Abstract
Background
Oxidative stress plays an important role in the development of atrial fibrillation (AF). Arginine derivatives including asymmetric dimethylarginine (ADMA) are central to nitric oxide metabolism and nitrosative stress. Whether blood concentrations of arginine derivatives are related to incidence of AF is uncertain.
Methods and Results
In 3310 individuals (mean age 58±10 years, 54% women) from the community-based Framingham Study we prospectively examined the relations of circulating levels of ADMA, L-arginine, symmetric dimethylarginine (SDMA), and the ratio of L-arginine/ADMA to incidence of AF using proportional hazards regression models. Over a median follow-up time of 10 years 247 AF cases occurred.
Using age- and sex-adjusted regression models, ADMA was associated with a hazard ratio of 1.15 per one standard deviation increase in loge-biomarker concentration (95% confidence interval 1.02 to 1.29, P=0.02) for AF, which was no longer significant after further risk factor adjustment (hazard ratio 1.09, 95% confidence interval 0.97 to 1.23, P=0.15). Neither L-arginine nor symmetric dimethylarginine was related to new-onset AF. A clinical model comprising clinical risk factors for AF (for age, sex, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, diabetes, hypertension treatment, myocardial infarction, and heart failure) (C-statistic: 0.781; 95% confidence interval, 0.753 to 0.808) was not improved by the addition of ADMA (0.782; 95% confidence interval, 0.755 to 0.809).
Conclusions
ADMA and related arginine derivatives were not associated with incident AF in the community after accounting for other clinical risk factors and confounders. Its role in the pathogenesis of AF needs further refinement.
Keywords: atrial fibrillation, ADMA, arginine derivatives, epidemiology, cohort, risk assessment
Graphical Abstract
The increasing prevalence of atrial fibrillation (AF) in the general population has resulted in substantial costs and public health impact.1–3 The risk of developing AF is incompletely understood;4–6 however, AF has been associated with elevated measures of oxidative stress.7
Oxidative stress is involved at different stages of the pathogenesis of left atrial injury, electrophysiological remodeling, and the development and perpetuation of AF.8–10 Nitric oxide (NO) is central to cardiovascular homeostasis and counteracts oxidative damage. Its bioavailability relies on the efficient generation from its precursor L-arginine by NO synthases. Asymmetric dimethylarginine (ADMA) is a product of irreversible posttranslational protein modification. After complete proteolysis, free ADMA is a competitive inhibitor of NO synthesis.11 ADMA’s congener symmetric dimethylarginine (SDMA) competes for cellular L-arginine uptake, fosters endothelial NO synthases uncoupling and increases superoxide anion production in response to vascular endothelial growth factor.12;13 ADMA has been associated with cardiovascular events in community-based and clinical samples.10;14 Furthermore, circulating levels of ADMA and L-arginine have been related to atrial and ventricular structure and function, and increased ADMA concentrations to heart failure.15;16 Circulating concentrations of ADMA are increased in experimental atrial tachyarrhythmias,17 severe postoperative arrhythmias18 and in AF.19;20
Despite limited electrophysiological evidence on the role of ADMA and arginine derivatives in the pathophysiology of AF,17;21 we hypothesized that endogenous L-arginine and its derivatives ADMA, SDMA, and L-arginine/ADMA ratio are related with long-term incidence of AF in a community-based sample.
Materials and Methods
Study sample
The present investigation was performed in the Framingham Offspring cohort, which was enrolled in the early 1970s with regular follow-up every four to eight years (N=5124).22 Participants (n=3532) who attended the sixth examination cycle (1995–1998) were eligible for analysis. For the present study attendees were excluded based on missing ADMA or L-arginine measurements (n=35), offsite visits (n=43), loss to follow-up (n=6), prevalent AF (n=112), serum creatinine greater than 2 mg/dL (n=17), or missing clinical covariates (n=9). Boston University Medical Center Institutional Review Board approved the study protocols and participants provided informed consent. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Clinical evaluations
The Framingham Heart Study cardiovascular health assessments include cardiac risk factor documentation during a physician-administered interview and physical examination. The participants reported their smoking habits and medications. Systolic and diastolic blood pressure measurements were obtained by a Heart Study physician. The average of two measurements obtained on the seated participant was used for analysis. The definition of diabetes mellitus comprised an elevated fasting blood glucose ≥126 mg/dL or the use of diabetes medication. Heart failure and myocardial infarction were diagnosed by the endpoint adjudication committee based on clinical and patient record data.23;24
Atrial fibrillation verification
On a routine base, the participants’ medical records were collected during follow-up. Participants were systematically asked if a physician had diagnosed AF in the health history update questionnaire. The final diagnosis of AF was considered present if documented by atrial fibrillation or atrial flutter on ECG tracings and information from hospital or outpatient records or Framingham Study clinic examinations. Incident AF cases were adjudicated by two Framingham cardiologists.4 We used events collected until December 31, 2012.
