Abstract
Data from three African field sites on Pan troglodytes demonstrate an unambiguous pattern of a slower growth rate in wild vs. captive chimpanzee populations. A revised dental growth chronology for chimpanzees is similar to estimated timing of Homo erectus and therefore has implications for interpreting life history in hominins.
The sequence and timing of dental emergence correlate with life history events in catarrhine primates, which include Old World monkeys, apes, and modern humans. The first permanent molar (M1) is particularly informative for comparing catarrhine life history because its full eruption correlates with the end of infancy and completion of 90–95% of brain growth in species studied so far (1, 2). The timing of M1 eruption provides a yardstick for comparing chimpanzees, modern humans (Homo sapiens), and fossil hominins.
Most ape samples traditionally used for evolutionary comparisons derive from captive chimpanzees (Pan troglodytes). Data on dental eruption sequences and timing, fusion of bone epiphyses, limb-length measurements, body mass, and timing of behavioral changes through life have been collected on captive chimpanzees of known age and sex from different facilities (3–9). Field observations on social and behavioral development suggest that wild chimpanzees take up to 3 years longer to mature compared with captive animals (10).
Somatic development of wild chimpanzees may be similarly prolonged. Four wild individuals at Mahale exhibit shorter limb lengths than captive chimpanzees of comparable age (11). Growth schedules from laboratory-reared chimpanzees reveal that they reach adult body mass and testicular maturity earlier than observed in field studies on wild chimpanzees at Mahale and Gombe (12). Other wild primates, like baboons, also may grow more slowly compared with their captive counterparts (13).
Here we report growth data on wild, immature chimpanzees of known age and sex from the Taï Forest, Ivory Coast, Gombe National Park, Tanzania, and Bossou, Guinea. Taï and Bossou (P. troglodytes verus) represent the western end of the species range, whereas Gombe (P. troglodytes schweinfurthii) lies at the eastern end. Data from these three field sites are consistent in pointing to a growth rate in wild populations that is slower than in captive animals and contribute to the evaluation of fossil hominin growth and life stages.
Materials and Methods
The chimpanzee skeletal sample from Taï consists of 12 immature individuals of various ages and 1 young adult, all of whose sex and dates of birth and death are recorded. Animals >10 years old (n = 5) are known only to year of birth, and their ages at death are therefore estimated to the half-year (10). Four immature males of known ages from Gombe are included (14, 15), along with a known-age immature male from Bossou (16). The entire sample of 18 individuals spans an age range of 1.8–16.5 years.
The degree of tooth emergence was noted on the upper deciduous canines (the last milk teeth to emerge) and six permanent maxillary teeth: central incisor (I1), lateral incisor (I2), canine, M1, second molar (M2), and third molar (M3). In this skeletal sample, partial emergence of a tooth is defined as the cusp tips surpassing the level of the bone (alveolus), as shown in Fig. 1. Full eruption in our sample is scored when the tooth crowns reach functional occlusion.
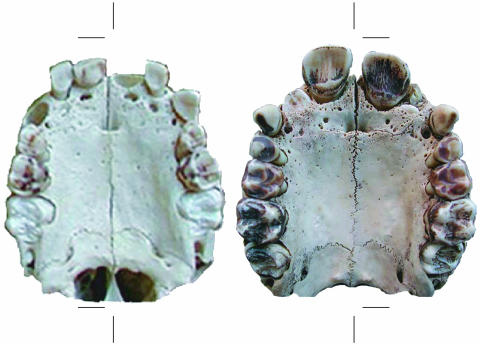
Fig. 1.
Females 3.8 years of age (Left) and 8.3 years of age (Right). Note the M1 just cresting the alveolar margin in the 3.8-year-old female. In the 8.3-year-old female, note the left I2 just cresting the alveolar margin and the right I2 and the M2 partial emergence.
Adults are defined as individuals having all the M3s fully erupted and a completely fused humeral head (17). For the associated skeletons, the degree of epiphyseal union of the proximal humerus was categorized in one of three ways: no fusion, partial fusion, or complete fusion.
The comparative data on dental emergence in captive chimpanzees are derived from a mixed longitudinal sample of oral examinations carried out over a 10-year period (n = 58) (8). The study recorded gingival emergences of the I1, I2, M1, and M2. No data on the emergence of the M3 were collected.
Two additional studies further illuminate the comparison of wild and captive chimpanzees. Three immature males from Gombe were studied during life, and the gingival emergence of selected teeth was observed and recorded by Pusey (18). Also, a study combining dental x-rays and oral examination of one captive chimpanzee revealed a time interval of 4–5 months from alveolar to gingival emergence in the M1 (19).
