Abstract
A 23-year-old black African male fell and bumped his head from a tackle while playing soccer. He subsequently became blind from optic neuritis. An MRI of the brain showed white-matter changes suggestive of acute disseminated encephalomyelitis (ADEM). MR spectroscopy of the brain showed a demyelination pattern. This case report brings to the fore unsettled questions about ADEM, among them being whether it can occur as a post-traumatic event.
Abbreviations: MRI, magnetic resonance imaging; ADEM, acute disseminated encephalomyelitis
Introduction
The term acute disseminated encephalomyelitis (ADEM) was first used in 1950 to describe an immune-mediated encephalomyelitis from infection, immunizations, and allergies (1). ADEM has a striking resemblance to multiple sclerosis in that it is also a white-matter (demyelinating) condition. Unlike multiple sclerosis, however, it tends to be monophasic, even though recurrences have been described. These recurrent forms blur the distinction between ADEM and multiple sclerosis (2). ADEM is a potentially serious condition that can cause encephalopathy and even demise of a subject. Its association with various infections is well described (3, 4). ADEM has also been described post vaccination (5). Poser linked central nervous system trauma to formation and even enlargement of multiple sclerosis plaques (6). ADEM, on the other hand, has not been described post head trauma. Howeer, Irani once mentioned a claim by "some investigators" that ADEM could occur "at the heels of trauma." He went on to state that there wasn’t much more to be said since this is not a well understood phenomenon (7). A literature search for a link between head trauma and ADEM came to naught. Nakamura et al described a 36-year-old man who presented with "multiple sclerosis like" white-matter changes post trauma, with optic neuritis and high cerebrospinal fluid (CSF) protein (8). The diagnosis of ADEM has some difficulties, since there are no specific biochemical markers for the condition.
Case report
A 23-year-old black African male patient fell and bumped his head from a tackle during a soccer game. He denied losing consciousness and did not recall being disoriented subsequently. No further details about the incident were available. Three days later, he suddenly became completely blind, with an unsteadiness of gait. He had dilated pupils bilaterally, with bilateral swelling of the discs. The features were suggestive of optic neuritis, a finding made in agreement with our ophthalmologists. The rest of the neurological examination revealed no abnormalities except for increased deep-tendon reflexes in the lower limbs. However, he had normal tone with flexor plantar responses and normal sensation. He had a cautious gait as a result of the visual impairment. An MRI exam (Figure 1A, Figure 1B, Figure 1C) showed large lesions in the white matter with no evidence of blood (as could have arisen from trauma).
Figure 1A.
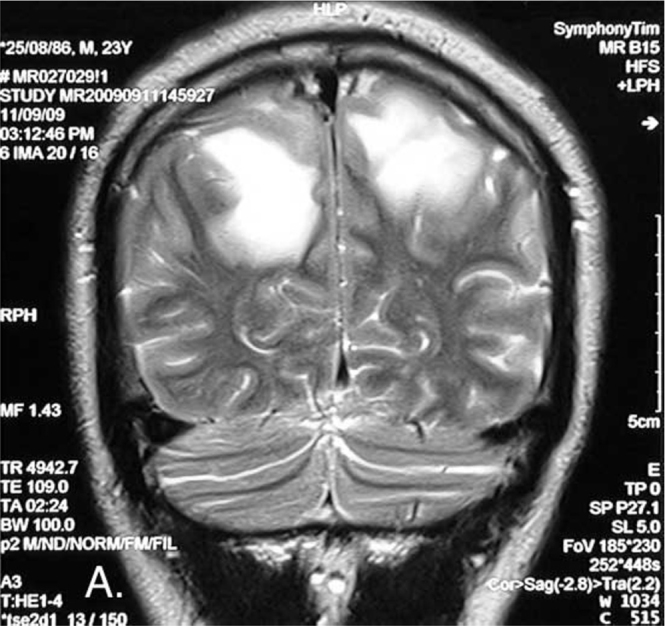
23-year-old male with post-traumatic acute disseminated encephalomyelitis. Coronal T2-weighted image shows parasagittal high-signal intensity lesions with vasogenic edema in white matter of both hemispheres.
Figure 1B.
