Radiation therapy (XRT) is an essential component in the management of many types of cancer 1. XRT of the thorax frequently places the heart in the radiation field, leading to cardiac injury 1–3. Improvements in early diagnosis and treatment have increased cancer survival, engendering the need to critically evaluate the long-term consequences of XRT 2–3. From a clinical standpoint, XRT-induced heart injury is heterogeneous. XRT-induced cardiomyopathy may be clinically apparent with an initial early injury or may be latent for many years before presenting with heart failure 1. From a pathologic standpoint, XRT induced cardiomyopathy is characterized by myocardial fibrosis in absence of left ventricular (LV) dilatation and associated with normal or reduced LV mass 1–3. Unfortunately, the incidence and pathophysiology of XRT-induced cardiomyopathy remains poorly described due to a lack of prospective data and a latency period that exceeds the follow-up duration of most clinical studies. Given the increasing recognition of XRT-induced heart disease and recent progress in murine cardiac imaging, we performed a systematic study of XRT-induced cardiomyopathy in the mouse.
Experiments followed guidelines on the humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996) and received local Institutional Animal Care and Use Committee approval. Three-months old C57BL/6J male mice (Jackson Laboratory, Bar Harbor, ME) were irradiated with a single 20 Gy dose of XRT (Group I, N=12). Anesthetized mice (pentobarbital 50 mg/kg) were shielded with lead plates leaving the thorax region exposed. XRT was administrated locally with a Trilogy linear accelerator equipped with state of the art guided radiation therapy (IGTR)(Varian, Medical system, Palo Alto, CA). An additional group of mice (Group II, N=6) underwent sham-irradiation. All mice were followed for 6 months (Figure, panel A).
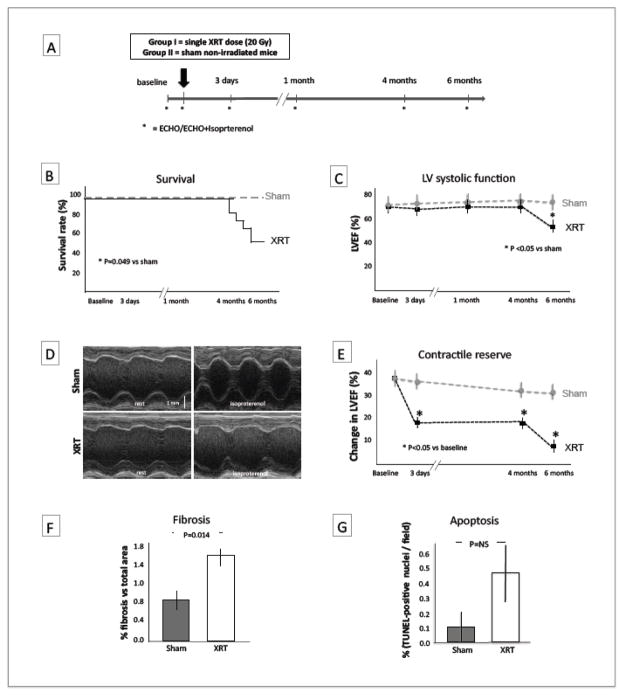
Figure.
The Experimental design is described in panel A: Group I (N=12) received a single thoracic radiation of 20 Gy at day 0, Groups II (N=6) sham non-irradiated mice were used as controls; an echocardiogram was performed at baseline (1 day prior to irradiation), 3 days, and 1, 4 and 6 months post irradiation; isoproterenol was administrated at 3 days, 4 and 6 months to measure contractile reserve. Panel B shows increased mortality in the XRT-treated group (p=0.049 vs sham non-irradiated). Panel C shows that XRT-treated mice had no significant change in LVEF up to 4 months and a significant drop in LVEF by 20% between 4 and 6 months (p<0.05 vs sham-non-irradiated). Panel D shows representative M-mode echocardiography recordings of the LV transverse mid-ventricular sections obtained before and after isoproterenol treatment in sham non-irradiated and XRT-treated mice. A M-Mode measurement of contractile reserve expressed as the change in LVEF measured before and after isoproterenol injection (a β-adrenergic agonist) is shown in panel E. The XRT-treated mice demonstrated a reduction in LV contractile reserve at 3 days, 4 and 6 months (p<0.05 vs sham-non irradiated). Interstitial myocardial fibrosis expressed as percentage of fibrotic areas on total area per field is shown in panel F, XRT-treated mice had a 2-fold increase in interstitial collagen deposition (p=0.014). Panel G shows a trend in the increment of TUNEL+ nuclei (indicated with and asterisk) reflecting apoptotic DNA fragmentation (p=NS).