Results
We converted our observations on alveolar emergence ages to estimated gingival emergence ages to provide a direct comparison between wild and captive data sets (Table 1). The upper deciduous canines in wild chimpanzees emerge within the range of the captive sample. However, the M1 lies at the late end of the captive range, the 90th percentile. The I1 falls at the edge of the captive sample, and the I2 lies entirely outside the range. For the M2, the wild chimpanzees are later and outside the recorded range for the captive sample.
Table 1. Maxillary gingival emergence times in years for wild chimpanzees, compared with captives.
| dC | M1 | I1 | I2 | M2 | C | M3 | |
|---|---|---|---|---|---|---|---|
| TMale1 | ≤1.5F | ||||||
| TMale2 | ≤1.8F | ||||||
| GMale1 | ≤2.3F | >2.6N | |||||
| TFemale1 | F | 4.1A | |||||
| TFemale2 | F | ≤4.9F | |||||
| TMale3 | F | F | >5.7N | ||||
| GMale2 | 6.3* | ||||||
| BMale1 | F | F | >6.5N | >6.5N | |||
| TFemale3 | F | F | ≤8.0O | r8.2E/8.6A | 8.2E | ||
| GMale3 | F | F | 7.0* | 7.4* | 8.4F | >8.5N | |
| TMale4 | F | F | 8.4E | 8.4E | 8.4E | >8.5N | |
| GMale4 | 10.1* | ||||||
| TFemale4 | F | F | ≤10.2F | ≤10.2F | ≤10.2F | r10.4E/10.8A | >10.8N |
| TFemale5 | F | F | F | F | F | ≤12.4E | 12.8N |
| TFemale6 | F | F | F | F | F | ≤12.4E | 12.4E |
| TMale5 | F | F | F | F | F | ≤13.4E | >13.8N |
| GMale5 | F | F | F | F | F | ≤12.9E | ≤12.7F |
| GMale6 | F | F | F | F | F | ≤13.1F | ≤13.1F |
| TMale6 | F | F | F | F | F | ≤14.2F | ≤14.2F |
| TFemale7 | F | F | F | F | F | F | ≤16.5F |
| Wild (range) | ≤1.5 | 4.1 (2.6 < × ≤ 4.9) | 6.3-8.4 (5.7 < × ≤ 10.2) | 7.4-8.6 (6.5 < × ≤ 10.2) | 8.2-8.4 (8.2 ≤ × ≤ 10.2) | 10.1-10.8 (8.5 < × ≤ 14.2) | 12.4 (10.8 < × ≤ 14.2) |
| Captive 10-90% range† | 0.8-1.4 | 2.7-4.1 | 4.7-6.5 | 5.3-6.9 | 5.3-7.3 | 7.9 | 10.5 |
Maxillary gingival emergence times are based on 4 months from alveolar margin to gingival emergence (19). When partially emerged, 1.5 months were subtracted from age at death to approximate gingival emergence time (based on 3 months from gingival emergence to full occlusion in faster developing crested langurs) (20). Fully occluded teeth were estimated to emerge at least 3 months before death. dC, upper deciduous canine; C, canine. Tooth score: N, not emerged; A, at alveolar margin; E, partial emergence; O, one side occluded and one side slightly below occlusion; F, full occlusion.
Direct observation (18).
Permanent canines are not as well documented in captive chimpanzees, but emergence seems to be at ≈8 years, although there are sex differences in growth patterns, particularly in crown formation times (8, 21). Among the wild chimpanzees, canines do not emerge until 2.5 years after the recorded age for captives.
Data on the appearance of the M3 are not available from captive studies, although, based on crown formation, its emergence has been estimated at ≈10.5 years old (22). The wild sample indicates an eruption point closer to 12.5 years of age, ≈2 years later.
Discussion
Emergence of the permanent teeth in wild chimpanzees is consistently later than 90% of the captive individuals. In many cases, emergence times are completely outside the known range recorded for captive chimpanzees.
Certain life history stages broadly coincide with the emergence times of teeth, particularly the molars. For example, the M1 tooth crown begins forming at birth (22), and its emergence coincides with behaviors indicating the transition to the juvenile period and implied independent feeding exclusively on adult foods (2). The emergence of the M2 and M3 signals behavioral changes during development, but the connection between dental and somatic growth and behavior is less well documented than for the M1. Exploring such connections in known-age individuals in wild populations provides a framework for inferring life history attributes from skeletal and therefore fossil remains.
