Abstract
We present a serologically proven case of WNV encephalitis in a young, pregnant woman with cranial and spinal MRI findings who was seen for asymmetric, flaccid paralysis of her extremities. Cranial MRI findings were nonspecific, as reported in reviews of West Nile virus encephalitis. Her spinal MRI displayed enhancement of the cauda equina described infrequently in the literature. Knowledge of the variable MRI appearance is important for the recognition and diagnosis of this disease.
Abbreviations: MRI, magnetic resonance imaging; WNV, West Nile virus; CT, computed tomography
Introduction
One of the Japanese encephalitis virus complexes of family flaviviridae, West Nile virus (WNV) is named for having been first isolated from a woman in the West Nile province of Uganda (1). WNV first arrived in North America as an epidemic in New York City in 1999 (2). The virus spread quickly and has become a seasonal epidemic that as of 2006 was reported in humans in nearly all continental states (3).
Case report
A 21-year old woman was transferred to our hospital for evaluation of worsening right-sided hemiparesis, fever of unknown origin, and altered mental status. Two weeks before, she was 39 weeks pregnant and developed low back pain and insomnia. She then developed right-leg paresis that gradually worsened until she was admitted to an outside hospital where she delivered a healthy term baby by Cesarean section. Her paresis spread to both lower extremities and gradually ascended to involve her right upper extremity and face. Her altered mental status worsened, and she developed a fever before transfer to our facility. The physical examination showed severe lower extremity weakness—worse on the right—with somnolence and an inability to follow commands. The differential diagnosis of acute flaccid paralysis was initially broad and included toxic exposure, epidural abscess, Guillain-Barré syndrome, and infectious forms of meningoencephalitis such as Japanese encephalitis, varicella zoster, enterovirus, lyme disease, and WNV (4).