IP=intraperitoneal, isopt=isoproterenol, XRT= XRT-treated mice, LVEF=left ventricular ejection fraction.
Transthoracic echocardiography was performed under light anesthesia (pentobarbital 50 mg/kg) to determine cardiac dimensions and function using the Vevo770 imaging system (VisualSonics Inc. Toronto, Ontario, Canada) equipped with a 30-MHz probe 4–5. The thickness of the posterior pericardium and of the mitral valve and aortic valve leaflets was also measured in M-mode, the presence of pericardial fluid was also determined. Finally, left ventricular ejection fraction (LVEF) was measured at rest and 3 minutes after injection with the β-adrenergic receptor agonist isoproterenol (10 ng/mouse, Sigma Aldrich), to measure contractile reserve, defined as percentage change of LVEF within 3 minutes after isoproterenol injection.
Tissue slides (5 μm), prepared from paraffin-embedded hearts collected at 6 months, were used for histological analysis. Collagen deposition was determined by Masson’s staining (Sigma Aldrich) and expressed as percentage fibrotic tissue in 5 random fields at 40X magnification using ImageJ software (rsbweb.nih.gov/ij/). DNA damage was detected by the TUNEL assay (Apo-Tag plus, Millipore, Billerica, MA) and expressed as percentage of the TUNEL positive cardiomyocytes on the total cardiomyocytes over 10 random fields.
Statistical analysis was performed using the SPSS 15.0 package for Windows. Continuous variables were expressed as mean and standard error. The T test for paired data was used to compare variables before and after treatment. Kaplan Meier survival curves were constructed and compared among different groups. The T test for unpaired data was used to compare 2 groups. Two-tailed P values <0.05 were considered significant.
XRT induced no significant changes in LVEF at 3 days, and 1 and 4 months. Contractile reserve, however, was reduced in the XRT-treated mice as early as 3 days post-XRT (p<0.05 vs baseline and sham non-irradiated mice).
Between 4 and 6 months, 6 of the 12 (50%) XRT-treated mice died, while no deaths occurred in the 6 sham non-irradiated group (Figure, panel B)(p=0.049). At 6 months, the surviving irradiated mice showed a significant reduction in LVEF (−14% [absolute reduction] and −20% [relative reduction], p<0.05 vs baseline and sham non-irradiated mice) and a further significant drop in contractility reserve at 6 months (p<0.01, for trend; p<0.05 vs baseline and vs sham non-irradiated mice, Figure, panels C-E) without LV dilatation or hypertrophy (Table). We detected no pericardial or valvular or regional wall motion abnormalities suggestive myocardial infarction in either group at echocardiography or at postmortem examination.
Table.
Echocardiographic parameters
| XRT | Baseline | 6 months | Sham | Baseline | 6 months |
|---|---|---|---|---|---|
| LVEDD (mm) | 3.5±0.2 | 3.6±0.2 | LVEDD (mm) | 3.5±0.2 | 3.6±0.3 |
| LVEF (%) | 68±4 | 56±4 * | LVEF (%) | 68±3 | 67±4 |
| LVM (mg) | 73±4 | 83±5 | LVM (mg) | 76±4 | 96±5 |
| HR (/min) | 312±42 | 426±60 | HR (/min) | 412±32 | 426±30 |
HR=heart rate; LVEDD=left ventricular end-diastolic diameter; LVEF=LV ejection fraction; LVM=LV mass; XRT=irradiation
P<0.05 vs baseline and vs sham.