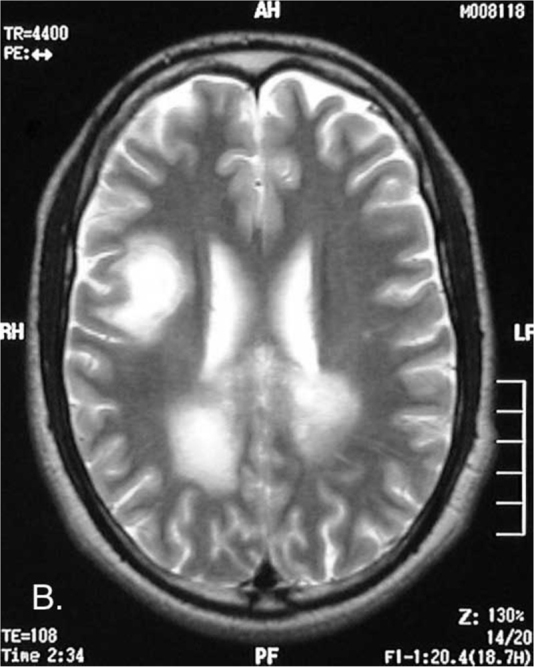
23-year-old male with post-traumatic acute disseminated encephalomyelitis. Axial T2-weighted image shows the high-signal parasagittal lesions with an additional lesion in the right parietal lobe.
Figure 1C.
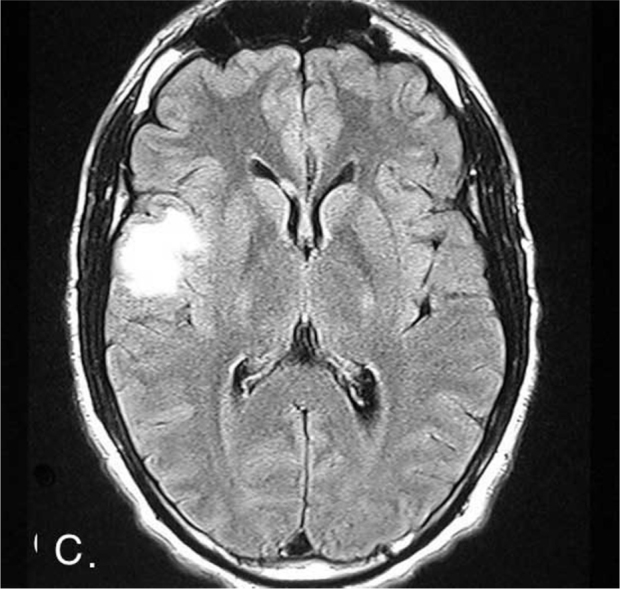
23-year-old male with post-traumatic acute disseminated encephalomyelitis. Axial FLAIR image showing the high-signal-intensity lesion in the right temporo-parietal region.
MR spectroscopy showed a markedly high choline with a reduced N-acetylaspartate (NAA)-to-creatine (Cr) ratio. There was also a lactate peak, shown as an inverted doublet (Fig. 2).
Figure 2.
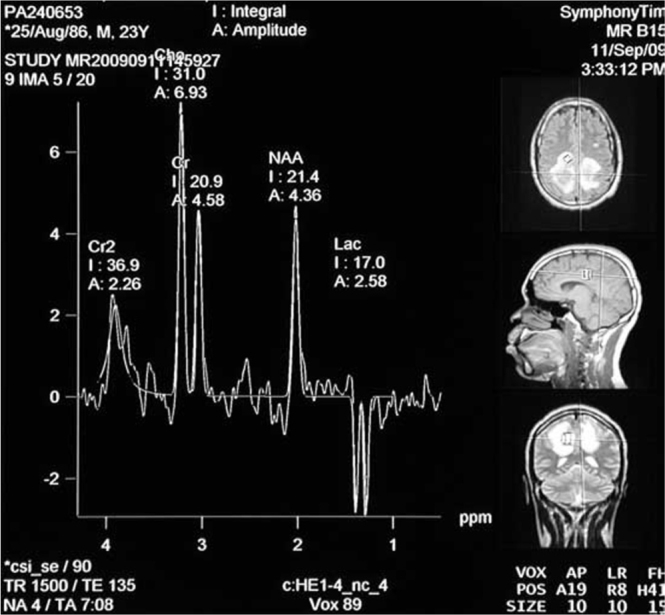
23-year-old male with post-traumatic acute disseminated encephalomyelitis. MR spectroscopy showing high choline, low NAA, and inverted doublet lactate peak.
