Abstract
von Willebrand factor (vWF) is a multimeric plasma glycoprotein with three tandem A domains. Domains A1 and A3 bind to platelet glycoprotein Ibα (GPIbα) and collagen, respectively. Domain A2 contains the Tyr-1605–Met-1606 bond that is cleaved by the metalloprotease ADAMTS13, and this reaction inhibits platelet thrombus growth. Fluid shear stress increases the rate of cleavage, suggesting that productive interaction with ADAMTS13 requires conformational changes within or near domain A2. The influence of the adjacent A1 and A3 domains was assessed by mutagenesis of a recombinant substrate consisting of domains A1A2A3. Deletion of domain A3 did not affect cleavage by ADAMTS13, whereas deletion of domain A1 increased the rate of cleavage ≈10-fold. Similar effects were observed with plasma ADAMTS13 and recombinant ADAMTS13 truncated after the spacer domain. Digestion of A1A2A3 by plasma ADAMTS13 was enhanced to a similar extent by a recombinant mutant fragment of platelet GPIbα that binds with high affinity to domain A1 or by heparin. Heparin also increased the digestion of purified plasma vWF. Neither GPIbα nor heparin increased the cleavage of substrate A2A3 that lacks domain A1. The results suggest that vWF domain A1 inhibits the cleavage of domain A2, and that inhibition can be relieved by interaction of domain A1 with platelet GPIbα or certain glycosaminoglycans. Thus, binding of vWF to its major physiological ligands may promote the feedback inhibition of platelet adhesion by stimulating the cleavage of domain A2 by ADAMTS13 independent of fluid shear stress.
The von Willebrand factor (vWF) is a multimeric plasma glycoprotein that plays an essential role in tethering platelets to the injured vessel wall. This process is mediated by interactions between domain A1 of vWF subunits and glycoprotein Ibα (GPIbα) on the platelet surface, and the largest vWF multimers support platelet adhesion most efficiently (1). vWF is secreted into the blood by endothelial cells as unusually large multimers (2, 3) with especially high affinity for GPIbα (4). Unusually large multimers are converted into a series of smaller multimers by the metalloprotease ADAMTS13 (5), a member of the “a disintegrin and metalloprotease with thrombospondin” repeats family of proteases (6–10). ADAMTS13 cleaves the Tyr-1605–Met-1606 bond in the central domain, A2, of vWF (11, 12) and thereby regulates vWF-dependent platelet adhesion. Mutations in vWF that increase its susceptibility to cleavage by ADAMTS13 cause a congenital bleeding disorder, von Willebrand disease type 2A (13–15). Conversely, congenital or acquired deficiency of ADAMTS13 causes life-threatening disseminated microvascular thrombosis or thrombotic thrombocytopenic purpura (TTP) (16–18), which indicates the biological importance of this proteolytic reaction.
The cleavage of vWF by ADAMTS13 in vitro is stimulated by high fluid shear stress or by mild denaturation of vWF with urea or guanidine hydrochloride, suggesting that conformational changes in vWF are necessary to expose the domain A2 cleavage site or ADAMTS13-binding sites (11, 12). Besides facilitating the cleavage of vWF by ADAMTS13, fluid shear stress also promotes vWF binding to platelets (19). Binding sites on vWF for platelet GPIb and collagen are in domains A1 and A3, respectively, flanking the ADAMTS13 cleavage site in domain A2. The tandem arrangement of these functionally important sites suggests that domain A1 or A3 might limit access of ADAMTS13 to domain A2.
To assess the role of the vWF A1 and A3 domains in substrate recognition by ADAMTS13, we characterized recombinant proteins based on the A1A2A3 segment of vWF. The results indicate that domain A1 inhibits the cleavage of the adjacent domain, A2, and that platelet GPIbα can act as a cofactor to relieve this inhibition. Therefore, the binding of platelets to vWF may regulate proteolysis by ADAMTS13.
Methods
Baculovirus Constructs. A cDNA fragment encoding vWF domains A1A2A3 was amplified by PCR by using pSVHvWF1 (20), primer 1 (5′-caccatggagcagaagctgatctccgaggaggacctggaggacatctcggaaccgccgttgcac-3′), and primer 2 (5′-gtgatggtgagcggccgccactccagagcacagtttgtggag-3′). A 117-bp fragment encoding a His6 tag and an Avi tag for biotinylation (Avidity, Denver) was amplified from a plasmid encoding GPIbα (1–289) with a His6 tag and Avi tag as the template, primer 3 (5′-ctccacaaactgtgctctggagtggcggccgctcaccatcac-3′, complement of primer 2), and primer 4 (5′-ttattcgtgccattcgattttctgagcctcgaag-3′). The two products were combined and cloned into the directional TOPO cloning vector pENTR/D-TOPO (Gateway cloning technology, Invitrogen) to generate an entry clone according to the manufacturer's instructions. The insert encodes a c-myc epitope (MEQKLISEEDL; the epitope is underlined), vWF amino acid residues Glu-1260–Gly-1874 (domains A1A2A3), and the amino acid sequence VAAAHHHHHHKLPAGGGLNDIFEAQKIEWHE (His-tag and Avi-tag sequences are underlined). Similar constructs lacking domain A1 (Glu-1260–Gly-1479) and/or domain A3 (Glu-1673–Gly-1874), or with the mutation R1597Q in domain A2, were made by a PCR-based method using the restriction endonuclease DpnI (Stratagene) (21) and primer 5 (5′-gctgatctccgaggaggacctgccggggctcttgggggtttcgac-3′), primer 6 (5′-gctgcagaggtgctgctccggagtggcggccgctcaccatcacc-3′), or primer 7 (5′-gtcagccagggtgaccaggagcaggcgcccaac-3′), respectively. The vWF signal peptide (Met-1–Cys-22) was amplified from pSVHvWF1 with primer 8 (5′-ctttaagaaggagcccttcaccatgattcctgccagatttgccg-3′) and primer 9 (5′-cctcggagatcagcttctgctcacaaagggtccctggcaaaatg-3′) and inserted into each clone by PCR (22). The DNA sequence of clones was confirmed, and bacmid expression vectors were made by recombination with pDEST8 and LR clonase (Invitrogen). Baculovirus stocks were made and amplified in Spodoptera frugiperda Sf9 cells.