XRT-treated mice had a 2-fold increase in myocardial interstitial fibrosis compared to sham non-irradiated mice at 6 months (p=0.014), reflecting XRT-induced injury. XRT-treated mice showed a 4-fold increase in TUNEL-positive cells (reflecting DNA fragmentation) compared to the sham non-irradiated mice, but the difference did not reach statistical significance.
Here we describe a mouse model of radiation-induced cardiomyopathy characterized by a latent phase of injury lasting several months in which the LVEF is normal but the mouse has significantly impaired contractile reserve, and a subsequent phase of reduced LVEF without evidence of LV enlargement of hypertrophy and in the presence of further impairment of contractile reserve, increased interstitial cardiac fibrosis and sudden death, a phenotype of overt cardiomyopathy. The absolute reduction in LVEF of 14% is likely highly significant, as in clinical trials using chemotherapy agents with cardiotoxicity an absolute decrease in LVEF of 5–10% is associated with an >4-fold increase risk of symptomatic heart failure and cardiac death 6.
Other animal models of XRT-induced cardiomyopathy have been reported 7. In the most commonly used rat model, toxicity presents with acute pericarditis and effusion that may be lethal, but is generally not evident when doses <30 Gy are administrated 8.
A recent study confirmed that XRT increases the long-term risk of death due to heart failure in women that received XRT for the treatment of a left-sided breast cancer (>50% higher risk) than those treated for a right-sided breast cancer 9, and patients with XRT-induced cardiomyopathy have a reduction in peak oxygen consumptions of a degree consistent with severe heart failure 10.
The mechanisms leading to the late cardiomyopathy and sudden death are not completely understood but previous investigations suggest that an acute inflammatory response occurs early after radiation promoting tissue fibrosis 11. The early decline in LV contractile reserve in our model is consistent with an early injury. From a clinical standpoint, the results of this study indicate that while the injury to the heart occurs immediately or very early after irradiation, a long latent clinical phase may occur. Consequently, many patients who have received chest irradiation in the past may have clinically latent disease and be at risk for late occurrence of heart failure or sudden death. It becomes clear that strategies are needed that are designed to prevent or limit the initial injury and/or the progression of the disease.
Acknowledgments
This study was supported by a grant from the Massey Cancer Center’s collaborative and multidisciplinary pilot project program to Dr. Abbate. Dr. Van Tassell is supported by an institutional K12. The study has been also in part supported by funds deriving from the VCU Pauley Heart and Massey Cancer centers and the Victoria Johnson Research Laboratories.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
EM, PG, DAG and AA designed the study and drafted the manuscript. EM and XD equally contributed to carry out studies. ST and BVT participated in the completion of the studies and analysis of the data. HK carried out the immunohistochemistry experiments. NV and CMB contributed to the final drawing up of the manuscript. All the authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205–11. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 2.Healy Bird BRJ, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–81. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 4.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–83. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 5.Gardin JM, Adams DB, Douglas PS, Feigenbaum H, Forst DH, Fraser AG, Grayburn PA, Katz AS, Keller AM, Kerber RE, Khandheria BK, Klein AL, Lang RM, Pierard LA, Quinones MA, Schnittger I American Society of Echocardiography. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiography. 2002;15:275–290. doi: 10.1067/mje.2002.121536. [DOI] [PubMed] [Google Scholar]
- 6.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz-Hector S. Radiation-induced heart disease: review of experimental data on dose response and pathogenesis. Int J Radiat Biol. 1992;61:149–60. doi: 10.1080/09553009214550761. [DOI] [PubMed] [Google Scholar]
- 8.Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–8. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, Mousannif A, Lê MG, Labbe M, Arriagada R, Jougla E, Chavaudra J, Diallo I, Rubino C, de Vathaire F. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57:445–52. doi: 10.1016/j.jacc.2010.08.638. [DOI] [PubMed] [Google Scholar]
- 10.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest therapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 11.Krüse JJ, Zurcher C, Strootman EG, Bart CI, Schlagwein N, Leer JW, Wondergem J. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol. 2001;58:303–11. doi: 10.1016/s0167-8140(00)00327-3. [DOI] [PubMed] [Google Scholar]