Chimpanzees. M1 emergence in wild chimpanzees occurs at ≈4 years of age, which corresponds closely to the behavioral studies at Taï, Gombe, and Mahale that classify infancy as lasting from 0 to 4 or 5 years (10, 14, 23).
Field researchers consistently identify three life stages after infancy: a juvenile stage from ≈5 to 10 years, an adolescent period from ≈10 to 13 years (females) or 10 to 15 years (males), and adulthood (sometimes further divided into young, prime, and old adult) (10, 14, 23). Long-term observations on chimpanzees at Taï, Gombe, and Mahale suggest correlations between dental development and behavioral events.
The I1, I2, and M2 emerge during the juvenile period, between ≈6.3 and 8.4 years. Juveniles still spend the majority of their time with their mothers and are at only about half their adult body size but can feed and travel independently (10).
Canine emergence at ≈10–11 years is associated with the beginning of the adolescent behavioral period. Adolescents spend less time with their mothers while developing social skills and physical attributes related to reproduction. Females begin having sexual swellings and mate regularly. Testes descend in the adolescent males, who become more aggressive and congregate with adult males (10, 14, 23).
The M3 emerges at ≈12.5 years in wild chimpanzees, which is before behavioral adulthood. Proximal humeral fusion occurs after M3 emergence, between 13.4 and 16.5 years; these two anatomical features together best approximate the adult life stage defined according to behaviors in the wild. Females make the transition to adulthood at ≈14 years with first reproduction (10, 14, 23). Males take longer; at ≈16 years, they exhibit social posturing, border patrolling, and integration into the male hierarchy (10, 14, 23).
Fossil Hominins. Dental development serves as a basis for reconstructing maturation rates and life history events in fossils. As the tooth forms, the layers of enamel record daily and weekly growth; these data in turn provide a basis for estimating age of tooth formation and emergence (24). The wild chimpanzee data reported here serve as a comparison with two specimens assigned to Homo erectus. Based on incremental markings in enamel and dentine in the posterior dentition of Sangiran S7-37 from Java, gingival emergence of the M1 is estimated at ≈4.4 years and the M2 at 7.6 years (24). For an African Homo erectus, KNM-WT15000 “Turkana Boy,” emergence of the M1 is estimated at ≈4 years and an age at death at closer to 8 years (24) (rather than 12 years based on a more human-like model of development) (25). Wild chimpanzees also have M1 emergences at ≈4 years, suggesting that H. erectus has rates of growth similar to chimpanzees. (Table 1). Our data further support an age at death for KNM-WT15000 between 8.5 and 10.8 years (24, 25) because the M2 are fully erupted, which occurs in wild chimpanzees as early as 8.5 years of age, and the maxillary canines are unerupted, which occurs as late as 10.8 years of age.
The sample size for estimating dental development in fossils is admittedly small. However, when combined with the wild chimpanzee data, we are persuaded that the developmental pattern of H. erectus is not intermediate between chimpanzees and modern humans, as Bogin (26) posits, based on a captive chimpanzee M1 eruption point of 3.1 years. By comparison with a wild chimpanzee behavior, KNM-WT15000 would have died as a juvenile, still in close association with the mother but eating adult foods and traveling independently. Perhaps other fossil hominin species life history patterns exhibit more rapid dental growth and earlier maturation than previously recognized, as studies on Neanderthal dentition and development suggest (27, 28).
Acknowledgments
We thank the Ministry of the Environment and Forests, the Ministry of Research of Côte d'Ivoire, the Directorship of the TaïNational Park and the Swiss Research Centre CSRS in Abidjan for Taï specimens; Gustl Anzenberger, Karin Isler, and the Institut of Anthropology at the University of Zürich for curation; Dr. Jane Goodall and the Tanzanian Government, especially the Director of Tanzania National Parks and Director of Wildlife, and Anthony Collins and the Tanzanian Field Staff at Gombe for the Gombe material; Dr. Yuzuru Hamada for dental information for the Bossou specimen; Chris Dean, Jay Kelley, and Gary Schwartz for discussions on dental development; three anonymous reviewers for their comments; and Sara Keene, Jerold Lowenstein, and Marissa Sousa for their insightful comments on the manuscript. This work was supported by the Leakey Foundation and the University of California, Santa Cruz, Faculty Research Committee and Social Sciences Division.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: I1, central incisor; I2, lateral incisor; M1, first permanent molar; M2, second molar; M3, third molar.