Recombinant Proteins. Sf9 cells were cultured in 6-liter bottles at 27°C with shaking in 750 ml of protein-free Sf-900 II medium (Invitrogen) to a density of 2 × 106 per ml. Virus stocks were added at a multiplicity of infection of 5, and cultures were incubated for 96 h. Media were dialyzed against PBS and incubated overnight with 5 ml of cobalt beads (Taylon, Clontech). Proteins were eluted with a gradient of 0–0.5 M imidazole in PBS. Fractions were concentrated by ultrafiltration and desalted by chromatography on a column of P-10 (Bio-Rad). Avi-tagged proteins were biotinylated with Bir-A biotinylase (Avidity) and purified by adsorption onto monomeric avidinagarose (Pierce) and elution with 2 mM biotin in PBS. Products were dialyzed against 20 mM Tris·HCl, pH 7.4/150 mM NaCl.
ADAMTS13 mutants with C-terminal truncations after the metalloprotease domain (del6) or the spacer domain (del2) were expressed in Sf9 cells as reported (23). Recombinant GPIbα with the point mutations G233V and M239V and recombinant vWF A1 domain were expressed in High Five cells and Escherichia coli BL21 (DE3), respectively, and were purified as reported (24).
Purification of Anti-ADAMTS13 IgG. Plasma was obtained from healthy volunteers and from a patient with autoimmune TTP, with informed consent under a protocol approved by the Institutional Review Board of Washington University. IgG was purified from 0.3-ml plasma samples by adsorption onto 15 μlof protein A Sepharose 4 Fast Flow (Amersham Pharmacia) and elution with 100 μl of 0.1 M glycine-HCl, pH 3.0. The eluate was neutralized immediately with 3.5 μl of 1 M Tris·HCl, pH 8.0.
ADAMTS13 Assays. For assays with plasma ADAMTS13, reactions contained a final concentration of 10% (vol/vol) plasma, 0.3 μM substrate, 5 mM Tris·HCl (pH 8.0), 2.5 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefabloc SC, Sigma-Aldrich), and 10 mM BaCl2, with or without 1.0 M urea, and were incubated at 37°C. Control reactions contained 10 mM EDTA or 6.25% (vol/vol) IgG purified from normal plasma or TTP plasma. ADAMTS13 mutants del6 and del2 were assayed similarly, by using 25% (vol/vol) of conditioned medium as the enzyme source. To investigate the effect of A1-ligand binding, reactions were supplemented with 3 μM GPIbα(G233V/M239V), 0.1 mg/ml monoclonal anti-GPIbα antibody 6D1 (25), 3 or 30 μg/ml heparin (porcine intestine, 150 units/mg, Sigma), 5 μg/ml botrocetin, or 0.2 mg/ml heat-treated BSA.
Reactions were mixed with three volumes of sample buffer (62.5 mM Tris·HCl, pH 6.8/15 mM EDTA/25% glycerol/2% SDS/0.01% bromophenol blue) and analyzed by SDS/PAGE and Western blotting with horseradish peroxidase (HRP)-conjugated monoclonal anti-c-myc antibody (Invitrogen) or HRP-conjugated NeutrAvidin (Pierce) and the chemiluminescent ECL detection system (Amersham Pharmacia).
In some assays, purified plasma vWF (0.1 mg/ml) was treated with 50 mM Hepes-Na (pH 7.4), 150 mM NaCl, and 1.23 M guanidine-HCl and diluted to 2 μg/ml in 50 mM Hepes-Na (pH 7.4), 150 mM NaCl, 0.25 mM ZnCl2, 5 mM CaCl2, various concentrations of heparin, EDTA, and 0.1 unit/ml plasma ADAMTS13. After 1 h at 37°C, reactions were stopped with sample buffer and analyzed by SDS/PAGE and Western blotting with HRP-conjugated polyclonal anti-vWF (DAKO) (26).
Densitometric analysis of Western blots was performed by using nih image 1.62 (which can be accessed at http://rsb.info.nih.gov/nih-image). The fraction of substrate digested was calculated according to the formula fraction = product/(product plus undigested).
Results
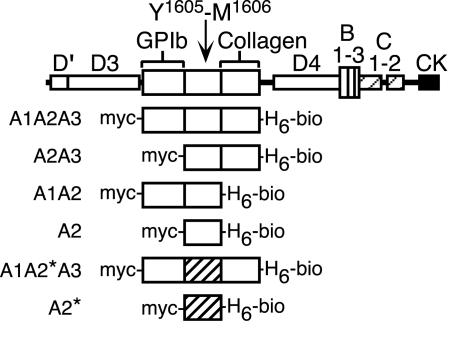
ADAMTS13 Substrates. Six variants of human vWF were designed to evaluate the influence of domains A1 and A3 on the susceptibility of the adjacent domain, A2, to digestion by ADAMTS13 (Fig. 1). To facilitate the detection of products, a c-myc tag was added to the N terminus, and a His6 tag and Avi tag were appended to the C terminus. The Avi-tag sequence can be biotinylated enzymatically in vitro, which allows purification of the product by avidin affinity chromatography and sensitive detection of substrate fragments by Western blotting. In the absence of high fluid shear stress, the native vWF domain A2 sequence requires mild denaturation for efficient cleavage by ADAMTS13 (11, 12). To avoid the use of denaturants, the R1597Q mutation was introduced into the A2 domain of two constructs. This mutation causes von Willebrand disease type 2A and enables vWF to be digested by ADAMTS13 without denaturants or fluid shear stress (15).