Biomarker determination
Fasting blood samples were obtained routinely, processed immediately and stored at -80°C. Measurements of the plasma arginine derivatives ADMA, L-arginine, and SDMA was performed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with high quality and validity of biomarker determination as described previously.25 C-reactive protein (high sensitivity CRP, Dade Behring assay) and B-type natriuretic peptide (BNP, ShionoRIA, Shionogi Inc, Osaka, Japan) were measured by routine methods as reported earlier.26 Mean inter-assay coefficients of variation were ≤5% for arginine derivatives and a maximum of 12.2% for BNP. The validity of determination in specimens stored for several years has been shown previously.14
Statistical Analyses
For analyses, arginine derivatives were natural logarithmically transformed and standardized (mean of 0 and standard deviation of 1). Kaplan Meier survival curves were drawn for arginine derivatives categorized into quartiles. In multivariable-adjusted proportional hazards regression models ADMA, L-arginine, and SDMA were related to incident AF.27 The proportional hazards assumption was confirmed using a Kolmogorov-type supremum test.28 The non-linear association of biomarkers with incident AF was assessed by including restricted cubic splines of biomarker with 3 knots at 5th, 50th, and 95th percentiles into the Cox regression model. The model was also adjusted on clinical covariates and other biomarkers. SAS macro %RCT_Reg was used for fitting the model.29 Multivariable models were adjusted for AF risk factors that have been reported consistently in association with incident AF:4;5;30;31 age, sex, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, diabetes, hypertension treatment, myocardial infarction, and heart failure. Since natriuretic peptides and CRP have consistently been related to AF, additional models included BNP and CRP.32 Renal function is related to both arginine derivative concentrations and incident AF.11;33 Therefore, we estimated glomerular filtration rate using the formula suggested by Chronic Kidney Disease Epidemiology Collaboration34 and included it as a covariate and comparator besides clinical variables.
For biomarker selection we applied a stepwise procedure to select arginine derivatives associated with AF using a conservative two-sided significance threshold of P<0.01 for entry and retention in the model.35 We forced in age, sex, and clinical covariates. We present regression coefficients per standard deviation (SD) increase in loge-transformed arginine derivatives. Further, we assessed χ2 values and C-statistics to describe discrimination of the arginine derivatives separately in addition to the baseline model including the clinical variables (age, sex, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, diabetes, hypertension treatment, myocardial infarction, and HF).36
We performed secondary analyses excluding individuals with diabetes because of previously recognized associations of arginine derivatives and diabetes.14
Analyses were conducted using SAS version 9.3 (Cary, North Carolina, http://www.sas.com/presscenter/guidelines.html). We assumed a two-sided P<0.05 as statistically significant.
This work was supported by NIH/NHLBI contract HHSN268201500001I, N01-HC-25195 (RSV), and NIH grants 2R01HL092577 and R01HL128914 (EJB, PTE); HL064753. HL076784, AG028321 (EJB), 1 RO1HL71039 (RSV); NIH Research career award K24 HL04334 (RSV) K24HL105780, (PTE), K24 DK080140 (JBM); and K23HL114724 (SAL); Established Investigator Award from the American Heart Association 13EIA14220013 (PTE) and by the Fondation Leducq 14CVD01 (PTE); a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 (SAL); an American Diabetes Association Career Development Award (JBM), Deutsche Forschungsgemeinschaft (German Research Foundation) Research Fellowship SCHN 1149/3-1, Federal Ministry of Education and Research 01ZX1408A (RBS). Marie Curie Intra-European Fellowship for Career Development 623127(DA). This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 648131) (RBS). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Participant Characteristics
The mean age of the total sample was 58±10 years, and 54% were women. Data on 3310 participants were available for analyses with a median follow-up time of 10 years, of whom 247 developed new-onset AF. Baseline characteristics by AF status are provided in Table 1, characteristics by ADMA quartiles are shown in Supplementary Table 1. Individuals who developed AF were older on average and more frequently men. The classical risk factor burden was higher in participants with AF. Diabetes was observed in 20.6% in participants with incident AF whereas in non-AF individuals only 8.5% had a diagnosis of diabetes. Median (25th/75th percentile) ADMA concentrations were 0.57 (0.48, 0.65) micromol/L in individuals who developed AF and 0.53 (0.46, 0.61) micromol/L in participants without AF.
