Abstract
Alexithymia is characterized by difficulties in different domains of emotion processing, especially in relation to negative emotions. Nevertheless, its causal mechanisms remain elusive. Reduced anticipation of negative emotional events might be one such mechanism because it enables the individual to prepare to respond effectively to coming events. To test this, changes in skin conductance response (SCR) were recorded during classical fear conditioning in sixty participants with high (HA), medium (MA) and low (LA) levels of alexithymia. Two coloured squares were presented, one was reinforced with a mild electrical stimulation (CS+) while the other was never reinforced (CS−). Critically, despite all groups showing higher SCR to CS+ compared to CS−, SCR to CS+ was lower and extinguished earlier in HA compared to MA and LA. These differences appeared to be attributable neither to differences in the intensity of stimulation received, nor to SCR to the stimulation itself. Groups showed comparable SCR to CS− as well. Therefore, HA exhibited decreased anticipation of the occurrence of a negative emotional event. Disruption of this mechanism may then compromise effective emotion recognition, emotional response and response regulation, which characterise HA, and represent a unifying causal mechanism underlying the difficulties in emotion processing of this group.
Alexithymia is a personality trait characterised by difficulties in identifying and describing feelings and discriminating between feelings and bodily sensations of emotional arousal1,2. Although considered a subclinical phenomenon, higher prevalence of high levels of alexithymia than the general population have been found in a number of conditions, including anxiety, depression, eating disorders and substance abuse, leading to the hypothesis that HA might represent a risk factor for developing such pathologies3. In addition, the incidence of high alexithymia appears higher also in autism compared to the general population4, and there is consistent evidence showing that the emotional difficulties observed in autism may in fact be due to co-occurring alexithymia5,6,7. Given this, it seems crucial to understand the role played by high alexithymia in emotion processing.
Research is producing a large body of literature on the differences exhibited by individuals with high levels of alexithymia (HA) as compared to individuals with low levels of alexithymia (LA), especially in relation to negative emotions. For example, HA are less accurate in recognizing emotional faces presented for a brief period of time8,9, rate the expression of fearful faces as less intense10, and fail to remap fear on their own somatosensory system11. Moreover, decreased activation of the amygdala has been reported during the processing of emotional stimuli in HA12,13, which seems to be specific to negative stimuli, such as sad faces, fearful bodies or observation of pain in others14,15,16,17. They also show impairments in emotional response regulation18 appearing less able to recur to reappraisal as a strategy to regulate emotions19. Furthermore, HA exhibit decreased empathic concern leading to more inclination towards utilitarian decisions in moral dilemmas20.
Although relating to distinct aspects of emotion processing, the difficulties of HA could be partly caused by a unifying underlying mechanism. Reduced anticipation of emotional events might be hypothesised to be one such mechanism. In fact, the anticipation of emotional events is a crucial adaptive mechanism, which enables the individual to prepare to respond effectively to the coming events. Through learning processes, individuals become able to attribute an emotional value to previously neutral cues and, as a consequence, anticipate an emotional event from cues in the environment, which have now become predictors of its occurrence21,22. By anticipating the coming emotional event, individuals not only can form a cognitive representation of the coming event per se but also of its consequences. The former then enables faster recognition and response to the emotional event, the latter optimal emotional response regulation and decision making23. Therefore, despite being a low level process, learning to anticipate emotional events might be crucial for effective emotion processing. Disruption of this process has been observed in psychopathologies related to emotion processing, such as depression, anxiety or psychopathy24,25,26. Similarly, this could be true also for HA.
Classical fear conditioning has been extensively used to study the process of learning to anticipate the occurrence of negative emotional events27. There, a neutral stimulus is paired with an aversive unconditioned stimulus (UCS), which elicits innate emotional responses, named unconditioned response (UR). After repeated pairing of the two stimuli, the individual learns to anticipate the occurrence of UCS at the presentation of the neutral stimulus, which becomes a conditioned stimulus (CS). In the end, the sole presentation of CS elicits an anticipatory response in preparation to the occurrence of UCS, called conditioned response (CR). Both UR and CR are marked by physiological changes in autonomic nervous system activity and increased skin conductance response (SCR) represents one of them28,29. Higher SCR in response to CS signals increased expectations regarding the occurrence of UCS following the presentation of CS indicating that the association between CS and UCS has been learnt30. Additionally, changes in subjective affective experience accompany the physiological changes and higher arousal and lower pleasantness are generally reported by participants at the presentation of CS compared to a neutral stimulus31.
Therefore, classical fear conditioning could be used to test whether HA present a reduced response in anticipating the occurrence of negative emotional events. To this end, sixty participants with HA, LA and medium levels of alexithymia (LA), as measured by the 20-item Toronto Alexithymia Scale (TAS-20)32, completed a classical fear conditioning task with partial reinforcement33,34. On each trial, one of two coloured squares was presented on a computer screen for 6 seconds followed by an inter trial interval of 12 seconds. The task included 40 trials (20 for each stimulus) divided in three blocks: habituation, acquisition and extinction. During habituation (4 trials) none of the stimuli was reinforced to ensure there were no baseline differences in response to the stimuli. During acquisition (16 trials) one stimulus was reinforced with a mild electric stimulation (UCS) on 80% of trials (CS+) while the other was never reinforced (CS−). During extinction (20 trials) no stimulation was administered. Changes in SCR were recorded continuously during the experiment as a somatic indicator of the degree of anticipation of the UCS. In addition, to assess subjective experience, participants reported the level of anxiety and fear experienced at the presentation of each CS during the experiment. Finally, because anxiety is known to affect SCR in classical conditioning25, levels of anxiety were measured with the State-Trait Anxiety Inventory35 and correlations between levels of anxiety and differential SCR response were explored to exclude an effect of anxiety on results. HA were hypothesised to show decreased anticipation of the electrical stimulation following the presentation of CS+, hence exhibit lower SCR to CS+ compared to MA and LA. No differences between MA and LA were expected.