Fig. 1.
Structure of vWF and recombinant ADAMTS13 substrates. The vWF subunit has five kinds of structural domains including A–D, and cystine knot (CK). Domain A1 binds platelet GPIbα, and domain A3 binds collagen. Domain A2 contains the Tyr-1605–Met-1606 bond cleaved by ADAMTS13. Truncation mutants were constructed as indicated with an N-terminal c-myc tag (myc-) and a C-terminal His6 tag (H6) and Avi tag biotinylation site (-bio). Two constructs contain a mutant A2* domain (hatched) with the substitution R1597Q.
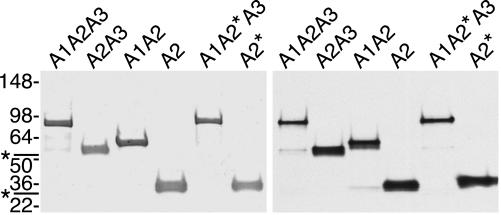
All recombinant vWF substrates were monomeric when analyzed by SDS/PAGE under nonreducing conditions, excluding the possibility of disulfide-mediated oligomerization or aggregation. Under reducing conditions, they have an apparent molecular mass ≈6–17 kDa greater than their calculated mass (Fig. 2), due at least partly to glycosylation. The A1A2A3 segment of human plasma vWF (27) is O-glycosylated on Ser-1263, Thr-1468, Thr-1477, Ser-1486, Thr-1487, and Thr-1679 and N-glycosylated on Asn-1515 and Asn-1574. Digestion of the recombinant vWF proteins with N-glycanase increased their mobility upon SDS/PAGE, suggesting that Sf9 cells also use at least the N-glycosylation sites. Additional digestion with sialidase, β(1→4)galactosidase, β-N-acetylglucosaminidase, and O-glycanase increased the mobility of substrate A1A2A3 only slightly, suggesting that Sf9 cells either use few of the O-glycosylation sites or modify them with oligosaccharides that are resistant to glycosidase digestion (data not shown). Substrates A1A2*A3 and A2* that contain the R1597Q mutation have slightly slower mobility on SDS/PAGE than the corresponding substrates containing a wild-type A2 domain (Fig. 2). The reason for this difference is unknown. A2 and A2* expressed in E. coli are indistinguishable upon SDS/PAGE, suggesting that when expressed in Sf9 cells the two sequences may acquire different posttranslational modifications. If so, the difference is not removed by digestion with N-glycanase and O-glycanase (data not shown).
Fig. 2.
Purified recombinant ADAMTS13 substrates. Proteins were analyzed by SDS/PAGE on 4–15% gradient gels and stained with Coomassie blue (Left) or visualized by Western blotting with NeutrAvidin-HRP (Right). Substrates containing the A1 domain (A1A2A3, A1A2, and A1A2*A3) contain a minor species that is marked by an asterisk (at left). Positions of standards (kDa) are shown at left. In each case the apparent molecular mass (A1A2A3, 86.7 kDa; A1A2, 61.2 kDa; A2A3, 57.0 kDa; A2, 32.5 kDa; A1A2*A3, 89.7 kDa; and A2*, 36.5 kDa) is slightly larger than the molecular mass calculated from amino acid sequence (A1A2A3, 72.5 kDa; A1A2, 51.1 kDa; A2A3, 47.6 kDa; A2, 26.2 kDa; A1A2*A3, 72.5 kDa; and A2*, 26.2 kDa).
Substrates containing the A1 domain (e.g., A1A2A3, A1A2*A3, and A1A2) contain minor species that are barely visible in Fig. 2 and are marked with an asterisk in all figures. These minor species seem to reflect a small amount of proteolysis between domains A1 and A2 during the biosynthesis or purification of the proteins.
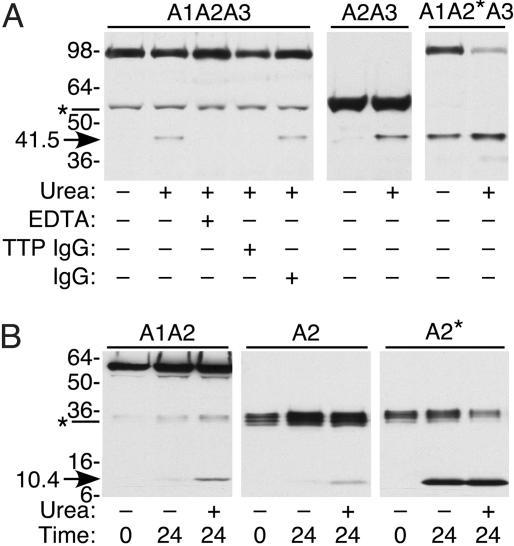
Cleavage of Substrates by Plasma and Recombinant ADAMTS13. Recombinant substrates were incubated with normal plasma ADAMTS13, and products were visualized by SDS/PAGE and Western blotting with avidin, which binds to the biotinylated C-terminal Avi tag of each protein. After overnight incubation, substrate A1A2A3 was cleaved to a small extent, but only in the presence of urea. Cleavage was inhibited by EDTA or by anti-ADAMTS13 IgG from a patient with TTP, confirming that the product was produced specifically by ADAMTS13 activity (Fig. 3A). The cleavage of substrates A1A2, A2A3, A1A2*A3, A2, and A2* also was inhibited by EDTA or by anti-ADAMTS13 IgG (data not shown).
Fig. 3.