Table 1.
Clinical and biomarker characteristics by AF status at follow-up
| Clinical Characteristics | Incident AF (N=247) | Non-AF (N=3063) |
|---|---|---|
| Age, years | 66±9 | 58±9 |
| Women, No (%) | 101 (41) | 1682 (55) |
| Height, cm | 168±10 | 167±9 |
| Weight, kg | 81.9±18.6 | 78.3±16.9 |
| Current smoking, No (%) | 41 (16.6) | 470 (15.3) |
| Systolic blood pressure, mm Hg | 136±21 | 128±18 |
| Diastolic blood pressure, mm Hg | 74±11 | 76±9 |
| Hypertension treatment, No (%) | 129 (52.2) | 774 (25.3) |
| Diabetes, No (%) | 51 (20.6) | 260 (8.5) |
| History of myocardial infarction, No (%) | 26 (10.5) | 90 (2.9) |
| History of heart Failure, No (%) | 7 (2.8) | 11 (0.4) |
| Biomarkers | ||
| ADMA (micromol/L) | 0.57 (0.48, 0.65) | 0.53 (0.46, 0.61) |
| L-arginine (micromol/L) | 80 (68, 92) | 76 (65, 90) |
| SDMA (micromol/L) | 0.4 (0.34, 0.48) | 0.38 (0.33, 0.45) |
| L-arginine/ADMA ratio | 143 (115, 172) | 145 (121, 173) |
| B-type natriuretic peptide (pg/mL) | 22 (8.3, 46) | 7.6 (4, 16.5) |
| C-reactive protein (mg/L) | 2.86 (1.29, 6.4) | 1.97 (0.9, 4.55) |
| Glomerular filtration rate (mL/min/1.73m2) | 82 (69, 93) | 85 (73, 100) |
Binary variables are expressed as n (%). Continuous variables are expressed as mean±SD or as median [25th, 75th percentile] for biomarkers.
ADMA stands for asymmetric dimethylarginine; SDMA, symmetric dimethylarginine.
Arginine derivatives and AF incidence
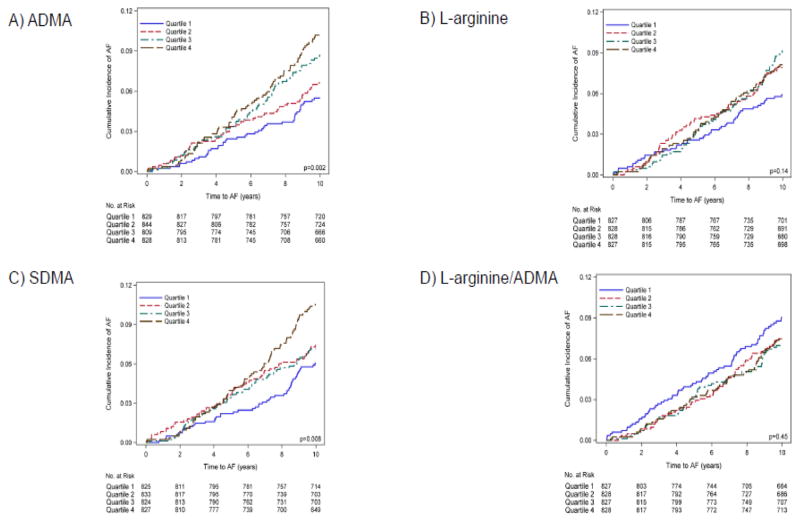
Figure 1 shows unadjusted cumulative AF incidence curves for quartiles of arginine derivative concentrations. Increasing ADMA and SDMA concentrations were related to incident AF with highest risk in the upper quartile. Neither L-arginine nor L-arginine/ADMA ratio showed a clear pattern of association with the outcome. The p values for non-linear association on all biomarkers were not statistically significant, ranging from 0.17 to 0.82.