Contrast-enhanced computed tomography (CT) of the head was normal, although limited by patient motion. Lumbar puncture with cerebrospinal fluid (CSF) analysis included 46 nucleated cells consisting of neutrophils (59%), lymphocytes (30%), and macrophages (11%). The protein was normal, but the glucose was elevated. Before her transfer to our hospital, the patient had been receiving empiric therapy with antiviral and antibiotic medications. CSF analysis was most consistent with a viral etiology—specifically WNV because of its known association with acute, flaccid paralysis (5, 6).
The patient then underwent contrast-enhanced MRI of the brain with T1-weighted, T2-weighted, T2-FLAIR, diffusion-weighted imaging (DWI), and postcontrast T1-weighted sequences. MRI of the entire spine was performed using axial and sagittal T2-weighted, and pre- and post-contrast T1-weighted sequences. Brain MRI demonstrated increased signal on T2-weighted and T2-FLAIR images in the bilateral caudate nuclei and putamen, as well as the posterior thalamus. There was no abnormal diffusion restriction to suggest infarction, and no abnormal parenchymal or leptomeningeal enhancement (Figs. 1A and B). Spine MRI demonstrated no extrinsic compression, abnormal signal, or enhancement of the spinal cord; however, there was prominent enhancement of the ventral nerve roots of the cauda equina (Fig. 2).
Figure 1.
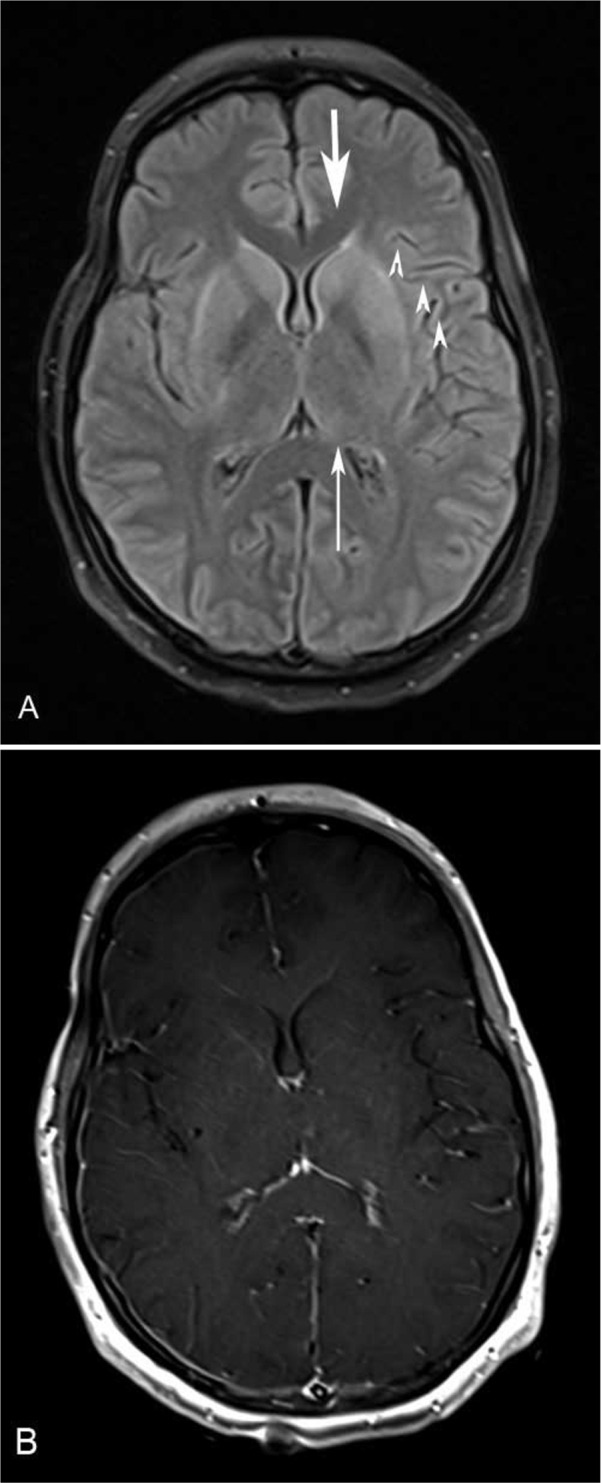
21-year-old woman with West Nile virus. A. Axial FLAIR image demonstrates increased signal in the bilateral caudate nuclei (thick arrow), putamen (arrowheads), and posterior thalami (thin arrow). B. Axial postcontrast T1-weighted image demonstrates no abnormal enhancement.
Figure 2.
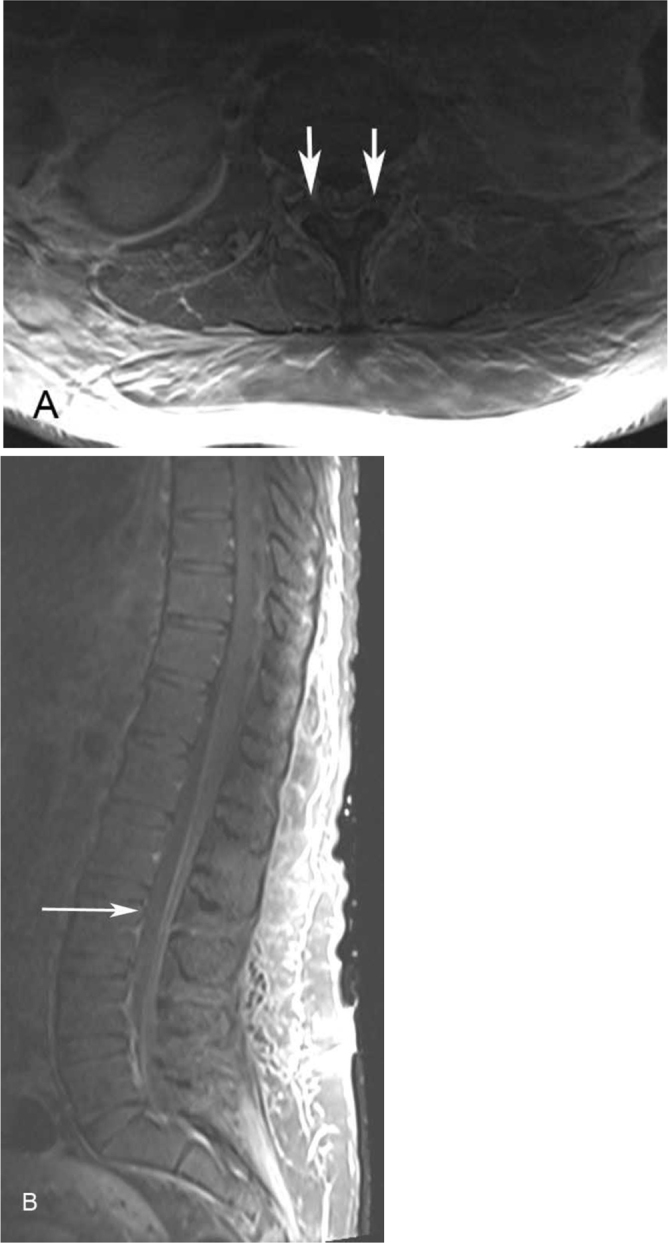
21-year-old woman with West Nile virus. Axial postcontrast T1-weighted (A) and sagittal postcontrast T1-weighted, fat-saturated (B) images demonstrate abnormal enhancement of the ventral nerve roots of the cauda equina (arrows) but otherwise no abnormal signal or enhancement.