Cleavage by plasma ADAMTS13. Substrates were incubated with normal plasma, and C-terminal products were detected by Western blotting with NeutrAvidin-HRP. (A) Substrates that contain domain A3. Substrate A1A2A3 was incubated with plasma ADAMTS13 overnight. No 41.5-kDa product (arrow) was detected in the absence of urea (lane 1 versus lane 2). Digestion was inhibited by EDTA (lane 3) or by IgG from a patient with autoimmune TTP (lane 4), but not by IgG from normal plasma (lane 5). Both A2A3 and A1A2*A3 were cleaved more efficiently with than without urea. (B) Substrates that lack domain A3. Substrates A1A2 and A2 were cleaved more efficiently with than without urea, generating a 10.4-kDa product (arrow). Substrate A2* was cleaved well in the absence of urea. Asterisks at left mark minor species in substrate A1A2A3 (A) or A1A2 (B) generated by proteolysis during protein expression or purification.
In the absence of urea, all substrates containing a wild-type A2 domain (A1A2A3 and A2A3 in Fig. 3A; A1A2 and A2 in Fig. 3B) were resistant to ADAMTS13, whereas substrates A1A2*A3 and A2* were cleaved readily (Fig. 3). These results are consistent with the finding that recombinant full-length vWF R1597Q is degraded rapidly by plasma ADAMTS13, without a need for fluid shear stress or denaturant (15).
Similar substrates with a wild-type A2 domain (e.g., A1A2A3 or A2) or a mutant A2* domain (e.g., A1A2*A3 or A2*) gave C-terminal digestion products with indistinguishable electrophoretic mobility (Fig. 3). Therefore, the small difference in apparent mass between A1A2A3 and A1A2*A3, or between A2 and A2*, may be due to differences in posttranslational modification on the N-terminal side of the Tyr-1605–Met-1606 bond.
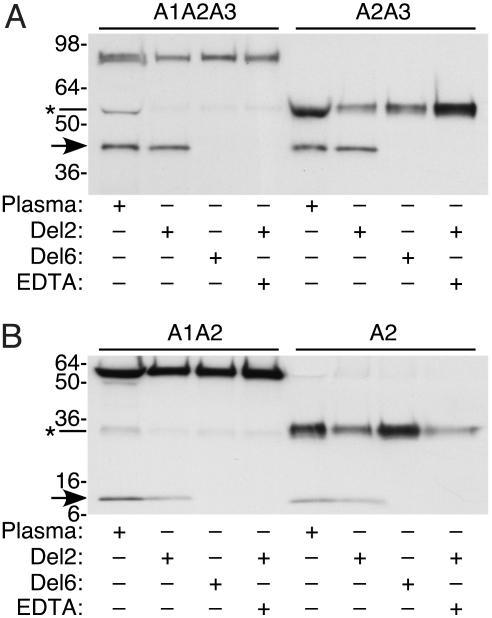
Mature ADAMTS13 consists of a metalloprotease domain, a disintegrin domain, a thrombospondin-1 repeat, a cysteinerich and spacer domain, seven more thrombospondin-1 repeats, and two CUB domains (named for domains shared by complement components Clr/s, urinary epidermal growth factor, and bone morphogenetic protein-1). In a previous study, ADAMTS13 truncated after the spacer domain (del2) and full-length ADAMTS13 had comparable activity toward plasma vWF, whereas ADAMTS13 truncated after the metalloprotease domain (del6) was inactive (23). Similar results were obtained when these truncated proteases were assayed for activity toward recombinant vWF fragments (Fig. 4). ADAMTS13 del2 cleaved all substrates into products indistinguishable from those produced by plasma ADAMTS13, whereas del6 did not cleave any substrates. These results suggest that structures C-terminal to the spacer domain are not required for ADAMTS13 to cleave either intact vWF or the isolated A2 domain under the conditions tested in vitro.
Fig. 4.
Cleavage by recombinant ADAMTS13. Substrates were incubated for 24 h with 1 M urea and plasma ADAMTS13, truncated recombinant ADAMTS13 del2 or del6, or del2 plus EDTA. C-terminal products (arrows) were detected by Western blotting with NeutrAvidin-HRP. Asterisks mark minor species in substrates A1A2A3 and A1A2 that lack the A1 domain and were generated by proteolysis during the expression or purification of these substrates. ADAMTS13 del6 cleaved none of the substrates, whereas ADAMTS13 del2 cleaved all substrates and EDTA inhibited the reaction. Products made by plasma ADAMTS13 and recombinant ADAMTS13 del2 had indistinguishable electrophoretic mobility.
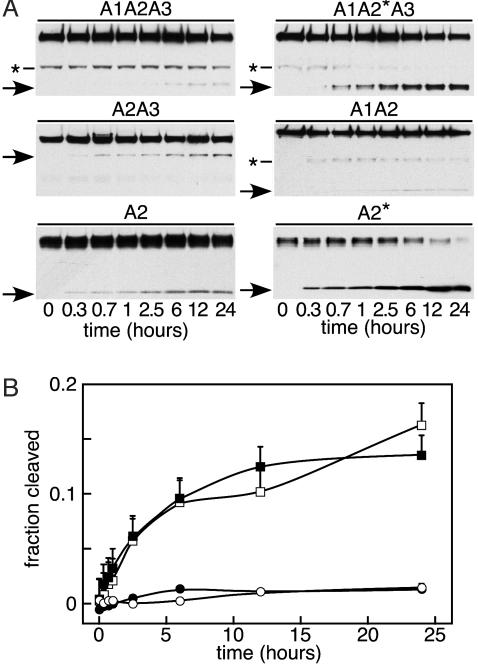
Time Course of Substrate Cleavage. The rate of cleavage was decreased by the presence of a wild-type A2 domain and also by the presence of the A1 domain (Fig. 5A). For example, substrates A1A2*A3 and A2* were cleaved more rapidly than substrates A1A2A3 and A2, respectively. Also, substrates A1A2A3 and A1A2 were cleaved slowly compared with substrates A2A3 and A2 (Fig. 5A), and densitometric analysis supported this interpretation (Fig. 5B). Substrates without domain A1 (A2A3 and A2) were digested ≈10-fold more rapidly and more completely than substrates containing domain A1 (A1A2A3 and A1A2), but the presence of domain A3 did not influence the rate or extent of reaction. Furthermore, addition of 7.8 μM free domain A1 did not inhibit the cleavage of substrate A2A3 or A2 (data not shown), suggesting that domain A1 inhibits the reaction by protecting either the ADAMTS13 cleavage site or possibly a distinct ADAMTS13-binding site on an adjacent A2 domain.
