Abstract
Purpose
To report the therapeutic efficacy of an accidentally injected intralenticular sustained-release dexamethasone implant in a patient with macular edema secondary to branch retinal vein occlusion and shortly discuss the management strategy of this rare complication.
Methods
Complete ophthalmological examination and optical coherence tomography imaging were performed at each visit.
Results
The implant accidentally caused a posterior capsular tear during the procedure and was injected into the crystalline lens because of an involuntary head movement of the patient. Since the anterior segment was normal, and the resultant cataract and implant itself did not obscure the visual axis, the decision was made to observe the patient with intralenticular implant, preserve the therapeutic effect and avoid reinjection. The macular edema resolved within time, while visual acuity did not show significant improvement due to an increase in lens opacification. The patient underwent phacoemulsification surgery at 7 months after the injection with implantation of posterior chamber IOL into the capsular bag.
Conclusion
Inadvertent injection of sustained-release intravitreal dexamethasone implant into the crystalline lens is an uncommon but possible complication that is mostly caused by surgeon inexperience, improper technique and uncontrolled head movement during the procedure. Once this complication occurs, early phacoemulsification and repositioning of the implant into the vitreous is the frequently preferred management strategy. However, remarkable decrease in macular edema and visual acuity improvement can also be achieved without an immediate surgical intervention.
Key Words: Intralenticular dexamethasone implant, Macular edema, Vein occlusion
Introduction
The dexamethasone intravitreal implant (Ozurdex®; Allergan, Inc., Irvine, Calif., USA) is a rod-shaped biodegradable sustained-release implant containing 700 µg preservative-free dexamethasone in the Novadur solid polymer drug delivery system and designed to be delivered with a 22-gauge needle. It has been approved by the US Food and Drug Administration as a first-line therapy for the treatment of diabetic macular edema, macular edema secondary to retinal vein occlusion and noninfectious uveitis affecting the posterior segment. The ZERO study which was designed to test reliability and safety of intravitreal Ozurdex injections reported no intraoperative lens injuries [1]. However, a few authors have described the accidental injection of Ozurdex into the crystalline lens [2, 3, 4].
We hereby report the therapeutic efficacy of an accidentally injected intralenticular sustained-release dexamethasone implant and shortly discuss the management strategy of this rare complication.
Case Presentation
A 53-year-old man with macular edema secondary to branch retinal vein occlusion presented with a central macular thickness (CMT) of 525 µm, a best-corrected visual acuity (BCVA) of 48 ETDRS letters and an intraocular pressure (IOP) of 13 mm Hg in the right eye. He was scheduled for intravitreal implantation of Ozurdex®. The implant was injected via the pars plana route 3.5 mm from the limbus following the topical anesthesia with proparacaine. However, the implant accidentally caused a posterior capsular tear and was injected into the crystalline lens because of an involuntary head movement of the patient during the procedure (fig. 1). Slit-lamp examination revealed that the implant had penetrated the posterior lens capsule and was lodged in the posterior cortex of the lens with a resultant mild posterior cortical cataract. Since the anterior segment was normal, and the resultant cataract and implant itself did not make a substantial effect on BCVA, the decision was made to observe the patient with intralenticular implant, preserve the therapeutic effect and avoid reinjection.
Fig. 1.
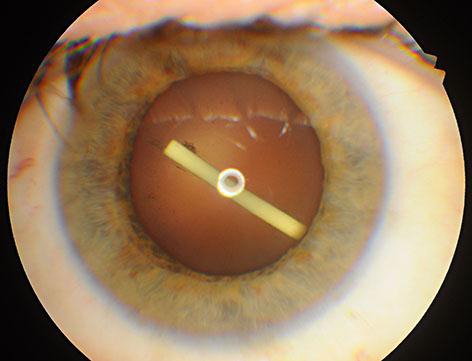
Intralenticular location of Ozurdex immediately after the injection.
CMT and BCVA were 253 µm and 58 letters at the first month, 262 µm and 56 letters at the third month and 254 µm and 48 letters at the sixth month (fig. 2). Macular edema resolved within time, while BCVA did not show significant improvement due to an increase in lens opacification (fig. 3). IOP was within normal limits during follow-up. The patient underwent phacoemulsification surgery at 7 months after the injection with implantation of posterior chamber IOL into the capsular bag. A gentle hydrodissection-hydrodelineation was performed, and low phaco parameters were used during the surgery of the cataract with a damaged posterior capsule. Posterior capsular fibrosis was encountered in the area of the posterior capsular defect during the surgery. After the cataract surgery, the IOL was well centered with a no anterior chamber reaction, and BCVA improved to 63 letters with a normal IOP and a normal foveal contour. The fellow eye was completely normal.
Fig. 2.
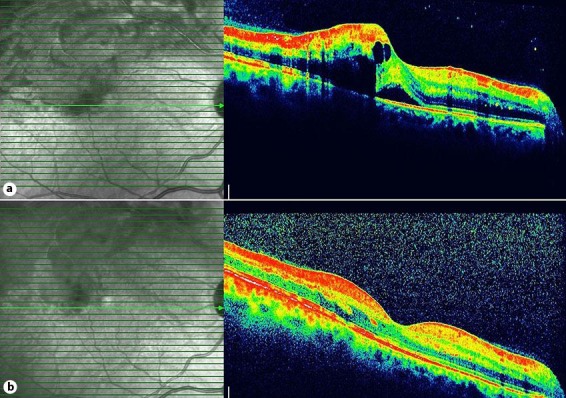
Optical coherence tomography of the right eye. a Before the injection. b Improvement of the macular edema 6 months after the injection.