Figure 1.
Cumulative atrial fibrillation (AF) incidence curves by quartile of arginine derivatives for A) ADMA, B) L-arginine, C) SDMA, and D) L-arginine/ADMA ratio. ADMA stands for asymmetric dimethylarginine, SDMA for symmetric dimethylarginine.
In age- and sex-adjusted Cox regression analyses loge-ADMA was related to new-onset AF (hazard ratio per standard deviation (SD) increment, 1.15; 95% confidence interval, 1.02 to 1.29, P=0.02) (Table 2). After adjustment for other clinical variables, ADMA was not significantly associated with AF (P=0.15). In multivariable stepwise regression analysis that included arginine derivatives, BNP, CRP, and estimated glomerular filtration rate in parallel with clinical risk factors, only BNP was associated with incident AF (hazard ratio per SD increment in loge BNP after model selection, 1.22; 95% confidence interval, 1.14 to 1.29; P<0.0001) (Table 3).
Table 2.
Age- and sex-adjusted (upper row) and additionally risk factor-adjusted (lower row) proportional hazards regression models for atrial fibrillation examining each biomarker separately.
| Variable | Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|
| ADMA | 1.15 | 1.02 | 1.29 | 0.02 |
| 1.09 | 0.97 | 1.23 | 0.15 | |
|
| ||||
| L-arginine | 1.10 | 0.97 | 1.24 | 0.12 |
| 1.10 | 0.97 | 1.24 | 0.13 | |
|
| ||||
| SDMA | 1.02 | 0.90 | 1.15 | 0.76 |
| 1.00 | 0.89 | 1.13 | 0.95 | |
|
| ||||
| L-arginine/ADMA ratio | 0.97 | 0.85 | 1.11 | 0.71 |
| 1.01 | 0.88 | 1.16 | 0.85 | |
Hazard ratios are provided per one standard deviation increase in loge-biomarker concentration. Models are adjusted for age, sex, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, diabetes, hypertension treatment, myocardial infarction, and heart failure.
ADMA denotes asymmetric dimethylarginine; SDMA, symmetric dimethylarginine.
Table 3.
Multivariable-adjusted Cox proportional hazards models for arginine derivatives associated with incident AF on the left and result from stepwise selection on the right side
| Variable | Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| ADMA | 1.10 | 0.79 | 1.52 | 0.58 |
| L-arginine | 1.06 | 0.73 | 1.55 | 0.76 |
| SDMA | 0.96 | 0.83 | 1.11 | 0.56 |
| L-arginine/ADMA | 1.02 | 0.65 | 1.59 | 0.94 |
| B-type natriuretic peptide | 1.23 | 1.15 | 1.31 | <0.0001 |
| C-reactive protein | 1.06 | 0.96 | 1.18 | 0.24 |
| eGFR | 1.12 | 0.98 | 1.28 | 0.10 |
Hazard ratios are per one standard deviation increase in loge-biomarker concentration. Models included all biomarkers simultaneously and adjusted for age, sex, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, diabetes, hypertension treatment, myocardial infarction, and heart failure simultaneously.
CI denotes confidence interval; eGFR denotes estimated glomerular filtration rate.
ADMA, L-arginine, SDMA, or the ratio of L-arginine/ADMA did not significantly improve the C statistic of the model comprising clinical variables (Supplementary Table 2). The lowest χ2 statistic was observed for the model containing ADMA, (χ2=5.892; P=0.75).
In pre-specified secondary analyses, we excluded 311 (9.4%) individuals with diabetes mellitus at baseline (Supplementary Table 3). Results of the regression analyses remained similar to the data in the total cohort. ADMA showed only borderline significance with incident AF in the risk factor-adjusted model (HR per SD increment in loge ADMA, 1.13; 95% CI, 0.99, 1.29; P, 0.08). The stepwise selection procedure again revealed BNP as the only biomarker significantly related to new-onset AF (Supplementary Table 4).
Discussion
In a prospective, community-based cohort with a mean follow up of ten years, we observed a weak association of ADMA with new-onset AF in age- and sex-adjusted models, but the relation was no longer statistically significant after adjustment for standard clinical AF risk factors. Neither L-arginine nor any of the other examined arginine derivatives were significantly associated with AF incidence.