Fig. 5.
Time course of substrate cleavage. (A) Substrates (0.3 μM) were incubated with plasma ADAMTS13 and 1 M urea for the indicated times. The C-terminal cleavage products (arrows) were detected by Western blotting with NeutrAvidin-HRP. Asterisks at left mark minor species in substrates A1A2A3, A1A2*A3, and A1A2 that lack the A1 domain. (B) Densitometric analysis of substrate cleavage by plasma ADAMTS13. The fraction of substrate cleaved was determined by densitometry as described in Methods. Results are shown for substrates A1A2A3 (○), A1A2 (•), A2A3 (□), and A2 (▪). Bars represent the SE for three independent experiments.
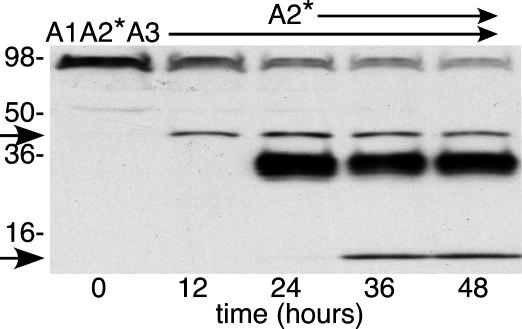
After ≈12 h, many reactions seemed to reach a plateau (Fig. 5), which is consistent with instability of ADAMTS13 or with heterogeneity of the substrate in susceptibility to proteolysis. Enzyme instability was excluded by the finding that plasma ADAMTS13 is active for >24 h at 37°C in the presence of 1 M urea (Fig. 6). Similar results were obtained for plasma ADAMTS13 in the absence of urea and for ADAMTS13 del2 with or without urea. Substrates with a mutant A2* domain could be cleaved completely, even in the absence of urea (data not shown). Therefore, the limited cleavage of other substrates in the presence of urea may reflect structural heterogeneity caused by variable denaturation of wild-type domain A2.
Fig. 6.
Stability of ADAMTS13. Plasma ADAMTS13 was incubated at 37°C for 48 h with 1 M urea and substrate A1A2*A3; at 24 h, substrate A2* was added. Products were analyzed by Western blotting with NeutrAvidin-HRP at 12-h intervals. The 40.5-kDa species (arrow) is the product of A1A2*A3 cleavage; the 10.5-kDa species (arrow) is the product of A2* cleavage.
Cofactor Activity of GPIbα and Heparin. The vWF A1 domain inhibited cleavage of the adjacent A2 domain (Fig. 5B), suggesting that the reaction might be influenced by ligands that bind the A1 domain including platelet GPIbα, heparin, and botrocetin. Ristocetin also binds within or near the vWF A1 domain and might influence cleavage of the A2 domain; however, ristocetin causes vWF to precipitate (28), which effectively prevents the analysis of product generation by ADAMTS13 (data not shown).
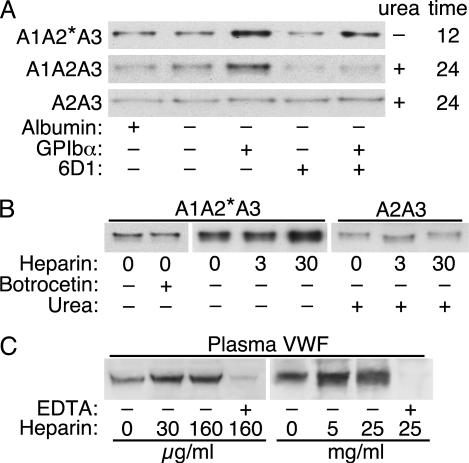
The effect of GPIbα binding was assessed with a recombinant fragment of GPIbα containing two mutations that cause platelet-type pseudo-von Willebrand disease, G233V and M239V (29, 30). These mutations increase the affinity of GPIbα for the vWF A1 domain so that binding occurs with a Kd of ≈600 nM (24), without the need for a cofactor such as ristocetin (31, 32). GPIbα(G233V/M239V) stimulated the cleavage of substrates A1A2*A3 (without urea) and A1A2A3 (with urea) (Fig. 7A). The stimulation by GPIbα(G233V/M239V) was blocked partially by a substoichiometric concentration of anti-GPIbα antibody 6D1, which inhibits A1-GPIbα binding (25). In contrast, GPIbα(G233V/M239V) did not stimulate the digestion of substrate A2A3, which lacks the GPIbα-binding site located on domain A1. GPIbα also increased the digestion of A1A2A3 by truncated ADAMTS13 del2 (data not shown). These results suggest that GPIbα-A1 binding enhances the digestion of vWF by ADAMTS13.
Fig. 7.
