Abstract
Background
Synthetic cathinones, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), serve as a substrate or blocker at monoaminergic transporters, respectively, and produce locomotor stimulant effects in rodents. The present study investigated in rats the effects of repeated exposure to 4-MMC, MDPV, or mixtures of the two on the induction of locomotor sensitization and expression of cross-sensitization to cocaine.
Methods
Seventy-two male Sprague-Dawley rats received daily intraperitoneal injections of saline, MDPV (0.5 mg/kg), 4-MMC (0.5, 1.0, or 2.0 mg/kg) or mixtures of 0.5 mg/kg MDPV + 4-MMC (0.5, 1.0, or 2.0 mg/kg) for seven consecutive days. Locomotor activity was recorded on days 1 and 7 and again after an acute injection of 5 mg/kg cocaine following a 10 day drug washout period.
Results
Rats injected with 0.5 mg/kg MDPV, 0.5, 1.0, or 2.0 mg/kg 4-MMC, or 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV displayed time-dependent increases in horizontal activity that were augmented on day 7 compared to day 1. In addition, rats pretreated with 0.5 mg/kg MDPV, 2.0 mg/kg 4-MMC, or mixtures of 4-MMC + MDPV displayed an enhanced response to cocaine.
Conclusions
Locomotor responses sensitize to MDPV and to certain mixtures of MDPV and 4-MMC following repeated dosing. Furthermore, previous exposure to these substances may produce cross-sensitization to the locomotor stimulant effects of cocaine. Considered together with recent findings that 4-MMC and MDPV have different sites of action, but both influence monoaminergic functioning, further investigations utilizing a variety of behavioral assays may prove informative regarding the abuse liability of synthetic cathinone mixtures.
Keywords: 4-methylmethcathinone; 4-MMC; mephedrone; 3,4-methylenedioxypyrovalerone; MDPV; synthetic cathinones; bath salts; cocaine; rats; locomotor sensitization; cross-sensitization
1. INTRODUCTION
Experimental investigations of the physiological and behavioral effects of synthetic cathinones (cathinone derivatives) have increased in direct response to their international popularity among recreational drug users at the turn of the 21st century. Since their emergence into the public domain, widespread media attention and toxicology reports have detailed numerous instances of untoward side effects and fatalities associated with the consumption of these drugs (e.g., Ross et al., 2012; Wood et al., 2010a, 2010b; Torrance and Cooper, 2010). For example, in 2011, synthetic cathinones were involved in over 20,000 emergency room visits in the United States (The DAWN Report, 2013). Amid these reports, three of the cathinone derivatives, 4-methylmethcathinone (4-MMC, mephedrone), 3,4-methylenedioxypyrovalerone (MDPV), and methylone, were placed on the Schedule I list of controlled substances on October 21, 2011, and an additional 10 derivatives were temporarily added March 7, 2014 (Drug Enforcement Agency (DEA), 2011, 2016). Despite legislative efforts devoted to criminalizing the sale, possession, and recreational use of certain synthetic cathinones, these drugs are the third most frequently identified (following synthetic cannabinoids and phenethylamines) new psychoactive substances reported to the United Nations Office on Drugs and Crime (UNODC, 2014).
Recreational users of 4-MMC and MDPV have reported their psychological effects to be similar to those of MDMA and cocaine (Winstock et al., 2010; 2011; Ross et al., 2012; Johnson and Johnson, 2014). Also similar to the amphetamines, untoward psychological effects of frequent heavy use include paranoia, hallucinations, aggressive/violent behavior, excited delirium, and psychosis (Wood et al., 2010a, 2010b; Ross et al., 2012; German et al., 2014). Psychoactive “bath salt” use is associated with increased risk-taking behaviors, such as unprotected sex, putting individuals at risk for human immunodeficiency virus and other sexually-transmitted diseases (Johnson and Johnson, 2014). Further, a majority of synthetic cathinone users have reported poly-substance abuse with cocaine, MDMA, alcohol, tobacco, and/or cannabis (for review, Prosser and Nelson 2012; Johnson and Johnson, 2014), which may further increase risks to health and safety.