Evidence exists to support the hypothesis that ADMA plays a role in the pathophysiology of AF. Experimental data show that manifest AF produces an environment of increased oxidative stress and NO consumption.37;38 Elevated blood concentrations of ADMA in AF indicate endothelial dysfunction and a prothrombotic milieu39 that may account for adverse events in AF.40 After restoration of sinus rhythm, circulating ADMA concentrations decrease,17;41 and higher concentrations predict the recurrence of AF after ablation.41;42
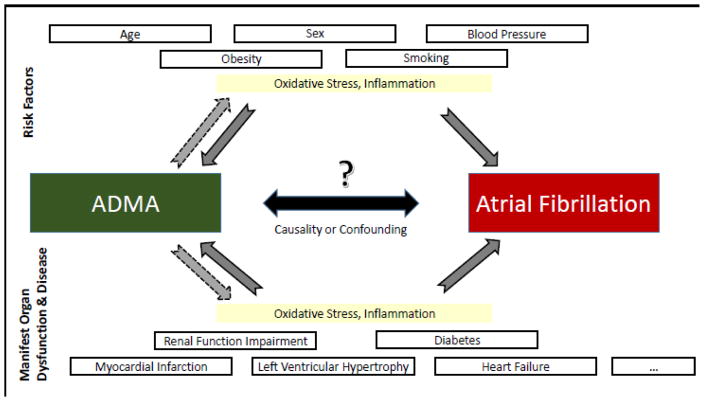
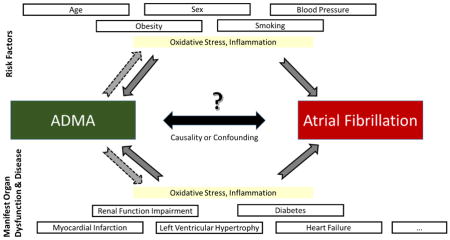
In our study, the association of higher ADMA concentrations with new-onset AF was not observed after adjustment for known AF risk factors. Thus, the risk mirrored by circulating ADMA appears to be mediated/explained by classical cardiovascular risk factors in our sample. Most risk markers related to cardiovascular disease and AF have been correlated with increased circulating ADMA concentrations (Figure 2) and an unfavorable imbalance of arginine derivatives.14 ADMA and L-arginine have been associated with age, sex, body mass index, smoking, blood pressure, diabetes, and renal impairment.11;43;44 In contrast to other, mostly smaller clinical samples that reported statistically significant associations of ADMA and AF phenotypes,17;40;45 we applied rigorous adjustment for clinical risk factors that have been correlated with ADMA.
Figure 2.
Risk factors, predisposing conditions and diseases that have been related to atrial fibrillation (AF)11;14–16 and elevated circulating ADMA concentrations. Whether such covariates confound the association of ADMA with AF is uncertain. ADMA stands for asymmetric dimethylarginine.
Whether ADMA exerts AF-specific mechanisms or is a mere bystander correlated with cardiovascular risk factors remains to be elucidated. Some evidence exists that the pathophysiology of ADMA may be more specific for AF than other supraventricular arrhythmias. In a small study of AF patients, ADMA concentrations were increased more than in other supraventricular tachycardias.46 We speculate on potential pathophysiological mechanisms that may explain the relation of clinical risk factors, ADMA, and AF risk (Figure 2). Another example that may link the pathophysiology of ADMA and AF is a study in healthy individuals that reported acute infusion of ADMA increased arterial stiffness.47 An increased burden of cardiovascular disease risk factors may increase ADMA concentrations and thus may result in higher vascular stiffness, which in turn may increase risk for new-onset AF.48
Arginine derivatives have been shown to be correlated with prevalent diabetes in prior data from the Framingham Heart Study, and effect modification was observed by diabetes status with differential association with mortality in individuals without diabetes only.49 Therefore, we performed pre-specified secondary analyses excluding individuals with diabetes mellitus. The relation with incident AF did not change substantively. In risk factor adjusted models, ADMA reached borderline significance with incident AF, but BNP remained the only biomarker selected into the final model.
Strengths and Limitations
Small associations that could help to understand pathophysiological conditions underlying ADMA and the other arginine derivatives in relation to AF may have been missed in the analyses we present due to the modest number of cases of AF and focus on circulating biomarker concentrations.