Effect of GPIbα(G233V/M239V), heparin, and botrocetin on substrate cleavage by ADAMTS13. (A) Substrates were incubated with normal plasma ADAMTS13 in the presence or absence of 0.2 mg/ml BSA, 3 μM GPIbα(G233V/M239V), 0.1 mg/ml monoclonal anti-GPIbα antibody 6D1, or 1 M urea, as indicated. The 40.5-kDa C-terminal cleavage products were detected by Western blotting with NeutrAvidin-HRP. (B) Substrate A1A2*A3 was incubated with normal plasma ADAMTS13 and the indicated concentrations (μg/ml) of heparin, 5 μg/ml botrocetin, or 1 M urea. (C) Plasma vWF was incubated with plasma ADAMTS13 and heparin, in the presence or absence of 10 mM EDTA. The 340-kDa product (26) is shown.
Botrocetin is a snake venom protein that binds vWF domain A1 adjacent to the GPIbα-binding site, and botrocetin promotes vWF-platelet binding by forming a ternary complex of botrocetin, vWF A1 domain, and GPIbα (33). Botrocetin did not stimulate cleavage of substrate A1A2*A3 (Fig. 7B), indicating that only certain ligands of domain A1 can modulate the activity of ADAMTS13. Botrocetin also did not potentiate or inhibit the increased cleavage observed in the presence of GPIbα (data not shown).
Heparin is a negatively charged glycosaminoglycan that binds to an electropositive face of the vWF A1 domain with an EC50 of ≈100 μg/ml (34). Addition of 3 μg/ml heparin had a minimal effect, but addition of 30 μg/ml heparin substantially enhanced the digestion of substrate A1A2*A3 (Fig. 7B). Heparin did not affect the digestion of substrate A2A3, suggesting that heparin enhances the cleavage of substrate A1A2*A3 by binding to domain A1. Heparin also enhanced the digestion of purified multimeric plasma vWF (Fig. 7C).
Discussion
Fluid shear stress or mild denaturation increases the susceptibility of vWF to digestion by ADAMTS13, suggesting that stretching of vWF causes conformational changes that expose the cleavage site or another ADAMTS13-binding site, or both (11, 12, 15). Modeling of the A2 domain suggests that the ADAMTS13-sensitive Tyr-1605–Met-1606 bond lies buried within the core β-sheet of the native structure (35), which is consistent with a need for protein unfolding before cleavage. Also, the von Willebrand disease type 2A mutation R1597Q within the A2 domain increases the rate of vWF cleavage by ADAMTS13, probably by interfering with the normal folding of the domain (15). However, the determinants of vWF recognition need not be limited to exposure of the cleavage site, and some studies of recombinant vWF domain A2 suggest a role for multiple interactions between vWF and ADAMTS13 (36). When expressed in E. coli, the refolded A2 domain was resistant to ADAMTS13 in the absence of denaturants, as is full-length, multimeric vWF. A smaller vWF73 construct that included only Asp-1596 to Arg-1668 was cleaved rapidly, but removal of another 9 amino acid residues from the C-terminal end of vWF73 made it completely resistant again (36). Therefore, structures missing from vWF73 normally inhibit access to the Tyr-1605–Met-1606 bond, and the segment Glu-1660–Arg-1668 may contribute to an auxiliary ADAMTS13-binding site.
Most studies have focused on domain A2, but the adjacent A1 and A3 domains also might participate in vWF recognition by ADAMTS13. Domain A1 binds platelet GPIbα and heparin, and domain A3 binds collagen; the proximity of these binding sites to domain A2 suggests that their ligands could regulate ADAMTS13 activity, independent of fluid shear stress. In fact, vWF domain A3 recently was proposed to serve as a docking site for ADAMTS13 on vWF (5), although the binding affinity and significance for vWF cleavage were not determined.
To investigate the influence of domains A1 and A3 on substrate cleavage, a series of recombinant vWF domain A2 constructs with or without the adjacent A1 and A3 domains (Fig. 1). The presence of domain A1 markedly decreased the rate and extent of substrate cleavage, whereas the presence of domain A3 had no significant effect (Fig. 5), and the addition of excess free domain A1 did not inhibit the cleavage of substrate A2A3 or A2. As expected (15), substrates with the mutation R1597Q in the A2 domain (A1A2*A3 and A2*) were cleaved much more rapidly than substrates with a wild-type A2 domain, but an inhibitory effect of domain A1 was still apparent. The results suggest that features of domain A1 inhibit substrate cleavage by ADAMTS13, perhaps by stabilizing the native conformation of domain A2 or by impairing the productive binding of ADAMTS13.
Although these experiments did not demonstrate a contribution of domain A3 to vWF cleavage by ADAMTS13 (5), the conditions used may not be optimal for this purpose. For example, ligands that bind to domain A3 such as collagen might regulate ADAMTS13 activity toward an adjacent A2 domain.
The inhibition of substrate cleavage by domain A1 was apparent even in the presence of urea (Fig. 5), suggesting that, in addition to the unfolding of domain A2, optimal digestion by ADAMTS13 might require a regulatory interaction specific to domain A1 such as ligand binding. Deficiency of ADAMTS13 causes TTP (16–18), which suggests that intravascular plateletvWF aggregates are important ADAMTS13 substrates in vivo, but a direct role of GPIbα-A1 binding in regulating ADAMTS13 activity has not been assessed. Recombinant GPIbα(G233V/M239V) binds vWF domain A1 with relatively high affinity (24), and when it was added to reactions the digestion of vWF substrates containing domain A1 was enhanced, whereas digestion of substrate A2A3 lacking domain A1 was unaffected (Fig. 7). Furthermore, GPIbα-A1 binding facilitated the digestion of substrate A1A2A3 in the absence of urea, suggesting that the GPIbα-A1 interaction renders vWF susceptible to ADAMTS13 in the absence of conformational changes that could be induced by fluid shear stress.