Results
UCS intensity and peak and mean SCR to UCS
Univariate ANOVAs were used to evaluate differences in UCS intensity and mean and peak SCR to the UCS. Results showed no significant differences among the three groups in either UCS intensity (MLA = 4.49 μS, SDLA = 2.86 μS; MMA = 4.01 μS, SDMA = 2.52 μS; MHA = 3.33 μS, SDHA = 2.16 μS; F(2, 57) = 1.06, p = 0.353, partial η2 = 0.04), peak SCR in response to UCS (MLA = 1.24 μS, SDLA = 0.56 μS; MMA = 1.26 μS, SDMA = 0.56 μS; MHA = 1.14 μS, SDHA = 0.46 μS; F(2, 57) = 0.31, p = 0.736, partial η2 = 0.01) or mean SCR in response to UCS (MLA = 1.03 μS, SDLA = 0.48 μS; MMA = 1.08 μS, SDMA = 0.46 μS; MHA = 0.91 μS, SDHA = 0.38 μS; F(2, 57) = 0.80, p = 0.456; partial η2 = 0.03). On average, the intensity of the stimulation received by participants as well as the physiological response to it did not differ significantly among groups.
SCR during habituation
A 3 × 2 RM ANOVA was carried out to analyse habituation with group as between-subject variable (low, medium, high) and stimulus type as within-subject variable (CS−, CS+). Analysis on SCR showed no significant main effect of group (F(2, 57) = 0.71, p = 0.494, partial η2 = 0.02), stimulus (F(1, 57) = 0.84, p = 0.361, partial η2 = 0.01) or interaction (F(2, 57) = 0.38, p = 0.681, partial η2 = 0.01), confirming that at baseline there were neither within group nor between group differences in response to the two conditioned stimuli (Fig. 1).
Figure 1. Habituation.

Mean skin conductance response (SCR) to the two conditioned stimuli (CS−, CS+) during habituation as a function of alexithymia group. Error bars represent standard errors.
SCR after habituation
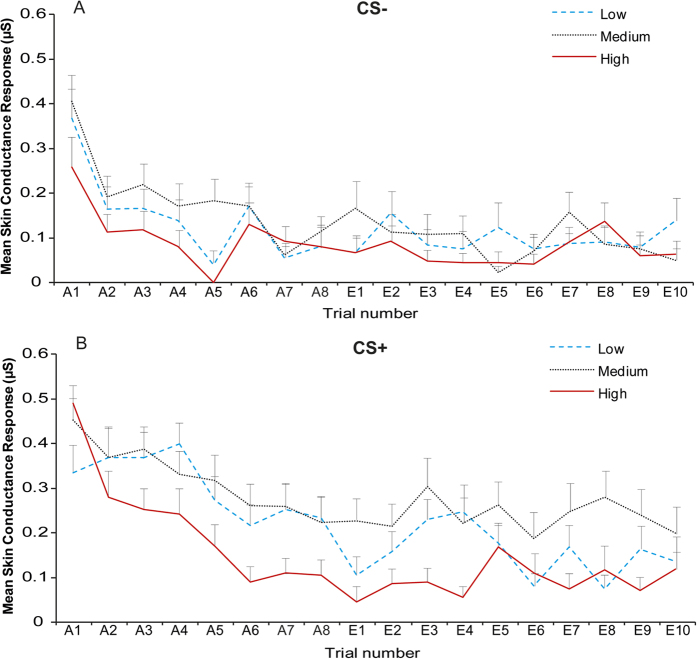
Figure 2 shows the mean SCR of the groups to CS− (panel A) and CS+ (panel B) for each trial of acquisition and extinction. A 3 × 2 × 3 RM ANOVA was carried out to analyse SCR in the phases following habituation with group as between-subject variable (low, medium, high) and stimulus (CS−, CS+) and phase (acquisition, early extinction and late extinction) as within-subject variables. Analysis on SCR showed significant stimulus by phase by group interaction (F(4,114) = 3.64, p = 0.008, partial η2 = 0.11). This interaction was further explored conducting separate ANOVAs for acquisition and extinction.
Figure 2. Skin conductance response during acquisition and extinction.
Trial by trial mean skin conductance response (SCR) to the two conditioned stimuli (panel A: CS−; panel B: CS+) as a function of alexithymia group. Error bars represent standard errors.
SCR during acquisition
During acquisition, a significant stimulus by group interaction (F(2, 57) = 3.26, p = 0.046, partial η2 = 0.10) was found, indicating that groups differed in SCR to the two conditioned stimuli during acquisition. Newman-Keuls test showed that despite all groups showing significant difference in response to CS+ as compared to CS− (MLACS− = 0.12 μS, SDLACS− = 0.12 μS; MLACS+ = 0.30 μS, SDLACS+ = 0.19 μS; p < 0.001; MMACS− = 0.16 μS, SDMACS− = 0.13 μS; MMACS+ = 0.31 μS, SDMACS+ = 0.17 μS; p < 0.001; MHACS− = 0.09 μS, SDHACS− = 0.07 μS; MHACS+ = 0.18 μS, SDHACS+ = 0.13 μS; p = 0.007), there was a significant difference between groups in response to CS+. Specifically, HA had significantly lower SCR compared to LA (p = 0.007) and MA (p = 0.015). No difference was found in SCR to CS+ between LA and MA or in response to CS− among any of the groups (all p > 0.262; Fig. 3). Therefore, all groups showed differential SCR to CS+ compared to CS−. However, SCR to CS+ exhibited by HA was significantly lower than SCR exhibited by the other two groups. On the contrary, responses to CS− were comparable among groups.