Despite the prevalence of poly-substance use among recreational “bath salt” users, the majority of preclinical investigations to date have only assessed the effects of single constituents. Undoubtedly, such investigations are a necessary first step to explicating the psychopharmacology of these substances. There is now sufficient evidence regarding the neurochemical and behavioral effects of individual synthetic cathinones to warrant studying their combined effects. The current study represents the first known attempt to characterize the behavioral effects of mixtures containing MDPV with variable doses of 4-MMC. These two chemicals were selected for the current study because there is already substantial published research on the behavioral effects of each individual substance.
The popularity of 4-MMC and MDPV among users may be partly attributable to their neuropharmacological effects. Previous research has demonstrated that 4-MMC shares similarities with MDMA in its potency and selectivity at membrane monoamine transporters (Baumann et al., 2012; Kehr et al., 2011; Rickli et al., 2015). Electrophysiological studies revealed that 4-MMC produces dopamine-releasing effects at hDAT (Cameron et al., 2013a, 2013b; Rickli et al., 2015). Furthermore, MDPV produces neurochemical actions similar to cocaine (i.e., ineffective as a monoamine releaser), inhibiting dopamine transporter activity by blocking reuptake (Cameron et al., 2013a, 2013b). Therefore, consistent with the aforementioned reports of comparable psychological effects, current evidence indicates 4-MMC and MDPV possess neurochemical profiles similar to MDMA and cocaine, respectively.
In addition to efforts devoted to examining the in vitro neurochemical effects of 4-MMC and MDPV, researchers have evaluated the behavioral effects of these compounds. It is well established that repeated and intermittent exposure to certain drugs produces progressive increases in locomotor and stereotyped movements, a phenomenon termed “behavioral sensitization” (see Steketee and Kalivas, 2011). In addition, it is suggested that sensitization is mediated by neuroadaptive changes in dopaminergic mesocorticolimbic and glutamatergic pathways (Vanderschuren and Kalivas, 2000), substrates implicated in drug abuse and chemical dependencies. Recent studies have demonstrated locomotor sensitization in rats following repeated exposure to 4-MMC (Gregg et al., 2013a, 2013b; Shortall et al., 2013; Lisek et al., 2012).
When this study was initiated, there were no published reports demonstrating locomotor sensitization to MDPV. Nonetheless, previous studies have revealed dose-dependent increases in locomotor activity following injections of MDPV in mice (e.g., Fantegrossi et al., 2013; Marusich et al., 2012) and rats (e.g., Aarde et al., 2013; Baumann et al. 2013), and only one known study has assessed locomotor sensitization to a mixture of drugs that included a synthetic cathinone (4-MMC) and d-amphetamine (Berquist et al., 2015). The present study investigated in rats the induction of locomotor sensitization with concurrent exposure to 4-MMC and MDPV in comparison to each substance alone, and subsequently assessed cross-sensitization to cocaine. The results are suggestive that MDPV and certain low dose mixtures of the 4-MMC and MDPV produce locomotor sensitization and can enhance locomotor responses to cocaine.
2. MATERIALS AND METHODS
2.1 Subjects, apparatus, and drugs
Seventy-two adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were pair-housed in polycarbonate cages with corncob bedding (Harlan Teklad, Conrad, Iowa) in a temperature and humidity controlled vivarium maintained on a 12:12 hour light-dark cycle (lights on at 0700). Animals had ad libitum access to standard rodent chow (Purina® 5001, Richmond, Indiana) and deionized water in their home cages. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2013) and were approved by the Institutional Animal Care and Use Committee at Western Michigan University.
Locomotor activity was assessed in eight custom-designed, acrylic open field chambers (40.5 cm × 40.5 cm × 40.5 cm). Each chamber was housed within an Accuscan automated activity monitoring system equipped with infrared emitters and detectors connected to a microprocessor with associated Versamax® software programmed to analyze beam breaks and determine various measures of activity (Accuscan Instruments, Inc., Columbus, OH, USA).
(±)-Mephedrone-hydrochloride (4-methylmethcathinone, 4-MMC), 3,4-methylenedioxypyrovalerone-hydrochloride (MDPV), and cocaine-hydrochloride were provided by the National Institute on Drug Abuse (NIDA) drug control supply program (Bethesda, MD). All drugs were prepared in 0.9% bacteriostatic sodium chloride and delivered to rats via intraperitoneal injections in a 1 ml/kg volume. Drug mixtures were injected as a single bolus. Drug doses were calculated based on the weights of the salts.