However, we had good statistical power to show moderate effect sizes that we assumed to have potential clinical relevance, e.g., we had 80% power to detect an association with incident AF for a biomarker with a hazard ratio of 1.16 per standard deviation change, at alpha=0.05. In addition, we may have missed AF cases that were not captured by our careful follow-up due to the often intermittent and unrecognized nature of the disease. Furthermore, a single biomarker measurement at baseline may not account for intra-individual variability and changes over time that may more closely reflect the risk of developing AF. We applied LC-MS/MS for the biomarker measurements, regarded as the gold standard.25 In addition, the Framingham sample is mostly comprised of white individuals of European ancestry, and associations may vary according to race/ethnic groups.50 Further, it needs to be considered that circulating biomarker concentrations may not adequately reflect atrial pathophysiology and atrial endothelial dysfunction and oxidative stress at the tissue level. Similar to myeloperoxidase another marker of oxidative stress, whose atrial tissue concentrations are strongly related to AF51 but no significant association with systemic measurements was observed,52;53 circulating arginine derivatives may not adequately mirror the local burden and its long-term changes in relation to AF. Since ADMA has repeatedly been demonstrated to be related to ventricular and atrial arrhythmias17;18 and AF19;20 a lack of predictive ability for incident AF does not preclude a significant role of ADMA and arginine derivatives in pathophysiological causative pathways.
We conclude that circulating ADMA is not strongly associated with new-onset AF, and hence is not suited as a biomarker for risk prediction of AF. L-arginine and SDMA, though pathophysiologically and from clinical observations plausible, are not predictive of long-term incidence of AF in a community-based cohort. Their role at the electrophysiological and tissue level needs to be further elucidated.
Supplementary Material
Acknowledgments
We are grateful to all Framingham Offspring participants, medical records and data management staff who enabled this work.
Abbreviations
- ADMA
asymmetric dimethylarginine
- AF
atrial fibrillation
- BNP
B-type natriuretic peptide
- CI
Confidence Interval
- CRP
C-reactive protein, CRP
- HR
Hazard ratio
- NO
nitric oxide
- SD
standard deviation
- SDMA
symmetric dimethylarginine
Footnotes
Disclosures
Dr. Ellinor is the PI on a grant from Bayer HealthCare to the Broad Institute related to AF genetics and therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–7. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 3.Bennell MC, Qiu F, Micieli A, Ko DT, Dorian P, Atzema CL, et al. Identifying predictors of cumulative healthcare costs in incident atrial fibrillation: a population-based study. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.114.001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially and Geographically Diverse Population: the CHARGE-AF Consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–51. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leftheriotis DI, Fountoulaki KT, Flevari PG, Parissis JT, Panou FK, Andreadou IT, et al. The predictive value of inflammatory and oxidative markers following the successful cardioversion of persistent lone atrial fibrillation. Int J Cardiol. 2009;135:361–9. doi: 10.1016/j.ijcard.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Nishijima Y, Sridhar A, Bonilla I, Velayutham M, Khan M, Terentyeva R, et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res. 2011;91:71–9. doi: 10.1093/cvr/cvr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e53–e59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 11.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 12.Feliers D, Lee DY, Gorin Y, Kasinath BS. Symmetric dimethylarginine alters endothelial nitric oxide activity in glomerular endothelial cells. Cell Signal. 2015;27:1–5. doi: 10.1016/j.cellsig.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 14.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieb W, Benndorf RA, Benjamin EJ, Sullivan LM, Maas R, Xanthakis V, et al. Plasma asymmetric dimethylarginine, L-arginine and left ventricular structure and function in a community-based sample. Atherosclerosis. 2009;204:282–7. doi: 10.1016/j.atherosclerosis.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–30. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 17.Goette A, Hammwohner M, Bukowska A, Scalera F, Martens-Lobenhoffer J, Dobrev D, et al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int J Cardiol. 2012;154:141–6. doi: 10.1016/j.ijcard.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Maas R, Dentz L, Schwedhelm E, Thoms W, Kuss O, Hiltmeyer N, et al. Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine predict adverse events in patients undergoing noncardiac surgery. Crit Care Med. 2007;35:1876–81. doi: 10.1097/01.CCM.0000277038.11630.71. [DOI] [PubMed] [Google Scholar]