Figure 3. Acquisition.
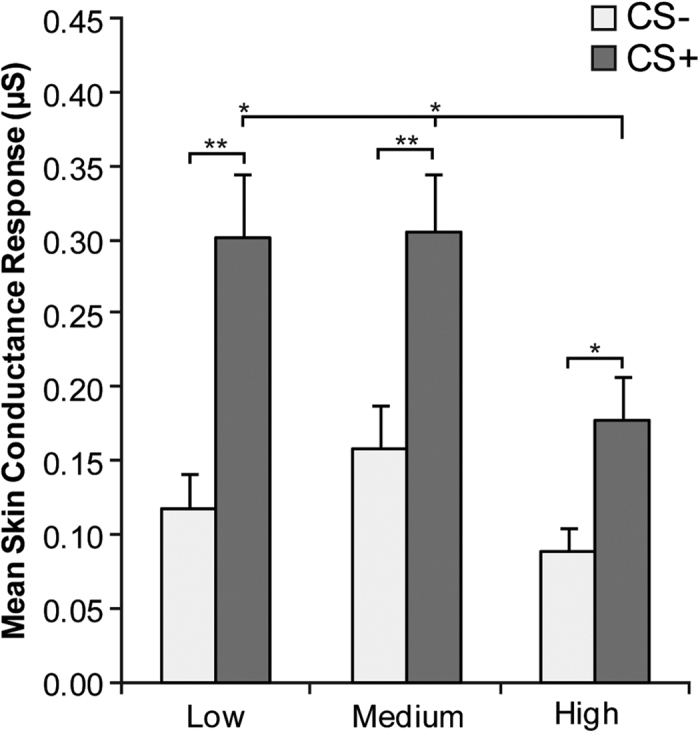
Mean skin conductance response (SCR) to the two conditioned stimuli (CS−, CS+) during acquisition as a function of alexithymia group. Error bars represent standard errors. Significant differences are indicated as follows: *p < 0.05; **p < 0.001.
The main effect of group (F(2, 57) = 3.26, p = 0.046, partial η2 = 0.10) and stimulus (F(1, 57) = 84.74, p < 0.001, partial η2 = 0.60) were also significant. However, these were secondary to the interaction described above.
Given that difference in SCR between CS+ and CS− may be influenced by the levels of anxiety, correlations between these two variables were explored to exclude a significant contribution of anxiety to the results. Neither trait nor state anxiety correlated significantly with difference in SCR to the two conditioned stimuli (all p > 0.292).
SCR during extinction
Extinction was divided in two blocks, early and late to investigate the role of time in the extinction of the conditioned response. A 3 × 2 × 2 RM ANOVA was carried out to analyse extinction with group as between subject variable and stimulus type and time (early, late) as within-subject variables. Nevertheless, analysis showed that time did not play a significant role in extinction. Neither a significant main effect nor interaction of time with the other factors was found (all p > 0.183).
On the contrary, there was a significant stimulus by group interaction (F(2, 57) = 5.53, p = 0.007, partial η2 = 0.16). Newman-Keuls test showed that only LA and MA maintained a significantly higher SCR to CS+ compared to CS− (MLACS− = 0.10 μS, SDLACS− = 0.15 μS; MLACS+ = 0.15 μS, SDLACS+ = 0.14 μS; p = 0.037; MMACS− = 0.10 μS, SDMACS− = 0.09 μS; MMACS+ = 0.24 μS, SDMACS+ = 0.20 μS; p < 0.001; Fig. 4).
Figure 4. Extinction.

Since the factor time did not interact significantly with the other factors, the figure shows mean skin conductance response (SCR) to the two conditioned stimuli (CS−, CS+) during extinction as a function of alexithymia group collapsing early and late extinction blocks. Error bars represent standard errors. Significant differences are indicated as follows: *p < 0.05; **p < 0.001.
A main effect of stimulus was present (F(1, 57) = 24.30, p < 0.001, partial η2 = 0.30), although secondary to the interaction described above. Instead, the main effect of group resulted non significant (F(2, 57) = 2.63; p = 0.081, partial η2 = 0.08).
Also in this phase neither trait nor state anxiety correlated significantly with the difference in SCR between CS+ and CS− (all p > 0.616).
Subjective reports of anxiety and fear
3 × 2 RM ANOVAs were conducted on subjective reports of fear and anxiety experienced at the presentation of the conditioned stimuli for each phase of conditioning with group as between-subject variable (low, medium, high) and stimulus type as within-subject variable (CS−, CS+). Both the subjective report on anxiety and fear showed a main effect of stimulus (respectively: F(1, 57) = 170.41, p < 0.001, partial η2 = 0.75; F(1, 57) = 133.34, p < 0.001, partial η2 = 0.70). The reported anxiety to CS+ (MCS+ = 59.84%, SDCS+ = 21.39%) was higher than to CS− (MCS− = 23.56%, SDCS− = 18.29%; p < 0.001) as well as the reported fear to CS+ (MCS+ = 46.13%, SDCS+ = 23.05%) was higher than to CS− (MCS− = 13.41%, SDCS− = 13.22%; p < 0.001). In contrast, no significant main effect of group or interaction was found either for anxiety or fear.
