhout H, Janssen CR. Development and validation of an acute biotic ligand model (BLM) predicting cobalt toxicity in soil to the potworm Enchytraeus albidus. Soil Biology & Biochemistry. 2006;38:1924–1932. doi: 10.1016/j.soilbio.2005.12.014. [DOI] [Google Scholar]
- Lotfy SM, Mostafa AZ. Phytoremediation of contaminated soil with cobalt and chromium phytoremediation of contaminated soil with cobalt and chromium. Journal of Geochemical Exploration. 2014;144:367–373. doi: 10.1016/j.gexplo.2013.07.003. [DOI] [Google Scholar]
- Luo D, Zheng H, Chen Y, Wang G, Fenghua D. Transfer characteristics of cobalt from soil to crops in the suburban areas of Fujian Province, southeast China. Journal of Environmental Management. 2010;91:2248–2253. doi: 10.1016/j.jenvman.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Madrid F, Biasioli M, Ajmone – Marsan F. Availability and bioaccessibility of metals in fine particles of some urban soils. Archives of Environmental Contamination and Toxicology. 2008;55:21–32. doi: 10.1007/s00244-007-9086-1. [DOI] [PubMed] [Google Scholar]
- Marcic C, Hecho IL, Denaix L, Lespes G. TBT and TPhT persistence in a sludged soil. Chemosphere. 2006;65:2322–2332. doi: 10.1016/j.chemosphere.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Martin J. Use of acid rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Science. 1950;69:215–233. doi: 10.1097/00010694-195003000-00006. [DOI] [Google Scholar]
- McGrath SP, Micó C, Zhao FJ, Stroud JL, Zhang H, Fozard S. Predicting molybdenum toxicity to higher plants: estimation of toxicity threshold values. Environmental Pollution. 2010;158:3085–3094. doi: 10.1016/j.envpol.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Mendel R, Bittner F. Cell biology of molybdenum. Biochimica et Biophysica Acta. 2006;1763:621–635. doi: 10.1016/j.bbamcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mico C, Li HF, Zhao FJ, McGrath SP. Use of Co speciation and soil properties to explain variation in Co toxicity to root growth of barley (Hordeum vulgare L.) in different soils. Environmental Pollution. 2008;156(3):883–890. doi: 10.1016/j.envpol.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Garcia C, Hernandez T. Toxic effect of cadmium and nickel on soil enzymes and the influence of adding sewage sludge. European Journal of Soil Science. 2003;54:377–386. doi: 10.1046/j.1365-2389.2003.00533.x. [DOI] [Google Scholar]
- Nakamaru Y, Uchida S. Distribution coefficients of tin in Japanese agricultural soils and the factors affecting tin sorption behavior. Journal of Environmental Radioactivity. 2008;99:1003–1010. doi: 10.1016/j.jenvrad.2007.11.012. [DOI] [PubMed] [Google Scholar]
- National Research Council . A study of the isolation system for geological disposal of radioactive wastes. Washington, DC: National Academy Press; 1983. p. 345. [Google Scholar]
- Öhlinger R. Dehydrogenases activity with the substrate TTC. In: Schinner F, Öhlinger R, Kandele E, Margesin R, editors. Methods in soil biology. Berlin Heidelberg: Springer Verlag; 1996. p. 241. [Google Scholar]
- Onta H, Hattori T. Oligotrophic bacteria on organic debris and plant roots in paddy field. Soil Biology & Biochemistry. 1983;1:1–8. [Google Scholar]
- Orwin KH, Wardle DA. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biology & Biochemistry. 2004;36:1907–1912. doi: 10.1016/j.soilbio.2004.04.036. [DOI] [Google Scholar]
- Parkinson, D., Gray, F. R. G., Williams, S. T. (1971) Methods for studying the ecology of soil microorganisms. Blackweel Scientific Publications Oxford and Einburg, IBP Handbook. p 19–116.
- Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Critical Reviews in Toxicology. 2013;43:316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- Pendias AK, Pendias H. Trace elements in soils and plants. 3. Boca Raton: CRC Press; 2001. p. 505. [Google Scholar]
- Pyrzyńska K. Determination of molybdenum in environmental samples. Analytica Chimica Acta. 2007;590:40–48. doi: 10.1016/j.aca.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Renella G, Egamberiyeva D, Landi L, Mench M, Nanipieri P. Microbial activity and hydrolase activities during decomposition of root exudates released by an artificial root surface in Cd-contaminated soils. Soil Biology & Biochemistry. 2006;38:702–708. doi: 10.1016/j.soilbio.2005.06.021. [DOI] [Google Scholar]
- Shedd, K. B. (2013) Commodity report 2014: cobalt. United States Geological Survey (http://minerals.usgs.gov/minerals/pubs/commodity/cobalt/mcs-2014-cobal.pdf).
- StatSoft Inc. (2012). Statistica (data analysis software system). version 10.0. Available at www.statsoft.com.
- Sterckeman T, Douay F, Baize D, Fourrier H, Proix N, Schvartz C. Trace elements in soils developed in sedimentary materials from northern France. Geoderma. 2006;136:912–929. doi: 10.1016/j.geoderma.2006.06.010. [DOI] [Google Scholar]
- Tabatabai MA. Effects of trace elements on urease activity in soils. Soil Biology & Biochemistry. 1977;9(1):9–13. doi: 10.1016/0038-0717(77)90054-2. [DOI] [Google Scholar]
- Tewari RK, Kumar P, Sharma PN, Bisht SS. Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Science. 2002;162:381–388. doi: 10.1016/S0168-9452(01)00578-7. [DOI] [Google Scholar]
- USDI (2013) Mineral commodity summaries. USGS. Accessed on January 24, 2013. http://minerals.usgs.gov/minerals/pubs/mcs).
- Wang F, Yao J, Chen H, Russel M, Chen K, Qiuany Y, Zaray G, Bramanti E. Short-time effect of heavy metals upon microbial community activity. Journal of Hazardous Materials. 2010;173:510–516. doi: 10.1016/j.jhazmat.2009.08.114. [DOI] [PubMed] [Google Scholar]
- Welp G. Inhibitory effects of the total and water-soluble concentrations of nine different metals on the dehydrogenase activity of a loess soil. Biology and Fertility of Soils. 1999;30:132–139. doi: 10.1007/s003740050599. [DOI] [Google Scholar]
- Wyszkowska J, Kucharski J, Kucharski M. Microbiological and biochemical properties of soil depending on adenine and azotobacterina applied. Journal of Elementology. 2008;13(1):127–138. [Google Scholar]
- Wyszkowska J, Kucharski M, Kucharski J, Borowik A. Activity of dehydrogenases, catalase and urease in copper polluted soil. Journal of Elementology. 2009;14(3):605–617. [Google Scholar]
- Wyszkowska J, Kucharski M, Kucharski J. Activity of b-glucosidase, arylsulfatase and phosphatases in soil contaminated with copper. Journal of Elementology. 2010;15(1):213–226. [Google Scholar]
- Wyszkowska J, Borowik A, Kucharski M, Kucharski J. Effect of cadmium, copper and zinc on plants, soil microorganisms and soil enzymes. Journal of Elementology. 2013;18(4):769–796. [Google Scholar]
- Xian Y, Wanga M, Chen W. Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere. 2015;139:604–608. doi: 10.1016/j.chemosphere.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Zaborowska M, Wyszkowska J, Kucharski J. Using basalt flour and brown algae to improve biological properties of soil contaminated with cadmium. Soil & Water Research. 2015;10(3):181–188. doi: 10.17221/281/2014-SWR. [DOI] [Google Scholar]
- Zaborowska M, Wyszkowska J, Kucharski J. Maintenance of soil homeostasis under exposure to cadmium. Communication of Soil Science and Plant Analysis. 2015;46:2051–2069. doi: 10.1080/00103624.2015.1069311. [DOI] [Google Scholar]