2.2 Experimental procedures
Rats were randomly assigned to one of the following treatment groups: 0.5, 1.0, or 2.0 mg/kg 4-MMC (n=8, 8, 8, respectively), 0.5 mg/kg MDPV (n=8), 0.5, 1.0, or 2.0 mg/kg 4-MMC + 0.5 m/kg MDPV (n=8, 8, 8), or saline (n=16), with housed pairs assigned to the same treatment group. Doses were selected based on previous research demonstrating that in rats 0.5 and 1.0 mg/kg 4-MMC (Lisek et al., 2012), and 0.5 mg/kg MDPV (Aarde et al., 2013), induce increases in locomotor activity. Higher doses of MDPV were not used to avoid potential disruptive effects due to the combined stimulant actions of the drug mixtures tested. The seven-day dosing schedule employed was similar to the variable-dose paradigm used by Gregg et al. (2013a), however, in the present study, all rats received the same dose of their designated treatments on days 1 through 7, and a single dose of cocaine (or saline) after a 10 day drug washout period. All subjects were injected (i.p.) daily over seven consecutive days at approximately the same time of day. Locomotor activity was recorded on day 1 and day 7. On days 2 through 6, rats were injected and placed immediately back into home cages. Following a 10-day washout period, all drug-treated subjects (n=56) and 10 of the saline-treated subjects received cocaine (SAL-COC) (5 mg/kg), while six of the saline-treated controls received saline (SAL-SAL). Locomotor activity was recorded in a similar manner to days 1 and 7, as described below.
All testing occurred during the light phase of the light-dark cycle. Treatment groups and time of day during which locomotor activity was assessed were counterbalanced among animals. On each test day, rats were habituated to the test chambers for a 60 min period prior to injections while activity was recorded. Rats were briefly handled to receive injections and placed back into test chambers for an additional 60 min. Activity recording was turned off during injections and turned back on after all eight animals in each cohort were injected. Test chambers were cleaned with a 35% isopropyl alcohol solution between cohorts. Overhead lights were on during testing and a white noise generator (~70 dB) was used to mask any background noise.
2.3 Data Analysis
The locomotor activity measure was computed as horizontal activity counts. Horizontal counts were sequestered into five min intervals using Versamax® software (Accuscan Instruments, Inc., Columbus, OH). Infrared beam breaks on the X and Y axes were used to determine horizontal activity. Horizontal counts were plotted as treatment group means (± S.E.M.) for each 5 min time interval over each 120 min assessment period. Results from test days 1 and 7 were analyzed using separate two-factor repeated-measures analysis of variance (ANOVA) procedures for each treatment group, with time interval and treatment day as the within-subjects factors. Cumulative measures for the 60 min period following drug injections were also determined and analyzed using a two-factor split-plot ANOVA (treatment group x treatment day) with treatment day as the within-subjects factor.
Similarly, horizontal counts obtained on the COC challenge test day were plotted as treatment group means (± S.E.M.) for each 5 min time interval over the 120 min assessment period and analyzed using a two-factor split-plot ANOVA with time interval as the within-subjects factor. For all foregoing two-factor ANOVA procedures, the following decision process was used: if an omnibus ANOVA revealed a statistically significant interaction, simple main effects tests were computed using Holm-Sidak adjustments; contrariwise, if the two-factor ANOVA revealed at least one statistically significant main effect, but no interaction, group mean differences were further analyzed using Holm-Sidak multiple comparisons tests. P levels <.05 were identified as statistically significant. Statistical analysis and graphic displays were performed using GraphPad Prism Version 6.0 software (La Jolla, CA, USA).