Discussion
This study investigated whether HA presented reduced anticipation of negative emotional events. To this end, changes in SCR were recorded during classical fear conditioning to assess differences among LA, MA and HA in anticipating the occurrence of a negative emotional event by learning patterns of association between CS+ and UCS.
All Participants correctly associated CS+ and UCS, suggesting that they explicitly identified the stimulus that anticipated the negative emotional event. In addition, groups did not differ in the intensity of UCS received, SCR to it and emotional experience reported in response to presentation of CS+. On the contrary, results showed significant differences among HA and MA and LA in SCR during acquisition that exacerbated during extinction. Specifically, during acquisition all three groups learned the anticipatory value of CS+ in predicting UCS, as indicated by higher SCR to CS+ compared to CS−. However, the degree of physiological response elicited by the anticipation of UCS in HA was lower compared to MA and LA, as shown by significantly lower SCR to CS+. This reduced response intensified during extinction, when the differential SCR to CS+ extinguished in HA while it was maintained in MA and LA. This suggested that the response elicited by the anticipation of UCS disappeared as soon as the predictive value of CS+ was no more reinforced by the administration of UCS. Crucially, this result did not appear to be dependent solely on the reduced SCR to CS+ during acquisition. These differences between the groups were attributable neither to differences in the intensity of UCS, because all groups received comparable intensities of stimulation, nor to reactivity to UCS itself, because groups did not differ in mean or peak SCR amplitude to UCS. In addition, groups did not differ in their SCR during habituation, acquisition or extinction to CS−, indicating comparable physiological response to neutral stimuli as well. Therefore, although HA seem to learn to differentiate a neutral from a conditioned stimulus, they appear less responsive in anticipating the negative consequences of a conditioned stimulus compared to LA and MA. This becomes particularly evident once the conditioned stimulus ceased to be reinforced revealing a difficulty in maintaining the association learned over time. As soon as the conditioned stimulus was no more reinforced by the aversive stimulus, the emotional value that HA had learnt to attribute to the conditioned stimulus disappeared.
Physiological changes in the anticipation of negative emotional events have been proposed to be a crucial component of emotional experience36 and they have the adaptive function of guiding attention towards the source of the events preparing the organism to effectively identify, respond and regulate the response to such event22,28. Therefore, the anticipation of emotional events might be crucial for effective emotion processing. Results suggest that HA are less able to anticipate the coming emotional event and possibly its consequences, which would be crucial to allow rapid identification, response and regulation of the response to such event. This difference may represent a shared underlying mechanism contributing to the difficulties of this group in emotion processing, which are particularly evident in ambiguous contexts, such as the recognition of emotional stimuli during limited time constraints, decision making in moral dilemmas and emotional response regulation8,9,18,20.
Anticipating the emotional future seems to involve more complex mechanisms than just learning about the contiguity between CS+ and UCS30. In fact, the individual is required to learn the causal relationship between the CS+ and UCS. At each learning trial, UCS acts as teaching signal strengthening the response to CS+. The strength of this teaching signal is modulated by predictions regarding the occurrence of UCS following the presentation of CS+37. A brain circuit seems to be responsible for such process involving the periacqueductal gray, relaying the UCS teaching signal to the amygdala through indirect pathways via the thalamus, which then project to the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC). Once in the amygdala, the UCS teaching signal then modulates plasticity at CS+ input synapses strengthening the response to CS+37. The amygdala then sends an output to the regions that regulate activity in the autonomic nervous system, to generate changes in SCR38,39,40. Indeed, previous research has shown decreased activation of mPFC, ACC and amygdala during processing of negative emotional stimuli in HA16,41. Similarly, this circuit might be less active also during the anticipation of negative emotional events.
The lower physiological response in anticipation to UCS in HA was not reflected in lower subjective reports of fear and anxiety. HA reported comparable levels of anxiety and fear experienced at the presentation of CS+ to LA and MA. These data might seem to contrast with an influential account of alexithymia, which describes alexithymia as the emotional equivalent of blindsight42. According to this frame, alexithymia would be characterised by an intact physiological response and a deficit in emotion concept representation43. Nevertheless, the literature concerning this aspect has reported inconsistent results. Studies found both comparable44,45,46 and decreased47,48,49,50,51 physiological response to emotional stimuli together with no difference48, increased50,52,53 or decreased46 subjective reports of emotional experience. To reconcile these contrasting findings, the literature has hypothesised that alexithymia might be characterised by a decoupling between the subjective experience and physiological response to emotional stimuli52 and the present data would support this decoupling. However, the direction of this decoupling remains a matter for future investigation. In addition, it has been argued that processes generating the physiological response in fear conditioning interact with but are distinct from those that give rise to conscious feelings of fear and anxiety22,28. In fact, while the amygdala is a crucial structure in generating SCR to CS+, cortical areas seem to be involved in attributing meaning to interoceptive inputs to construct the experience of an emotion54. Speaking more broadly, the present data suggest that the processes giving rise to the explicit emotional experience might be partly dissociated from those giving rise to the physiological response to emotional stimuli. This dissociation has been observed in a number of other conditions. For example, patients with lesions to the amygdala have shown diminished55 or absent56 SCR to an aversively conditioned stimulus, despite intact unconditioned response and awareness about the association between conditioned and unconditioned stimulus. On the contrary, patients with split brain57, hemispatial neglect58 and affective blindsight59,60,61 have shown intact physiological response in the absence of awareness for emotional stimuli. Nevertheless, although physiological responses and awareness for emotion can be separated, somatic and interoceptive information regarding one’s own body is generally incorporated with semantic and contextual knowledge to generate an integrated representation of affective state62,63 and this might be the case for LA and MA. However, in HA the physiological and cognitive aspect of emotional experience may remain decoupled possibly contributing to their difficulties. Despite comparable cognitive aspects of emotional experience, lower physiological response in anticipation of emotional events alone might not be sufficient to prepare HA to effectively respond to emotional events.