A comprehensive toxicology and heavy-metal screening had negative results. No bacterial, acid-fast, and fungal organisms grew in CSF cultures. CSF serology tests for herpes simplex virus, cytomegalovirus, and enterovirus were also negative; however, a serology test was positive for WNV IgM in CSF, identifying the causative agent.
The patient improved following continued supportive care but retained residual lower-extremity weakness. She was discharged to an inpatient rehabilitation facility closer to her hometown. No followup was available.
Discussion
WNV infection is primarily an avian infection that is transmitted to humans by mosquitoes. More than 99% of people infected with WNV are asymptomatic; however, some patients suffer severe neuroinvasive disease (3). Patients who do present with encephalitis often have neurologic signs such as altered mental status, slurred speech, and motor weakness. Initially described in 1979, WNV-associated asymmetric flaccid paralysis has been presented in numerous cases and reviews (5, 6, 7, 8, 9). The pathology had been hypothesized to be a peripheral demyelinating process similar to Guillain-Barré syndrome, but a review by Sejvar et al. concluded that the process more likely involves anterior horn cells—a polio-like syndrome (10). A longitudinal study of affected patients found that many suffered long-term sequelae such as atrophy, tremor, and parkinsonism (11).
Brain MRI has been found to be nonspecific in WNV. An imaging review of 18 cases of WNV by Jeha et al. found abnormalities in 6 patients, with mostly T2 and FLAIR hyperintensities, in areas of the cortex and brainstem in isolation, or in the cortex and associated subcortical white matter (6). No deep gray-matter structures showed abnormality. Ali et al. reviewed 17 cases of WNV and more commonly found diffusion restriction on DWI that was thought to represent the earliest marker of viral encephalitis, but a single patient displayed increased T2 and T2-FLAIR signal throughout the brain including basal ganglia (12). Sejvar et al. reviewed 16 seropositive cases and found T2-hyperintense lesions in the basal ganglia of 2 severely ill patients (5). A review of 17 patients further reinforced this nonspecific pattern, with findings that ranged from cortical to brain-stem abnormalities and no cases of basal ganglia involvement (8).
Spine MRI is typically performed when patients present with motor weakness in cases of WNV, and often there is preferential enhancement of the ventral spine and cauda equina. These findings are nonspecific and can be seen in viral, tick-borne, and AIDS-related infections (WNV, polio, rabies, Lyme disease, rickettsia, and CMV), arachnoiditis, spinal-cord injury from compression or infarction, sarcoidosis, lymphoma, meningeal carcinomatosis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and transverse myelitis (13, 14, 15). In the case series by Jeha et al., 3 of 8 patients with weakness had spinal abnormalities that showed enhancement of cord parenchyma, cauda equina, or both (6). A single case displayed enhancement of cauda equina, as in our case, but without selective ventral involvement. Ali et al. imaged the spines of 3 patients who displayed motor weakness and found no consistent abnormalities; however, 2 of these patients’ abnormalities included enhancement of the cauda equina (12). In another series, the findings of 2 patients with acute flaccid paralysis included enhancement of the cauda equina (5). Similar enhancement of cauda equina and nerve roots were seen in 3 patients in the review by Petropoulou et al (8).
WNV has been previously described in pregnant women (16, 17). Research has primarily focused on intrauterine transmission of WNV. The Centers for Disease Control and Prevention reviewed the outcomes in 83 reported cases of WNV in pregnant women during a two-year period, and only 3 infants were found to have congenital defects possibly related to WNV infection (18). One case report describes ocular and neurologic complications in an infant who suffered intrauterine infection (19). However, a prospective study reinforced the earlier finding that intrauterine infection and sequelae are rare (20). Our patient’s child suffered no apparent complications, although his blood titers were not tested for exposure.
Identification of the causative agent in encephalitis is often delayed by necessary culture and lab-processing times. Therefore, patients presenting with signs and symptoms are often treated empirically to avoid complications. Although cranial MRI findings are nonspecific, spinal MRI findings can support the diagnosis. When ventral-cord or cauda-equina enhancement is present in a patient with signs of encephalomyelitis, WNV should be considered.
The differential diagnosis of patients with meningoencephalitis should include WNV due to its widespread prevalence. This case demonstrates the nonspecific cranial MRI findings typical of WNV encephalitis. Signs of paresis or paralysis should prompt MRI of the spine to evaluate for anterior myelitis. Ultimately, diagnosis rests with CSF serologies.