3. RESULTS
3.1 Induction of Sensitization
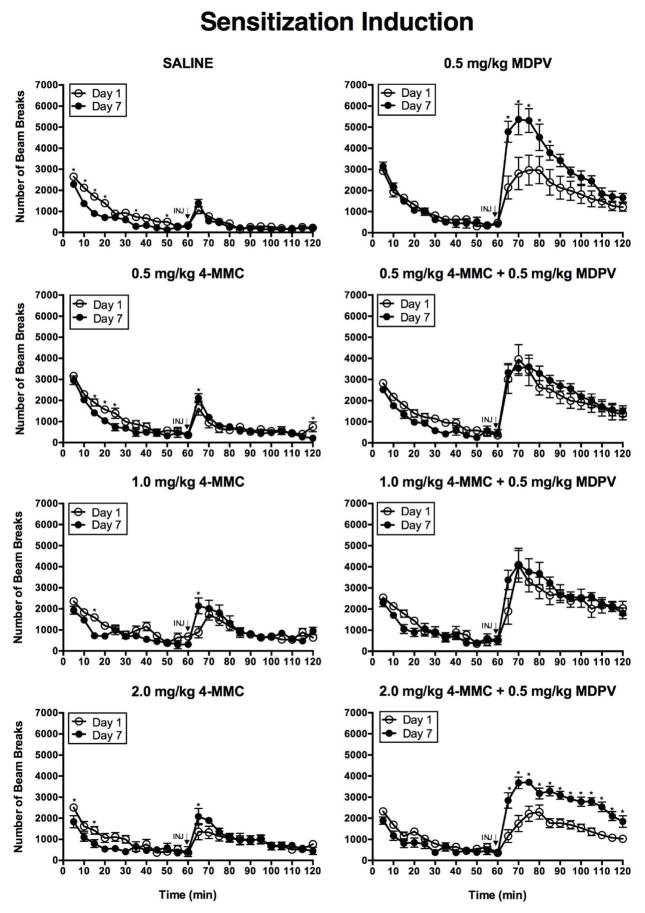
Figure 1 displays a comparison of horizontal activity on day 1 and day 7 depicted in five minute intervals over the 120 min assessment period for all treatment groups. Two-factor ANOVAs on the time course data revealed statistically significant interactions between treatment day and time interval in the saline, 0.5 mg/kg MDPV, 0.5, 1.0, or 2.0 mg/kg 4-MMC, and the 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV treatment groups (omnibus ANOVAs results not shown). Because the interaction was statistically significant in these groups, simple main effects tests were computed using Holm-Sidak adjustments. Figure 1 displays the results of the simple main effects tests comprising effects of treatment day at levels of time interval within each treatment group. In all but five treatment groups, drug-induced increases in activity were similar on day 1 and day 7. Rats injected with 0.5 mg/kg MDPV, 0.5, 1.0, or 2.0 mg/kg 4-MMC, or 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV displayed greater post-injection activity on day 7 compared to day 1 at particular time intervals, as indicated by the symbols displayed in figure 1. In animals treated with any dose of 4-MMC alone, statistically significant differences in activity between day 1 and day 7 were evident during the first 5 min post-injection interval, as well as at a few time intervals before injections. In the 0.5 mg/kg MDPV-treated animals, statistically significant differences were evident at all time intervals within the first 25 min after injection. In the animals treated with 2 mg/kg 4-MMC + 0.5 mg/kg MDPV, statistically significant differences in activity between day 1 and day 7 were observed at all post-injection time intervals.
Figure 1.
Time course of horizontal activity on test day 1 and test day 7 by treatment group. Each line graph depicts activity during the 60 min habituation period and subsequent 60 min post-injection period, with individual points representing group means (±S.E.M.) at each 5 min interval. [n=8 each drug treatment group, n=16 saline control group; INJ
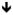 = injection; Asterisks (*) indicate significant differences (p < .05) between day 1 and day 7 horizontal beam breaks at specified time intervals.]
= injection; Asterisks (*) indicate significant differences (p < .05) between day 1 and day 7 horizontal beam breaks at specified time intervals.]