To conclude, the present study shows that HA are less able to anticipate the occurrence of negative emotional events compared to LA and MA. This indicates a disruption in HA in learning to attribute an emotional value to previously neutral stimuli and use them as cues to predict the emotional future. The ability to predict the emotional future has the adaptive function of guiding attention towards the source of the emotional event preparing the organism to effectively respond to it21,22,28. Anticipating the coming emotional event, individuals not only can form a cognitive representation of the coming event but also of its consequences. Therefore, disruption of this process may lead to difficulties in effective recognition, response and response regulation to emotional events, which characterise HA and may represent a unifying low level mechanism, which may underlie part of the difficulties in emotion processing of HA. As this represents the first evidence of disruption in emotional learning in HA, further research will be needed to clarify which aspect of emotional learning might be affected in HA and in what way this can impact higher level emotion processing.
Methods
Participants
Three-hundred university students completed the 20-item Toronto Alexithymia Scale (TAS-20)32. Depending on the score, students were classified as LA (TAS-20 ≤ 36), MA (36 < TAS-20 < 61) or HA (TAS-20 ≥ 61)64. Individuals from the three groups were randomly contacted and asked to participate in the study. Due to the high co-occurrence of alexithymia and depression65, participants completed the Beck Depression Inventory66, and were excluded in case their score was higher than the moderate/severe depression cut-off (i.e. 19; n = 5). Sixty-two university students with no history of neurological or psychiatric disorders completed the study. After the experimental task, explicit awareness of the contingency between CS and UCS was assessed. Two participants were removed from analysis due to failure in reporting the correct association between stimuli. The final sample included in the analysis consisted of 60 participants (22 males, 38 females; age M = 24.03, SD = 2.38 years old) divided in three groups: 20 LA participants (13 females; TAS-20 M = 30.42, SD = 3.79; age M = 24.67, SD = 2.83 years old); 20 MA participants (12 females; TAS-20 M = 46.10, SD = 6.18; age M = 24.06, SD = 1.80 years old); 20 HA participants (13 females; TAS-20 M = 63.63, SD = 2.39; age M = 23.35, SD = 2.32). A priori targets for sample size and data collection stopping rule were based on sample and effect sizes reported in the literature on classical fear conditioning (sample size around 17–19 participants per group as indicated by a recent meta-analysis67).
Because anxiety is known to affect SCR in classical conditioning25, levels of anxiety were measured with the State-Trait Anxiety Inventory35. Levels of anxiety in the three groups differed significantly both for state (F(2, 57) = 5.86, p = 0.005) and trait anxiety (F(2, 57) = 21.54, p < 0.001). Post-hoc Newman-Keuls test showed that for state anxiety LA (M = 33.84, SD = 6.29) had significantly lower levels of anxiety compared to MA (M = 40.22, SD = 7.32; p = 0.009) and HA (M = 39.79, SD = 6.08; p = 0.006), while for trait anxiety all groups differed significantly from each other with LA (M = 36.42, SD = 6.71) showing lower levels of trait anxiety compared to MA (M = 44.32, SD = 8.05; p = 0.001) and HA (M = 51.53, SD = 7.01; p < 0.001) and MA showing lower levels of trait anxiety compared to HA (p = 0.003). Correlations between levels of anxiety and differential SCR response were explored to exclude an effect of anxiety on results.
The study was designed and conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki and the institutional guidelines of the University of Bologna and was approved by the Ethics Committee of the Department of Psychology. All participants gave informed written consent to participation after being informed about the procedure of the study.
Stimuli
The task consisted in a classical differential fear conditioning paradigm with partial reinforcement33,34. Two isoluminant coloured squares represented the CS. The UCS consisted of a mild electric stimluation of 200 ms in duration generated by a Digitimer Stimulator (Model DS7, Digitimer Ltd., UK) administered to the inner wrist of the right hand, to which two electrodes were attached. The intensity of the stimulation was set with a standard workup procedure. It was initially set at 0.5 mA and increased of 1 mA until participants reported it as being highly uncomfortable but not painful.
Each trial consisted in the presentation of one CS in the centre of a computer screen (17″, refresh rate 60 Hz) for 6 seconds followed by an inter trial interval of 12 seconds during which a fixation cross was presented. The task included 40 trials (20 for each CS) divided in three blocks: habituation, acquisition and extinction. At the beginning of habituation, instructions appeared on the screen stating that two different images would be presented one at the time in the centre of the screen, no stimulation would be administered and the task of the participant would be to carefully observe the images. Habituation included 4 trials (2 for each CS) to ensure the absence of any baseline differences within and between groups in response to the images. At the beginning of acquisition similar instructions stated that the same two images would appear one at the time in the centre of the screen and that one of them might be paired with the stimulation. The task of participants remained to carefully observe the images. No information was given about contingencies between images and stimulation. Acquisition included 16 trials (8 for each CS). CS+ was reinforced in 80% of the trials (n = 6), while CS− was never reinforced. Extinction followed acquisition without any instructions. It included 20 trials (10 for each CS) and no stimulation was administered. Stimuli were presented in pseudorandomised order, no more than two presentations of the same stimulus occurred in a row34. The first two trials of acquisition always included one CS− and one CS+ presented randomly. The colour of the square associated to the CS+ and CS− was counterbalanced across participants.