This conclusion is consistent with the properties of vWF produced by cultured endothelial cells. When treated with histamine, endothelial cells secrete unusually large vWF species that form long fibers or “strings,” which remain bound to the cell surface (37). vWF strings have markedly increased affinity for platelet GPIbα (4), and they bind platelets tightly. When exposed to ADAMTS13, vWF strings are digested, and the platelets are released within seconds, even at very low fluid shear rates (37). The products of ADAMTS13 cleavage are smaller multimers typical of normal plasma that no longer bind spontaneously to platelets. The results of Fig. 7 indicate that the binding of a small N-terminal fragment of GPIbα to vWF domain A1 is sufficient to promote digestion of the adjacent domain A2 by ADAMTS13, and this mechanism may be important for reducing the size of potentially thrombogenic vWF strings secreted by endothelial cells in vivo, especially in the low shear environment of the venous circulation. The same process may contribute to the markedly increased proteolysis of vWF subunits observed in von Willebrand disease type 2B (38), a variant characterized by the spontaneous binding of plasma vWF multimers to platelet GPIbα (39).
Like platelet GPIbα, heparin and botrocetin also bind to vWF domain A1, but heparin stimulates the cleavage of substrate A1A2*A3, whereas botrocetin does not (Fig. 7B). A comparison of the binding sites for these ligands suggests an explanation for their different effects on substrate recognition by ADAMTS13. A strongly electronegative surface of GPIbα binds to a large electropositive surface on the side of vWF domain A1 (40), which could shield it from the nearby electronegative surface of vWF domain A2 (35). GPIbα-A1 binding might facilitate recognition by ADAMTS13 by contributing a new binding site on GPIbα, by exposing a cryptic binding site on domain A2, or by reducing unfavorable electrostatic interactions. Mutagenesis data indicate that heparin binds to domain A1 across the same positively charged surface that is covered by GPIbα (34), and therefore heparin or other glycosaminoglycans could plausibly have a similar effect on ADAMTS13 activity. In contrast, botrocetin binds a distinct positively charged surface at the top of domain A1 (33) so that the GPIbα-binding site of domain A1 might still interact functionally with ADAMTS13.
Further study will be required to determine how vWF domain A1 and its ligands influence ADAMTS13 activity. Nevertheless, these observations suggest an answer to the paradox that cleavage of vWF in vitro does not seem to depend on the C-terminal seven thrombospondin-1 repeats and two CUB domains of ADAMTS13 (23), even though these domains are conserved among fish, amphibians, birds, and mammals (ref. 41 and unpublished results). A ternary complex of vWF, ADAMTS13, and GPIbα may constitute a physiological assembly of substrate, enzyme, and cofactor, perhaps facilitated by high fluid shear stress. The interactions within such a complex could require the participation of C-terminal domains of ADAMTS13, which would not be apparent under typical assay conditions in vitro that exclude platelets. The function of these domains may only become apparent in the presence of platelet membrane glycoproteins. In addition, extracellular matrix or endothelial cells might contribute to the regulation of ADAMTS13 activity in vivo.
Acknowledgments
We thank Elodee A. Tuley for extensive technical assistance and Agnès Veyradier (Washington University School of Medicine) for critical reading of the manuscript, and Barry Coller (The Rockefeller University, New York) for antibody 6D1. X.L.Z. was supported in part by the Transfusion Medicine Fellowship program, Department of Pathology and Immunology, Barnes-Jewish Hospital, Washington University School of Medicine.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GPIbα, glycoprotein Ibα; HRP, horseradish peroxidase; TTP, thrombotic thrombocytopenic purpura; vWF, von Willebrand factor.
References
- 1.Sporn, L. A., Marder, V. J. & Wagner, D. D. (1987) Blood 69, 1531–1534. [PubMed] [Google Scholar]
- 2.Moake, J. L., Rudy, C. K., Troll, J. H., Weinstein, M. J., Colannino, N. M., Azocar, J., Seder, R. H., Hong, S. L. & Deykin, D. (1982) N. Engl. J. Med. 307, 1432–1435. [DOI] [PubMed] [Google Scholar]
- 3.Tsai, H. M., Nagel, R. L., Hatcher, V. B. & Sussman, I. I. (1989) Blood 73, 2074–2076. [PubMed] [Google Scholar]
- 4.Arya, M., Anvari, B., Romo, G. M., Cruz, M. A., Dong, J. F., McIntire, L. V., Moake, J. L. & Lopez, J. A. (2002) Blood 99, 3971–3977. [DOI] [PubMed] [Google Scholar]
- 5.Dong, J. F., Moake, J. L., Bernardo, A., Fujikawa, K., Ball, C., Nolasco, L., Lopez, J. A. & Cruz, M. A. (2003) J. Biol. Chem. 278, 29633–29639. [DOI] [PubMed] [Google Scholar]
- 6.Hurskainen, T. L., Hirohata, S., Seldin, M. F. & Apte, S. S. (1999) J. Biol. Chem. 274, 25555–25563. [DOI] [PubMed] [Google Scholar]