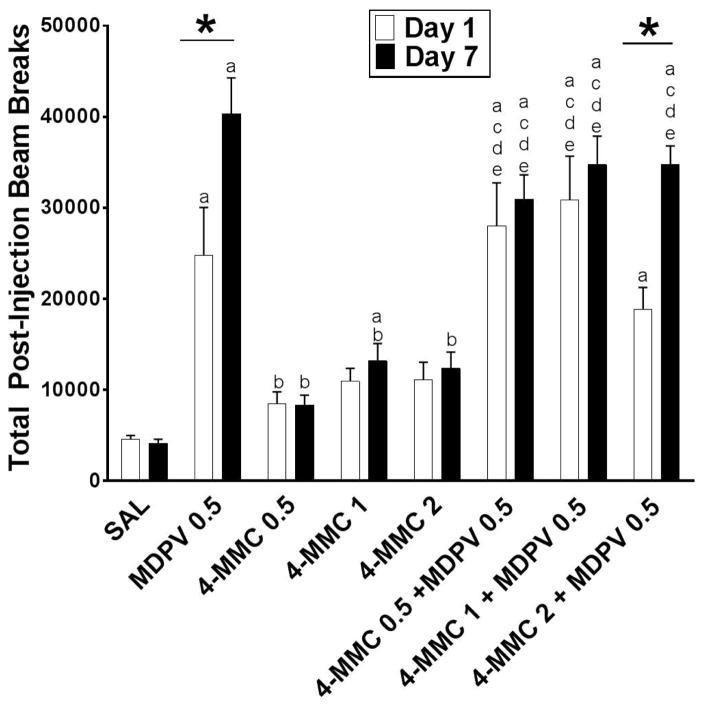
Figure 2 depicts the cumulative post-injection activity counts for the 60 min period following drug injections across treatment day and treatment groups. A two-factor split-plot ANOVA revealed statistically significant main effects of treatment day [F(1,64)=16.59, p=.0001] and of treatment group [F(7,64)=43.66, p<.0001], and an interaction between treatment day and treatment group [F(7,64)=3.58, p=.0026]. Because the interaction was statistically significant, simple main effects tests were computed using Holm-Sidak adjustments. These tests revealed that rats injected with 0.5 mg/kg MDPV or 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV displayed greater activity on day 7 compared to day 1 (indicated by asterisks in figure 2). Additionally, rats injected with 0.5 mg/kg MDPV, 1.0 mg/kg 4-MMC, or each of the drug mixtures displayed greater levels of activity on day 1 or day 7 compared to saline-treated rats (indicated by the letter a in figure 2).
Figure 2.
Cumulative horizontal activity following injections on test day 1 and test day 7 by treatment group. Each bar represents treatment group means (±S.E.M.) of the 60 min post-injection sums on day 1 (white bars) and day 7 (black bars). Asterisks (*) indicate significant within-group differences between treatment days (p < .05). Letters indicate significant between-group differences on each treatment day (a=saline, b=MDPV 0.5, c=4-MMC 0.5, d=4-MMC 1, e=4-MMC 2; p < .05)
3.2 Cross-Sensitization to Cocaine
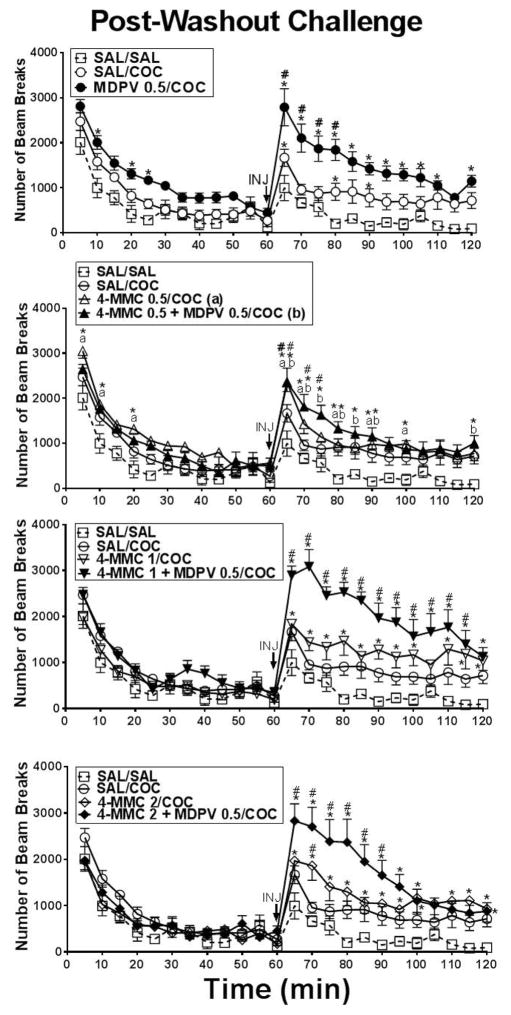
Figure 3 displays a comparison of horizontal activity on test day 17 (COC challenge) depicted in five minute intervals over the 120 min assessment period for all treatment groups. A two-factor ANOVA on these data revealed statistically significant main effects of treatment group [F(8,63)=5.76, p<.0001] and of time interval [F(23,1449)=93.10, p<.0001], and an interaction between treatment group and time interval [F(184,1449)=3.39, p<.0001]. Because the interaction was statistically significant, simple main effects tests were computed using Holm-Sidak adjustments. These tests revealed that rats previously treated with 0.5 mg/kg MDPV, 2.0 mg/kg 4-MMC, or any drug mixture displayed greater cocaine-induced activity compared to saline-saline (indicated by asterisks in figure 3) or to the saline-cocaine (indicated by pound sign in figure 3) treated rats at particular post-injection time intervals.