Skin conductance response recording
The SCR was recorded through two Ag/AgCl electrodes (TSD203 Model; Biopac Systems, USA), filled with isotonic hyposaturated conductant attached to the distal phalanges of the second and third finger of participants’ left hand and held with Velcro straps. The SCR signal was continuously recorded at 200 Hz and amplified using a DC amplifier (Biopac GSR100; Biopac Systems, USA) with 5 μS/V gain factor and 10 Hz low pass filter. The analogue signal was digitalized using the MP-150 digital converter (Biopac Systems, USA) and fed into AcqKnowledge 3.9 software (Biopac Systems, USA).
Assessment of subjective anxiety, fear and contingency awareness
At the end of the task, participants were asked to report the level of anxiety and fear experienced at the presentation of each CS during the experiment on separate visual analogue scales ranging from 0 (not at all) to 100 (extreme). The order of questions was balanced across participants.
Participants were also asked to indicate which of the two stimuli was associated with the stimulation to ensure explicit awareness of pairing between CS+ and UCS. Participants who failed to report the correct association were removed from analysis.
Procedure
The experiment took place in a sound attenuated room with dimmed light. Participants were seated in a chair in front of a computer monitor at ~70 cm distance. Once seated, the experimental procedure was explained and written informed consent was obtained from participants. Then SCR electrodes were attached and correct recording of the signal was ensured. Afterwards, the intensity of the stimulation was set and the task began. Following completion of the task, subjective reports were completed.
Data analysis
SCR data were analyzed using MATLAB (The MathWorks, Inc., USA) custom-made scripts33. SCR was calculated as the peak-to-peak amplitude difference of the largest deflection in the 0.5–4.5 sec latency window after stimulus onset. Regarding SCR to UCS, stimulus onset was represented by the time of stimulation administration, while regarding SCR to CS, stimulus onset referred to the time of CS appearance. The SCR was transformed into microsiemens (μS) and calculated for each trial. Minimum response criterion was 0.02 μS and smaller responses were encoded as zero. Square root transformation was conducted on raw SCR to normalize the data distribution and SCR were scaled to each subject’s maximal UCS response to account for interindividual variability34.
Both SCR to UCS and CS− were analysed to ensure that groups did not differ in their physiological response to the stimulation or in the anticipatory response to CS− but only to a conditioned stimulus that predicts an aversive event (i.e. CS+). Regarding SCR to UCS, both peak response and average response were analysed. For each participant, peak response represented the highest SCR in response to the six stimulations administered while mean response was the average of the SCRs to the six stimulations. Regarding the response to CSs, SCRs during the three phases of conditioning were analysed separately. Concerning habituation, all trials were included in the analysis. With regards to acquisition, the first two trials were not included in the analysis because participants learned the association between UCS and CS+ after its first pairing with the stimulation. Regarding extinction, all trials were included in the analysis but this phase was divided in two blocks (early and late extinction). Then, mean SCR of each participant was computed to produce four average scores representing the SCR of each subject during habituation, acquisition, early and late extinction. These were then averaged to obtain the SCR during the different phases for each alexithymia group.
Assumptions of normal distribution were verified. Several ANOVAs were then used to investigate differences among the three groups. Post hoc analyses were conducted with Newman-Keuls test. Significance threshold was p < 0.05.
Additional Information
How to cite this article: Starita, F. et al. Reduced anticipation of negative emotional events in alexithymia. Sci. Rep. 6, 27664; doi: 10.1038/srep27664 (2016).
Acknowledgments
We are grateful to Khatereh Borhani and Cristina Scarpazza for their help in participants screening and recruitment, Riccardo Paracampo and Marco Zanon for their help in setting up the experimental task, Sara Garofalo for her inputs on data analysis and Marina Cieri, Serena Pierantoni and Giulia Salvi for their assistance with data collection. This work was supported by FARB University of Bologna Grant (Protocol: RFBO 120993) to Elisabetta Làdavas.
Footnotes
Author Contributions All authors developed the study concept and contributed to the study design. Testing and data collection were performed by F.S., who also performed the data analysis and interpretation under the supervision of E.L. and G.d.P. F.S. drafted the manuscript and E.L. and G.d.P. provided critical revisions. All authors approved the final version of the manuscript for submission.