- 7.Levy, G. G., Nichols, W. C., Lian, E. C., Foroud, T., McClintick, J. N., McGee, B. M., Yang, A. Y., Siemieniak, D. R., Stark, K. R., Gruppo, R., et al. (2001) Nature 413, 488–494. [DOI] [PubMed] [Google Scholar]
- 8.Fujikawa, K., Suzuki, H., McMullen, B. & Chung, D. (2001) Blood 98, 1662–1666. [DOI] [PubMed] [Google Scholar]
- 9.Zheng, X., Chung, D., Takayama, T. K., Majerus, E. M., Sadler, J. E. & Fujikawa, K. (2001) J. Biol. Chem. 276, 41059–41063. [DOI] [PubMed] [Google Scholar]
- 10.Soejima, K., Mimura, N., Hirashima, M., Maeda, H., Hamamoto, T., Nakagaki, T. & Nozaki, C. (2001) J. Biochem. 130, 475–480. [DOI] [PubMed] [Google Scholar]
- 11.Tsai, H.-M. (1996) Blood 87, 4235–4244. [PubMed] [Google Scholar]
- 12.Furlan, M., Robles, R. & Lämmle, B. (1996) Blood 87, 4223–4234. [PubMed] [Google Scholar]
- 13.Dent, J. A., Berkowitz, S. D., Ware, J., Kasper, C. K. & Ruggeri, Z. M. (1990) Proc. Natl. Acad. Sci. USA 87, 6306–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons, S. E., Bruck, M. E., Bowie, E. J. W. & Ginsburg, D. (1992) J. Biol. Chem. 267, 4424–4430. [PubMed] [Google Scholar]
- 15.Tsai, H. M., Sussman, I. I., Ginsburg, D., Lankhof, H., Sixma, J. J. & Nagel, R. L. (1997) Blood 89, 1954–1962. [PubMed] [Google Scholar]
- 16.Furlan, M., Robles, R., Solenthaler, M., Wassmer, M., Sandoz, P. & Lammle, B. (1997) Blood 89, 3097–3103. [PubMed] [Google Scholar]
- 17.Furlan, M., Robles, R., Galbusera, M., Remuzzi, G., Kyrle, P. A., Brenner, B., Krause, M., Scharrer, I., Aumann, V., Mittler, U., et al. (1998) N. Engl. J. Med. 339, 1578–1584. [DOI] [PubMed] [Google Scholar]
- 18.Tsai, H. M. & Lian, E. C. (1998) N. Engl. J. Med. 339, 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri, Z. M. (1997) J. Clin. Invest. 99, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita, T. & Sadler, J. E. (1995) J. Biol. Chem. 270, 13406–13414. [DOI] [PubMed] [Google Scholar]
- 21.Makarova, O., Kamberov, E. & Margolis, B. (2000) BioTechniques 29, 970–972. [DOI] [PubMed] [Google Scholar]
- 22.Geiser, M., Cebe, R., Drewello, D. & Schmitz, R. (2001) BioTechniques 31, 88–90, 92. [DOI] [PubMed] [Google Scholar]
- 23.Zheng, X., Nishio, K., Majerus, E. M. & Sadler, J. E. (2003) J. Biol. Chem. 278, 30136–30141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura, S., Li, C. Q., Cao, Z., Wang, H., Wardell, M. R. & Sadler, J. E. (2000) J. Biol. Chem. 275, 7539–7546. [DOI] [PubMed] [Google Scholar]
- 25.Coller, B. S., Peerschke, E. I., Scudder, L. E. & Sullivan, C. A. (1983) Blood 61, 99–110. [PubMed] [Google Scholar]
- 26.Majerus, E. M., Zheng, X., Tuley, E. A. & Sadler, J. E. (2003) J. Biol. Chem. 278, 46643–46648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Titani, K., Kumar, S., Takio, K., Ericsson, L. H., Wade, R. D., Ashida, K., Walsh, K. A., Chopek, M. W., Sadler, J. E. & Fujikawa, K. (1986) Biochemistry 25, 3171–3184. [DOI] [PubMed] [Google Scholar]
- 28.Scott, J. P., Montgomery, R. R. & Retzinger, G. S. (1991) J. Biol. Chem. 266, 8149–8155. [PubMed] [Google Scholar]
- 29.Miller, J. L., Cunningham, D., Lyle, V. A. & Finch, C. N. (1991) Proc. Natl. Acad. Sci. USA 88, 4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell, S. D. & Roth, G. J. (1993) Blood 81, 1787–1791. [PubMed] [Google Scholar]
- 31.Miller, J. L. & Castella, A. (1982) Blood 60, 790–794. [PubMed] [Google Scholar]
- 32.Weiss, H. J., Meyer, D., Rabinowitz, R., Pietu, G., Girma, J. P., Vicic, W. J. & Rogers, J. (1982) N. Engl. J. Med. 306, 326–333. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda, K., Doggett, T. A., Bankston, L. A., Cruz, M. A., Diacovo, T. G. & Liddington, R. C. (2002) Structure (London) 10, 943–950. [DOI] [PubMed] [Google Scholar]
- 34.Rastegar-Lari, G., Villoutreix, B. O., Ribba, A. S., Legendre, P., Meyer, D. & Baruch, D. (2002) Biochemistry 41, 6668–6678. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins, P. V., Pasi, K. J. & Perkins, S. J. (1998) Blood 91, 2032–2044. [PubMed] [Google Scholar]
- 36.Kokame, K., Matsumoto, M., Fujimura, Y. & Miyata, T. (2004) Blood 103, 607–612. [DOI] [PubMed] [Google Scholar]
- 37.Dong, J. F., Moake, J. L., Nolasco, L., Bernardo, A., Arceneaux, W., Shrimpton, C. N., Schade, A. J., McIntire, L. V., Fujikawa, K. & López, J. A. (2002) Blood 100, 4033–4039. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman, T. S., Dent, J. A., Ruggeri, Z. M. & Nannini, L. H. (1986) J. Clin. Invest. 77, 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggeri, Z. M., Pareti, F. I., Mannucci, P. M., Ciavarella, N. & Zimmerman, T. S. (1980) N. Engl. J. Med. 302, 1047–1051. [DOI] [PubMed] [Google Scholar]
- 40.Huizinga, E. G., Tsuji, S., Romijn, R. A. P., Schiphorst, M. E., de Groot, P. G., Sixma, J. J. & Gros, P. (2002) Science 297, 1176–1179. [DOI] [PubMed] [Google Scholar]
- 41.Sadler, J. E. (2002) Proc. Natl. Acad. Sci. USA 99, 11552–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]