Figure 3.
Time course of horizontal activity plotted in 5 min intervals during the 60 min habituation period and 60 min immediately following 5 mg/kg cocaine or saline challenge after a 10 day washout. Individual points represent group means (±S.E.M.) at each 5 min interval. The SAL/SAL group (n=6) received a saline injection. The SAL/COC (n=10) and other treatment groups (n=8) received 5.0 mg/kg cocaine. The same SAL/SAL and SAL/COC control data are plotted in all four graphs for visual comparison to each treatment group. Symbols indicate statistically significant differences at particular time intervals. Asterisks (*) indicate statistical differences from the SAL/SAL treatment group and pound signs (#) indicate statistical differences (i.e., p < .05) from the SAL/COC treatment group.
4. DISCUSSION
This is the first known study to demonstrate the induction of locomotor sensitization to repeated daily exposure to MDPV or to a mixture comprising low doses of 4-MMC and MDPV in rats, as indicated by statistically greater levels of cumulative locomotor counts on day 7 compared to day 1. It is noteworthy that activity levels are generally comparable among the 0.5, 1.0, and 2.0 mg/kg 4-MMC treatment groups, and activity levels in these groups are lesser than the treatment group injected with 0.5 mg/kg MDPV or any of the drug mixtures. Indeed, the results of the simple main effects tests on cumulative horizontal counts displayed in Figure 2 suggests that 0.5 mg/kg MDPV was responsible for the observed increases in horizontal activity among the groups treated with drug mixtures, although this speculation may be best addressed with further research including additional doses of MDPV while 4-MMC dose is held constant, and perhaps isobolographic analysis. Moreover, in the 0.5, 1.0, or 2.0 mg/kg 4-MMC treatment groups, there are statistically greater levels of activity on day 7 compared to day 1 only during the first five minutes post-injection. By definition, these effects qualify as evidence of within-group sensitization, although such results may need to be interpreted with caution, given the lack of effects observed when the data were transformed into aggregate form (i.e., cumulative post-injection counts). Also apparent in the time-course activity data recorded during the induction phase are statistical differences in activity during the pre-injection (habituation) interval in several of the treatment groups. That is, in the saline-, 0.5, 1.0, or 2.0 mg/kg 4-MMC-treated rats, there are greater levels of activity on day 1 compared to day 7 at specific time intervals. It is possible that novelty of the test chambers may account for the differences observed during the pre-injection period, and the statistical analysis was not powerful enough to detect differences in the rats injected with 0.5 mg/kg MDPV (due to relatively greater levels of variability in activity).
It is unclear why the acute drug effects on day 1 differed among the drug mixture groups given the present data. Higher doses of 4-MMC mixed with 0.5 mg/kg MDPV could also be tested in future studies to further evaluate this relationship in more detail. In addition, inspection of the standard error bars in Figures 1 and 2 indicate that rats injected with 0.5 mg/kg MDPV displayed relatively greater levels of variability than rats injected with 4-MMC or saline. It is noteworthy that in the 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV-treated rats, the variability was lesser than in the other mixture groups, and the overall levels of variability observed during day 7 appear reduced in comparison to day 1. Together, these data suggest that greater variability in horizontal activity may be attributable to increased dopaminergic stimulation, which can then be modulated through other monoaminergic (e.g., serotonergic) systems; although, without supplemental neurochemical measures, this speculation awaits experimental validation. Moreover, open-field novelty may lead to greater variability in drug-treated subjects (note the variability was consistent in saline-injected rats across both treatment days). Future research investigating the effects of monoaminergic releasers and blockers on measures of variability seems warranted and may serve as an additional index of psychostimulant activity.