At no time did the patient exhibit a pyrexia. A lumbar puncture was not done due to a concern for raised intracranial pressures. There was no evidence of venous sinus thrombosis, and the lesions were strictly confined to the white matter. He was put on high doses of methylprednisolone (500mg intravenous daily for 5 days) and then tapered with oral steroids over 12 days. The patient’s vision improved to a point of seeing movement and the silhouette of objects and people. Later assessment showed a further improvement in which he was able to appreciate color. A full restoration of vision was never achieved, however. He developed some pallor of the disc, suggesting optic atrophy in spite of the steroids. He was found to be HIV-positive with a cluster of differentiation (CD) 4 count of 177 cells/mm3. Syphilis studies were nonreactive.
Discussion
The MRI showed white-matter changes of the same age that were reminiscent of ADEM. A choline rise is a feature of demyelination, albeit not specific (9). A lactate peak, even though a general feature of hypoxic brain conditions, has been described with ADEM (10). In multiple sclerosis, unlike with ADEM, optic-nerve involvement tends not to be simultaneous. Other pointers arguing against multiple sclerosis include the white-matter lesions of the same age, lesions > 2cm that are atypical for multiple sclerosis, occurring only with the rare tumefactive variant (11, 12). Multiple sclerosis is also very uncommon among black Africans, and the patient had no history of mixed lineage (13). The presence of Dawson’s fingers (multiple-sclerosis lesions around the ventricle-based brain veins) is a sign attributed to multiple sclerosis rather than ADEM. This finding, however, was in only 21% of a study population with multiple sclerosis (14).
In HIV-endemic regions like sub-Saharan Africa, it can be a challenge to prove causation by HIV as opposed to a coincidental association. In our neurology departmental database, ADEM constitutes less than 1% of the patient population. We believe that the presentation therefore is not as result of HIV.
Trauma to the brain can elicit an immune response, as has been shown by Chen et al. A rat model showed a marked increase in the CD 4 and CD 8 T-lymphocyte counts after brain injury (15). Interleukin 6 has also been shown to rise in traumatic brain injuries (16). Interleukin 6, which has a number of roles (including recruitment of T lymphocytes), has also been shown to be increased in patients with ADEM compared to their normal counterparts (17). ADEM is partly explained by a T-cell-mediated response that because of "molecular mimicry" recognizes foreign and self antigens and, as a result of this "cross recognition," causes demyelination (18). We postulate that an immune response could have been triggered by the brain trauma that subsequently resulted in the acute demyelination.
Conclusion
There seems to be a common pathway at a certain point in the way the brain reacts to insult, be it physical trauma or infection. As to whether that can result in ADEM is a matter to be resolved by further studies. A single case report can only introduce the topic. At this point, it cannot be said with any amount of certainty that this was a direct result of trauma. There are no biochemical markers for ADEM, the best available diagnostic tool being clinico-radiological. The diagnosis could be so difficult as to warrant a tissue biopsy for histology. A shortcoming in the workup was not getting CSF (this was due to a concern about high pressures). There are no CSF findings specific for ADEM; still, CSF aspiration would be useful for excluding meningitis and for searching for oligoclonal bands, the absence of which would favor ADEM rather than multiple sclerosis. This however is not an absolute finding, because oligoclonal bands have been described with ADEM (19). In the definition and explanation of ADEM, infections and vaccination seem to be the exclusive triggers of the condition. The case report is a call to investigate whether indeed ADEM could occur "at the heels of trauma." This could help in early diagnosis and management of this condition, which at times is fatal. A suggestion has been made that prospective studies on ADEM with a followup of at least 10 years are necessary to resolve some unanswered questions (20).
Acknowledgments
We thank Mr. Thabiso Nkosi for his assistance, which went beyond the line of duty.