References
- Sifneos P. E. The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother. Psychosom. 22, 255–262 (1973). [DOI] [PubMed] [Google Scholar]
- Taylor G. J., Bagby R. M. & Parker J. D. A. The Alexithymia Construct: A Potential Paradigm for Psychosomatic Medicine. Psychosomatics 32, 153–164 (1991). [DOI] [PubMed] [Google Scholar]
- Panaite V. & Bylsma L. M. In Encyclopedia of Human Behavior (Second Edition) (ed. Ramachandran V. S.) 92–99 (Academic Press, 2012). [Google Scholar]
- Bird G. & Cook R. Mixed emotions: the contribution of alexithymia to the emotional symptoms of autism. Transl. Psychiatry 3, e285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R., Brewer R., Shah P. & Bird G. Alexithymia, Not Autism, Predicts Poor Recognition of Emotional Facial Expressions. Psychol. Sci. 956797612463582, 10.1177/0956797612463582 (2013). [DOI] [PubMed] [Google Scholar]
- Heaton P. et al. Measuring the effects of alexithymia on perception of emotional vocalizations in autistic spectrum disorder and typical development. Psychol. Med. 42, 2453–2459 (2012). [DOI] [PubMed] [Google Scholar]
- Shah P., Hall R., Catmur C. & Bird G. Alexithymia, not autism, is associated with impaired interoception. Cortex. 10.1016/j.cortex.2016.03.021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg D. et al. Alexithymia and the Processing of Emotional Facial Expressions (EFEs): Systematic Review, Unanswered Questions and Further Perspectives. PLoS ONE 7, e42429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihme K. et al. Alexithymic features and the labeling of brief emotional facial expressions – An fMRI study. Neuropsychologia 64, 289–299 (2014). [DOI] [PubMed] [Google Scholar]
- Prkachin G. C., Casey C. & Prkachin K. M. Alexithymia and perception of facial expressions of emotion. Personal. Individ. Differ. 46, 412–417 (2009). [Google Scholar]
- Scarpazza C., di Pellegrino G. & Làdavas E. Emotional modulation of touch in alexithymia. Emot. Wash. DC 14, 602–610 (2014). [DOI] [PubMed] [Google Scholar]
- Jongen S. et al. An investigation of facial emotion recognition impairments in alexithymia and its neural correlates. Behav. Brain Res. 271, 129–139 (2014). [DOI] [PubMed] [Google Scholar]
- Moriguchi Y. & Komaki G. Neuroimaging studies of alexithymia: physical, affective, and social perspectives. Biopsychosoc. Med. 7, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel H. et al. Alexithymic features and automatic amygdala reactivity to facial emotion. Neurosci. Lett. 435, 40–44 (2008). [DOI] [PubMed] [Google Scholar]
- Pouga L., Berthoz S., de Gelder B. & Grèzes J. Individual differences in socioaffective skills influence the neural bases of fear processing: The case of alexithymia. Hum. Brain Mapp. 31, 1469–1481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M. et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46, 658–667 (2010). [DOI] [PubMed] [Google Scholar]
- van der Velde J. et al. Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neurosci. Biobehav. Rev. 37, 1774–1785 (2013). [DOI] [PubMed] [Google Scholar]
- Pollatos O. & Gramann K. Attenuated modulation of brain activity accompanies emotion regulation deficits in alexithymia. Psychophysiology 49, 651–658 (2012). [DOI] [PubMed] [Google Scholar]
- Swart M., Kortekaas R. & Aleman A. Dealing with Feelings: Characterization of Trait Alexithymia on Emotion Regulation Strategies and Cognitive-Emotional Processing. PLOS ONE 4, e5751 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil I. & Silani G. Reduced empathic concern leads to utilitarian moral judgments in trait alexithymia. Front. Psychol. 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally G. P. & Westbrook R. F. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learn. Mem. 13, 245–253 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. & Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 108, 483–522 (2001). [DOI] [PubMed] [Google Scholar]
- Bubic A. et al. Prediction, cognition and the brain. Front. Hum. Neurosci. 4, 25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbets P., Broek A. van den & Evers E. A. T. Fear conditioning and extinction in anxiety- and depression-prone persons. Memory 23, 350–364 (2015). [DOI] [PubMed] [Google Scholar]
- Duits P. et al. Updated Meta-Analysis of Classical Fear Conditioning in the Anxiety Disorders. Depress. Anxiety 32, 239–253 (2015). [DOI] [PubMed] [Google Scholar]
- Rothemund Y. et al. Fear conditioning in psychopaths: Event-related potentials and peripheral measures. Biol. Psychol. 90, 50–59 (2012). [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian Fear Conditioning. Annu. Rev. Neurosci. 24, 897–931 (2001). [DOI] [PubMed] [Google Scholar]
- LeDoux J. E. Coming to terms with fear. Proc. Natl. Acad. Sci. 111, 2871–2878 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A. & LeDoux J. E. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron 48, 175–187 (2005). [DOI] [PubMed] [Google Scholar]
- Li S. S. Y. & McNally G. P. The conditions that promote fear learning: Prediction error and Pavlovian fear conditioning. Neurobiol. Learn. Mem. 108, 14–21 (2014). [DOI] [PubMed] [Google Scholar]
- Tabbert K. et al. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc. Cogn. Affect. Neurosci. 6, 495–506 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. J., Bagby R. M. & Parker J. D. a. The 20-Item Toronto Alexithymia Scale: IV. Reliability and factorial validity in different languages and cultures. J. Psychosom. Res. 55, 277–283 (2003). [DOI] [PubMed] [Google Scholar]
- Garofalo S., Maier M. E. & di Pellegrino G. Mediofrontal negativity signals unexpected omission of aversive events. Sci. Rep. 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Levy I., Niv Y., LeDoux J. E. & Phelps E. A. From Fear to Safety and Back: Reversal of Fear in the Human Brain. J. Neurosci. 28, 11517–11525 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., Lushene R., Vagg P. R. & Jacobs G. A. Manual for the State-Trait Anxiety Inventory (1983). [Google Scholar]