References
- 1.Smith, B. H. (1989) Evolution (Lawrence, Kans.) 43, 683–688. [Google Scholar]
- 2.Smith, B. H. (1991) Am. J. Phys. Anthropol. 86, 157–174. [Google Scholar]
- 3.Kerley, E. R. (1966) Tulane Stud. Zool. 13, 71–82. [Google Scholar]
- 4.Gavan, J. A. (1971) in The Chimpanzee, ed. Bourne, G. H. (Univ. Park, Baltimore), Vol. 4, pp. 46–102. [Google Scholar]
- 5.Watts, E. S. (1971) Ph.D. thesis (Univ. of Pennsylvania, Philadelphia).
- 6.Tutin, C. E. G. (1994) in Chimpanzee Cultures, eds. Wrangham, R. W., McGrew, W. C., DeWaal, F. B. M. & Heltne, P. G. (Harvard Univ. Press, Cambridge, MA), pp. 181–194.
- 7.Nissen, H. W. & Riesen, A. H. (1964) Am. J. Phys. Anthropol. 22, 285–294. [DOI] [PubMed] [Google Scholar]
- 8.Conroy, G. C. & Mahoney, C. J. (1991) Am. J. Phys. Anthropol. 86, 243–254. [Google Scholar]
- 9.Leigh, S. R. & Shea, B. T. (1995) Am. J. Primatol. 36, 37–60. [DOI] [PubMed] [Google Scholar]
- 10.Boesch, C. & Boesch-Achermann, H. (2000) The Chimpanzees of the TaïForest: Behavioural Ecology and Evolution (Oxford Univ. Press, Oxford).
- 11.Kimura, T. & Hamada, Y. (1996) Primates 37, 237–251. [Google Scholar]
- 12.Hamada, Y., Udono, T., Teramoto, T. & Sugawara, T. (1996) Primates 37, 279–295. [Google Scholar]
- 13.Phillips-Conroy, J. & Jolly, C. J. (1988) Am. J. Primatol. 15, 17–29. [DOI] [PubMed] [Google Scholar]
- 14.Goodall, J. (1986) The Chimpanzees of Gombe: Patterns of Behavior (Harvard Univ. Press, Cambridge, MA).
- 15.Zihlman, A. L., Morbeck, M. E. & Goodall, J. (1990) J. Zool. (London) 221, 37–61. [Google Scholar]
- 16.Matsuzawa, T., Sakura, O., Kimura, T., Hamada, Y. & Sugiyama, Y. (1990) Primates 31, 635–641. [Google Scholar]
- 17.Bolter, D. R. & Zihlman, A. L. (2003) J. Zool. (London) 260, 99–110. [Google Scholar]
- 18.Pusey, A. (1978) Ph.D. thesis (Stanford Univ., Palo Alto, CA).
- 19.Kelley, J. & Smith, T. (2003) J. Hum. Evol. 44, 307–329. [DOI] [PubMed] [Google Scholar]
- 20.Wolf, K. E. (1984) Ph.D. thesis (Yale Univ., New Haven, CT).
- 21.Schwartz, G. T. & Dean, M. C. (2001) Am. J. Phys. Anthropol. 115, 269–283. [DOI] [PubMed] [Google Scholar]
- 22.Reid, D. J., Schwartz, G. T., Dean, C. & Chandrasekera, M. S. (1998) J. Hum. Evol. 35, 427–448. [DOI] [PubMed] [Google Scholar]
- 23.Nishida, T. (1990) The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies (Univ. of Tokyo Press, Tokyo).
- 24.Dean, M. C., Leakey, M. G., Reid, D., Schrenk, F., Schwartz, G. T., Stringer, C. & Walker, A. (2001) Nature 414, 627–631. [DOI] [PubMed] [Google Scholar]
- 25.Smith, B. H. (1993) in The Nariokotome Homo erectus Skeleton, eds. Walker, A. C. & Leakey, R. F. (Harvard Univ. Press, Cambridge, MA), pp. 195–220.
- 26.Bogin, B. (2003) in Patterns of Growth and Development in the Genus Homo, eds. Thompson, J. L., Krovitz, G. E. & Nelson, A. J. (Cambridge Univ. Press, New York), pp. 15–44.
- 27.Ramirez Rozzi, F. V. & De Castro, J. M. B. (2004) Nature 428, 936–939. [DOI] [PubMed] [Google Scholar]
- 28.Stringer, C. B., Dean, M. C. & Martin, R. D. (1990) in Primate Life History and Evolution, ed. deRousseau, C. J. (Wiley–Liss, New York), pp. 115–152.