Footnotes
Published: August 21, 2012
References
- 1.Hollidge BS, Gonzalez-Scarano F, Soldan SS. Arboviral encephalitides: transmission, emergence, and pathogenesis. J Neuroimmune Pharmacol. 2010;5(3):428–442. doi: 10.1007/s11481-010-9234-7. [PubMed] Epub 2010/07/24. doi: 10.1007/s11481-010-9234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344(24):1807–1814. doi: 10.1056/NEJM200106143442401. [PubMed] Epub 2001/06/16. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [PubMed] Epub 2007/09/21. doi: CID51212 [pii] 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 4.Marx A, Glass JD, Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiologic reviews. 2000;22(2):298–316. doi: 10.1093/oxfordjournals.epirev.a018041. [PubMed] Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 5.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–515. doi: 10.1001/jama.290.4.511. [PubMed] Epub 2003/07/24. doi: 10.1001/jama.290.4.511 290/4/511 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61(1):55–59. doi: 10.1212/01.wnl.0000073617.08185.0a. [PubMed] Epub 2003/07/09. [DOI] [PubMed] [Google Scholar]
- 7.Gadoth N, Weitzman S, Lehmann EE. Acute anterior myelitis complicating West Nile fever. Arch Neurol. 1979;36(3):172–173. doi: 10.1001/archneur.1979.00500390090012. [PubMed] Epub 1979/03/01. [DOI] [PubMed] [Google Scholar]
- 8.Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26(8):1986–1995. [PubMed] Epub 2005/09/13. doi: 26/8/1986 [pii] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas H, Wippold FJ., 2nd. West Nile virus: case report with MR imaging findings. AJNR Am J Neuroradiol. 2003;24(7):1376–1378. [PubMed] Epub 2003/08/15. [PMC free article] [PubMed] [Google Scholar]
- 10.Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Ewing D, Mazowiecki M. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11(7):1021–1027. doi: 10.3201/eid1107.040991. [PubMed] Epub 2005/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Pape J, Biggerstaff BJ. West Nile Virus-associated flaccid paralysis outcome. Emerg Infect Dis. 2006;12(3):514–516. doi: 10.3201/eid1203.050643. [PubMed] Epub 2006/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26(2):289–297. [PubMed] Epub 2005/02/15. doi: 26/2/289 [pii] [PMC free article] [PubMed] [Google Scholar]
- 13.Marjelund S, Jaaskelainen A, Tikkakoski T, Tuisku S, Vapalahti O. Gadolinium enhancement of cauda equina: a new MR imaging finding in the radiculitic form of tick-borne encephalitis. AJNR Am J Neuroradiol. 2006;27(5):995–997. [PubMed] Epub 2006/05/12. [PMC free article] [PubMed] [Google Scholar]
- 14.Yousem DM, Grossman RI. Neuroradiology: the requisites. 3rd ed. Mosby/Elsevier; Philadelphia, PA: 2010. xvii, 619 pp. [Google Scholar]
- 15.Coskun A, Kumandas S, Pac A, Karahan OI, Gulec M, Baykara M. Childhood Guillain-Barre syndrome. MR imaging in diagnosis and follow-up. Acta Radiol. 2003;44(2):230–235. doi: 10.1080/j.1600-0455.2003.00023.x. [PubMed] Epub 2003/04/16. [DOI] [PubMed] [Google Scholar]
- 16.Bruno J, Rabito FJ, Jr., Dildy GA., 3rd West nile virus meningoencephalitis during pregnancy. The Journal of the Louisiana State Medical Society : official organ of the Louisiana State Medical Society. 2004;156(4):204–205. [PubMed] Epub 2004/09/16. [PubMed] [Google Scholar]
- 17.Chapa JB, Ahn JT, DiGiovanni LM, Ismail MA. West Nile virus encephalitis during pregnancy. Obstetrics and Gynecology. 2003;102(2):229–231. doi: 10.1016/s0029-7844(03)00614-8. [PubMed] Epub 2003/08/09. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003-2004. Pediatrics. 2006;117(3):e537–e545. doi: 10.1542/peds.2005-2024. [PubMed] Epub 2006/03/03. doi: 10.1542/peds.2005-2024. [DOI] [PubMed] [Google Scholar]
- 19.Alpert SG, Fergerson J, Noel LP. Intrauterine West Nile virus: ocular and systemic findings. American Journal of Ophthalmology. 2003;136(4):733–735. doi: 10.1016/s0002-9394(03)00452-5. [PubMed] Epub 2003/10/01. [DOI] [PubMed] [Google Scholar]
- 20.Paisley JE, Hinckley AF, O'Leary DR, Kramer WC, Lanciotti RS, Campbell GL. West Nile virus infection among pregnant women in a northern Colorado community, 2003 to 2004. Pediatrics. 2006;117(3):814–820. doi: 10.1542/peds.2005-1187. [PubMed] Epub 2006/03/03. doi: 10.1542/peds.2005-1187. [DOI] [PubMed] [Google Scholar]