Fig. 3.
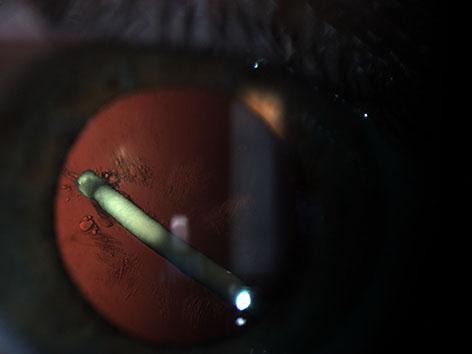
Intralenticular location of Ozurdex and cataract development 4 months after the injection.
Discussion
There are few reports of iatrogenic crystalline lens damage during intravitreal injection of Ozurdex [2, 3, 4]. Most of these recommend immediate phacoemulsification with removal of the implant. None of the above case reports described a therapeutic efficacy of a single in tralenticular Ozurdex. This report demonstrates the therapeutic efficacy of an inadvertently injected intralenticular Ozurdex. The therapeutic effect of intralenticular Ozurdex can be questionable since the implant was delivered into the capsular bag and the lens substance is not directly exposed to aqueous or blood circulation that might serve as a clearance route [4]. Nevertheless, in the present case, the implant is partially in the lens capsule and partially in the vitreous which can explain the effect on macular edema. A breach of the posterior capsule could have resulted in an inflammatory response. However, as a corticosteroid was introduced into the eye, and the inflammatory response was suppressed. An immediate surgical removal of the implant was postponed due to the absence of an anterior segment reaction and in order to prevent a loss of effectiveness of the drug delivery system. The implant continued to be effective in the eye. This conservative approach cannot necessarily be extrapolated to all cases, and a proactive approach will be more suitable for those with an increased IOP and if the anterior segment is not normal and the resultant cataract prevents visualization of the retina.
Another approach to intralenticular Ozurdex is to perform immediate phacoemulsification with IOL implantation and repositioning Ozurdex into the vitreous cavity [4]. This approach can cause further stress on the damaged posterior capsule with possible extension of the capsular tear. The repositioning of the implant into the vitreous cavity can be difficult because of the resistance exerted by the vitreous, especially in young patients, so an anterior vitrectomy can be necessary. A posterior capsulorhexis can be performed in order to allow in the bag implantation of the IOL or a 3-piece IOL can be implanted into the sulcus. Repositioning the implant into the vitreous cavity can cause implant split which can further cause changes in the drug release and a higher risk of glaucoma [5]. Another risk of this approach is the possible migration of the repositioned implant into the anterior chamber through the posterior capsule defect which may in turn cause corneal decompensation [6].
In conclusion, inadvertent injection of sustained-release intravitreal dexamethasone implant into the crystalline lens is an uncommon but possible complication that is mostly caused by surgeon inexperience, improper technique and uncontrolled head movement during the procedure. Once this complication occurs, early phacoemulsification and repositioning of the implant into the vitreous is the frequently preferred management strategy. However, remarkable decrease in macular edema and visual acuity improvement can also be achieved without an immediate surgical intervention. Postponement of the lens extraction and removal of the implant until the completion of its effect may prevent unnecessary reinjections. Additionally, fibrosis of the posterior capsule defect can enable an uneventful cataract surgery within the bag IOL implantation. This rare condition should be borne in mind during intravitreal injections, and ophthalmologists should be aware of this potential complication.
Statement of Ethics
Informed consent has been obtained from the patient.
Disclosure Statement
The authors declare that they have no proprietary interests or financial disclosure.
Footnotes
This article was presented at the 14th Euretina Congress, London, UK, September 11–14, 2014.
References
- 1.Schmitz K, Maier M, Clemens CR, Hohn F, Wachtlin J, Lehmann F, Bertelmann T, Rudiger K, Horn M, Bezatis A, Spital G, Meyer CH; German Retinal Vein Occlusion Group. Reliability and safety of intravitreal Ozurdex injections. The ZERO study (in German). Ophthalmologe. 2014;111:44–52. doi: 10.1007/s00347-012-2737-2. [DOI] [PubMed] [Google Scholar]
- 2.Berarducci A, Sian IS, Ling R. Inadvertent dexamethasone implant injection into the lens body management. Eur J Ophthalmol. 2014;24:620–622. doi: 10.5301/ejo.5000436. [DOI] [PubMed] [Google Scholar]
- 3.Coca-Robinot J, Casco-Silva B, Armadá-Maresca F, García-Martínez J. Accidental injections of dexamethasone intravitreal implant (Ozurdex®) into the crystalline lens. Eur J Ophthalmol. 2014;24:633–636. doi: 10.5301/ejo.5000439. [DOI] [PubMed] [Google Scholar]
- 4.Munteanu M, Rosca C. Repositioning and follow-up of intralenticular dexamethasone implant. J Cataract Refract Surg. 2013;39:1271–1274. doi: 10.1016/j.jcrs.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Roy R, Hegde S. Split Ozurdex implant: a caution. Can J Ophthalmol. 2013;48:e15–e16. doi: 10.1016/j.jcjo.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Pardo-López D, Francés-Muñoz E, Gallego-Pinazo R, Díaz-Llopis M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex®) Graefes Arch Clin Exp Ophthalmol. 2012;250:1703–1704. doi: 10.1007/s00417-011-1802-x. [DOI] [PubMed] [Google Scholar]