Footnotes
Published: September 26, 2012
References
- 1.Van Bogaert L. Post-infectious encephalomyelitis and multiple sclerosis, the significance of perivenous encephalomyelitis. J Neuropathol Exp Neurol. 1950;9(3):219–249. doi: 10.1097/00005072-195007000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Cohen O, Stener-Birmanns B, Biran I, Abramsky O, Honigman S, Steiner I. Recurrence of acute disseminated encephalomyelitis at the previously affected brain site. Arch Neurol. 2001;58(5):797–801. doi: 10.1001/archneur.58.5.797. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Allen SH, Malik O, Lipman MC, Johnson MA, Wilson MA. Acute demyelinating encephalomyelitis (ADEM) in a patient with HIV infection. The Journal of Infection. 2002;45(1):62–64. doi: 10.1053/jinf.2002.0982. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara H, Sakamoto S, Katsumata T, Katayama Y. Acute disseminated encephalomyelitis developed after mycoplasma pneumonia infection complicating subclinical measles infection. Intern Med. 2009;48(6):479–483. doi: 10.2169/internalmedicine.48.1740. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Huynh W, Cordato DJ, Kehdi LT, Masters LT, Dedousis C. Post vaccination encephalomyelitis: Literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poser CM. Trauma to the central nervous system may result in formation of or enlargement of multiple sclerosis plaques. Arch Neurol. 2000;57(7):1074–1077. doi: 10.1001/archneur.57.7.1074. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Irani D. Neuroimmunological lessons learned from acute disseminated encephalomyelopathy. Transverse Myelitis Association. 2006;1 http://www.myelitis.org/newsletters/j1/journal-1-11.htm article 11. Online: last accessed August 18, 2012. [Google Scholar]
- 8.Nakamura T, Ota K, Niwa N, Takeuchi M, Uchiyama S, Iwata M. Multiple cerebral white matter lesions following head trauma with eyeball contusion. Rinsho Shinkeigaku. 2004;44(2):108–110. [PubMed] [Japanese] [PubMed] [Google Scholar]
- 9.Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, McDonald WI. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117:49–58. doi: 10.1093/brain/117.1.49. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Ben Sira L, Miller E, Artzi M, Fatal-Valevski A, Constantini S, Ben Bashat D. H-MRS for the diagnosis of acute disseminated encephalomyelitis: insight into the acute disease stage. Paediatr Radiol. 2010;1(40):106–113. doi: 10.1007/s00247-009-1372-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Miller DH, Weinshenker BG, Filippi M. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult scler. 2008;14:1157–1174. doi: 10.1177/1352458508096878. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Thomsen K, Mandrekar J, Altintas A, Erickson BJ, König F, Giannini C, Lassmann H, Linbo L, Pittock SJ, Brück W. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008 Jul;131(Pt 7):1759–1775. doi: 10.1093/brain/awn098. [PubMed] Epub 2008 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean G, Bhigjee AI, Bill PL, Fritz V, Chikanza IC, Thomas JE, Levy LF, Saffer D. Multiple sclerosis in black South Africans and Zimbabweans. J Neurol Neurosurg Psychiatry. 1994;57(9):1064–1069. doi: 10.1136/jnnp.57.9.1064. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikaeloff Y, Adamsbaum C, Husson B. MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood. Brain. 2004;127(pt 9):1942–1947. doi: 10.1093/brain/awh218. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Chen KJ, Li W, Wang ZG. Relationship between cellular immune response and apoptosis of the brain neurons after brain trauma in rats. Nan Fang Yi Ke Xue Xue Bao. 2009;29(3):497–499. [PubMed] [Chinese] [PubMed] [Google Scholar]
- 16.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head trauma. Injury. 2007;38(12):1392–1400. doi: 10.1016/j.injury.2007.10.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Dale RC, Morovat A. Interleukin-6 and oligoclonal IgG synthesis in children with acute disseminated encephalomyelitis. Neuropediatrics. 2003;34:141–145. doi: 10.1055/s-2003-41281. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Sobel RA. Antiviral T-Cell Immunity + Anti-CNS Autoantibody = A model for human acute disseminated encephalomyelitis or multiple sclerosis relapse? Am J Pathol. 2007;170:436–438. doi: 10.2353/ajpath.2007.061098. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow study of 40 adult patients. Neurology. 2001;56(10):1313–1318. doi: 10.1212/wnl.56.10.1313. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelopathy: Current understanding and controversies. Semin Neurol. 2008 Feb;28(1):84–94. doi: 10.1055/s-2007-1019130. [PubMed] [DOI] [PubMed] [Google Scholar]