- Seth A. K., Suzuki K. & Critchley H. D. An interoceptive predictive coding model of conscious presence. Conscious. Res. 2, 395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally G. P., Johansen J. P. & Blair H. T. Placing prediction into the fear circuit. Trends Neurosci. 34, 283–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. C., Nguyen H. T. & Bandettini P. A. The role of the human amygdala in the production of conditioned fear responses. NeuroImage 26, 1193–1200 (2005). [DOI] [PubMed] [Google Scholar]
- Olsson A. & Phelps E. A. Social learning of fear. Nat. Neurosci. 10, 1095–1102 (2007). [DOI] [PubMed] [Google Scholar]
- Pape H.-C. & Pare D. Plastic Synaptic Networks of the Amygdala for the Acquisition, Expression, and Extinction of Conditioned Fear. Physiol. Rev. 90, 419–463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde J. et al. Alexithymia influences brain activation during emotion perception but not regulation. Soc. Cogn. Affect. Neurosci. nsu056, 10.1093/scan/nsu056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D., Ahern G. L., Schwartz G. E. & Kaszniak A. W. Is Alexithymia the Emotional Equivalent of Blindsight? Biol. Psychiatry 42, 834–844 (1997). [DOI] [PubMed] [Google Scholar]
- Lane R. D., Weihs K. L., Herring A., Hishaw A. & Smith R. Affective agnosia: Expansion of the alexithymia construct and a new opportunity to integrate and extend Freud’s legacy. Neurosci. Biobehav. Rev. 55, 594–611 (2015). [DOI] [PubMed] [Google Scholar]
- Bausch S. et al. Alexithymia and script-driven emotional imagery in healthy female subjects: no support for deficiencies in imagination. Scand. J. Psychol. 52, 179–184 (2011). [DOI] [PubMed] [Google Scholar]
- Easterbrooks M. A., Chaudhuri J. H. & Gestsdottir S. Patterns of emotional availability among young mothers and their infants: A dydaic, contextual analysis. Infant Ment. Health J. 26, 309–326 (2005). [DOI] [PubMed] [Google Scholar]
- Stone L. A. & Nielson K. A. Intact Physiological Response to Arousal with Impaired Emotional Recognition in Alexithymia. Psychother. Psychosom. 70, 92–102 (2001). [DOI] [PubMed] [Google Scholar]
- Bermond B., Bierman D. J., Cladder M. A., Moormann P. P. & Vorst H. C. M. The cognitive and affective alexithymia dimensions in the regulation of sympathetic responses. Int. J. Psychophysiol. 75, 227–233 (2010). [DOI] [PubMed] [Google Scholar]
- Franz M., Schaefer R. & Schneider C. Psychophysiological Response Patterns of High and Low Alexithymics Under Mental and Emotional Load Conditions. J. Psychophysiol. 17, 203–213 (2003). [Google Scholar]
- Neumann S. A., Sollers J. J., Thayer J. F. & Waldstein S. R. Alexithymia predicts attenuated autonomic reactivity, but prolonged recovery to anger recall in young women. Int. J. Psychophysiol. 53, 183–195 (2004). [DOI] [PubMed] [Google Scholar]
- Newton T. L. & Contrada R. J. Alexithymia and repression: contrasting emotion-focused coping styles. Psychosom. Med. 56, 457–462 (1994). [DOI] [PubMed] [Google Scholar]
- Pollatos O., Schubö A., Herbert B. M., Matthias E. & Schandry R. Deficits in early emotional reactivity in alexithymia. Psychophysiology 45, 839–846 (2008). [DOI] [PubMed] [Google Scholar]
- Eastabrook J. M., Lanteigne D. M. & Hollenstein T. Decoupling between physiological, self-reported, and expressed emotional responses in alexithymia. Personal. Individ. Differ. 55, 978–982 (2013). [Google Scholar]
- Pollatos O. et al. Differential effects of alexithymia subscales on autonomic reactivity and anxiety during social stress. J. Psychosom. Res. 70, 525–533 (2011). [DOI] [PubMed] [Google Scholar]
- Lindquist K. A., Wager T. D., Kober H., Bliss-Moreau E. & Barrett L. F. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci. 35, 121–143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K. S., LeDoux J. E., Spencer D. D. & Phelps E. A. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 15, 6846–6855 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115–1118 (1995). [DOI] [PubMed] [Google Scholar]
- Làdavas E., Cimatti D., Pesce M. D. & Tuozzi G. Emotional evaluation with and without conscious stimulus identification: evidence from a split-brain patient. Cogn. Emot. 7, 95–114 (1993). [Google Scholar]
- Tamietto M. et al. Once you feel it, you see it: Insula and sensory-motor contribution to visual awareness for fearful bodies in parietal neglect. Cortex 62, 56–72 (2015). [DOI] [PubMed] [Google Scholar]
- Bertini C., Cecere R. & Làdavas E. I am blind, but I ‘see’ fear. Cortex J. Devoted Study Nerv. Syst. Behav. 49, 985–993 (2013). [DOI] [PubMed] [Google Scholar]
- Cecere R., Bertini C., Maier M. E. & Làdavas E. Unseen Fearful Faces Influence Face Encoding: Evidence from ERPs in Hemianopic Patients. J. Cogn. Neurosci. 26, 2564–2577 (2014). [DOI] [PubMed] [Google Scholar]
- Tamietto M. et al. Unseen facial and bodily expressions trigger fast emotional reactions. Proc. Natl. Acad. Sci. 106, 17661–17666 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. F., Mesquita B., Ochsner K. N. & Gross J. J. The Experience of Emotion. Annu. Rev. Psychol. 58, 373–403 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H. D., Wiens S., Rotshtein P., Öhman A. & Dolan R. J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195 (2004). [DOI] [PubMed] [Google Scholar]
- Franz M., Schaefer R., Schneider C., Sitte W. & Bachor J. Visual Event-Related Potentials in Subjects With Alexithymia: Modified Processing of Emotional Aversive Information? Am. J. Psychiatry 161, 728–735 (2004). [DOI] [PubMed] [Google Scholar]
- Li S., Zhang B., Guo Y. & Zhang J. The association between alexithymia as assessed by the 20-item Toronto Alexithymia Scale and depression: A meta-analysis. Psychiatry Res. 227, 1–9 (2015). [DOI] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J. & Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961). [DOI] [PubMed] [Google Scholar]
- Fullana M. A. et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol. Psychiatry. 10.1038/mp.2015.88 (2015). [DOI] [PubMed] [Google Scholar]