- 19.Lim HS, Willoughby SR, Schultz C, Gan C, Alasady M, Lau DH, et al. Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol. 2013;61:852–60. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Stamboul K, Lorin J, Lorgis L, Guenancia C, Beer JC, Touzery C, et al. Atrial Fibrillation Is Associated with a Marker of Endothelial Function and Oxidative Stress in Patients with Acute Myocardial Infarction. PLoS ONE. 2015;10:e0131439. doi: 10.1371/journal.pone.0131439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Qu X, Liang Z, Chen W, Xia W, Song Y. Variance of DDAH/PRMT/ADMA pathway in atrial fibrillation dogs. Biochem Biophys Res Commun. 2008;377:884–8. doi: 10.1016/j.bbrc.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 22.Dawber T, Meadors G, Moore F., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: an epidemiological investigation of cardiovascular disease. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 24.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 25.Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Boger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:211–9. doi: 10.1016/j.jchromb.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR, Oakes D. Analysis of Survival Data. London, UK: Chapman and Hall; 1984. Analysis of Survival Data; p. 201. [Google Scholar]
- 28.Lin D, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 29.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 30.Shulman E, Kargoli F, Aagaard P, Hoch E, Di BL, Fisher J, et al. Validation of the Framingham Heart Study and CHARGE-AF Risk Scores for Atrial Fibrillation in Hispanics, African-Americans, and Non-Hispanic Whites. Am J Cardiol. 2016;117:76–83. doi: 10.1016/j.amjcard.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Pfister R, Bragelmann J, Michels G, Wareham NJ, Luben R, Khaw KT. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015;22:932–9. doi: 10.1177/2047487314544045. [DOI] [PubMed] [Google Scholar]
- 32.Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16:1426–33. doi: 10.1093/europace/euu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic Kidney Disease Is Associated With the Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123:2946–53. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–7. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 37.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–8. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 38.Han W, Fu S, Wei N, Xie B, Li W, Yang S, et al. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol. 2008;130:165–73. doi: 10.1016/j.ijcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Lim HS, Willoughby SR, Schultz C, Gan C, Alasady M, Lau DH, et al. Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol. 2013;61:852–60. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 40.Chao TF, Lu TM, Lin YJ, Tsao HM, Chang SL, Lo LW, et al. Plasma asymmetric dimethylarginine and adverse events in patients with atrial fibrillation referred for coronary angiogram. PLoS ONE. 2013;8:e71675. doi: 10.1371/journal.pone.0071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim HS, Willoughby SR, Schultz C, Chakrabarty A, Alasady M, Lau DH, et al. Successful catheter ablation decreases platelet activation and improves endothelial function in patients with atrial fibrillation. Heart Rhythm. 2014;11:1912–8. doi: 10.1016/j.hrthm.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Xia W, Qu X, Yu Y, Zhang X, Feng W, Song Y. Asymmetric dimethylarginine concentration and early recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin Electrophysiol. 2008;31:1036–40. doi: 10.1111/j.1540-8159.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 43.Maas R, Schulze F, Baumert J, Lowel H, Hamraz K, Schwedhelm E, et al. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clin Chem. 2007;53:693–701. doi: 10.1373/clinchem.2006.081893. [DOI] [PubMed] [Google Scholar]
- 44.Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–3. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 45.Lim HS, Willoughby SR, Schultz C, Chakrabarty A, Alasady M, Lau DH, et al. Successful catheter ablation decreases platelet activation and improves endothelial function in patients with atrial fibrillation. Heart Rhythm. 2014;11:1912–8. doi: 10.1016/j.hrthm.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Schultz CD, Rangneker G, Lim HS, Fraudeau A, Young G, Roberts-Thomson K, et al. Characterization of thrombogenic, endothelial and inflammatory markers in supraventricular tachycardia: a study in patients with structurally normal hearts. Clin Exp Pharmacol Physiol. 2014;41:551–7. doi: 10.1111/1440-1681.12256. [DOI] [PubMed] [Google Scholar]
- 47.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, et al. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–9. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, et al. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–15. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 49.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sydow K, Fortmann SP, Fair JM, Varady A, Hlatky MA, Go AS, et al. Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin Chem. 2010;56:111–20. doi: 10.1373/clinchem.2009.136200. [DOI] [PubMed] [Google Scholar]
- 51.Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–4. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–7. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnabel RB, Wild PS, Wilde S, Ojeda FM, Schulz A, Zeller T, et al. Multiple biomarkers and atrial fibrillation in the general population. PLoS ONE. 2014;9:e112486. doi: 10.1371/journal.pone.0112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.