Following a 10-day drug washout period, rats previously injected with 0.5 mg/kg MDPV, 2.0 mg/kg 4-MMC, or any of the drug mixtures displayed statistically greater levels of activity to the acute effects of 5 mg/kg cocaine than rats previously exposed to saline. Such results provide support for cross-sensitization to cocaine among rats exposed to the foregoing drugs and drug mixtures. Gregg et al. (2013b) observed that rats exposed to 15 mg/kg 4-MMC for 5 to 7 days showed cross-sensitization of repetitive movements to 15 mg/kg cocaine, however there was no evidence of cross-sensitization of stereotyped movements to this dose of 4-MMC. The present findings revealed no evidence of cross-sensitization to cocaine in rats treated with the two lowest doses of 4-MMC (0.5 or 1 mg/kg), but did reveal a single, post-injection time interval in which rats previously exposed to 2.0 mg/kg 4-MMC exhibited statistically greater levels of activity than rats that received saline during the induction phase. Notwithstanding cautious interpretation of these data, the current findings represent the first demonstration of locomotor cross-sensitization to cocaine following repeated exposure to 0.5 mg/kg MDPV, 2.0 mg/kg 4-MMC, and mixtures of 4-MMC + MDPV. Future studies could examine a wider range of doses with various synthetic cathinones to establish a dose-response relationship between these compounds and their potential to enhance sensitivity to other drugs. It would also be of interest to expose subjects to a range of cocaine doses and subsequently test for cross-sensitization to 4-MMC and/or MDPV to evaluate bidirectionality of sensitization between cocaine and synthetic cathinones.
The present experiment revealed that 0.5 mg/kg 4-MMC-treated rats did not show strong evidence of within-group locomotor sensitization. Although no direct comparisons to other studies are currently possible, a previous report by Lisek et al. (2012) demonstrated evidence for the expression of locomotor sensitization following repeated exposure to 0.5 mg/kg 4-MMC in male Sprague-Dawley rats. In that study, rats received daily injections of 0.5 mg/kg 4-MMC for five consecutive days immediately followed by a 10 day washout period. Locomotor activity was not recorded on days 1 through 5, which precludes evaluating whether 0.5 mg/kg 4-MMC produced within-group locomotor sensitization under the experimental conditions Lisek et al. (2012) had prepared. Nevertheless, on experimental day 16, the rats injected with 4-MMC revealed statistically greater ambulatory responses than saline-pretreated rats. In the present study, horizontal movements were recorded on day 1 and day 7, with daily injections occurring on days 2–6. As mentioned, activity was recorded during this initial phase of the study (i.e., induction phase) to evaluate within-group sensitization. Further, instead of evaluating the expression of sensitization to a dose of 4-MMC following a drug washout period as in Lisek et al. (2012), the present study evaluated the expression of cross-sensitization to cocaine. Future studies could include additional assessments of different drugs tested during post-washout periods or include a variety of synthetic cathinones.
Although there are currently no published reports on the behavioral effects of 4-MMC and MDPV in mixtures, a recent study utilizing in vitro electrophysiology techniques found that when 4-MMC and MDPV are applied to the dopamine transporter simultaneously, 4-MMC displays pharmacological activity more quickly at the target site than MDPV (Cameron et al., 2013b). This suggests that a mixture of 4-MMC + MDPV delivered concurrently would produce dopamine release first, followed by dopamine reuptake blockade. The authors of that report concluded that these combined pharmacological actions at the dopamine transporter indicate concurrent use of these synthetic cathinones may further augment dopaminergic neurotransmission, and thus pose greater health threats to substance users (Cameron et al., 2013b). The results of the present study tentatively support this conclusion, but may require further validation. Indeed, only the 2.0 mg/kg 4-MMC dose when combined with 0.5 mg/kg MPDV produced statistically significant increases in cumulative post-injection horizontal counts on day 7 compared to day 1. The lack of locomotor sensitization to the 0.5 mg/kg 4-MMC + 0.5 mg/kg MDPV and 1.0 mg/kg 4-MMC + 0.5 mg/kg MDPV mixtures may be due to 4-MMC’s actions at other monoaminergic transporter sites, such as the serotonin transporter (SERT) and norepinephrine transporter (NET) (e.g., see Baumann et al., 2012), effects similar to the pharmacological profile of MDMA. Future studies could assess the effects of selective dopamine or serotonin antagonists on 4-MMC- or MDPV-induced locomotor sensitization. Such research would further enhance our understanding of the potential neural mechanisms involved in mediating the locomotor sensitization produced by these drugs.
In sum, the current study established that 0.5 mg/kg MDPV and 2.0 mg/kg 4-MMC + 0.5 mg/kg MDPV produced statistically greater increases in locomotor activity in rats after repeated daily dosing for seven days. Further, MDPV alone, as well as mixtures of MDPV and 4-MMC, induced cross-sensitization to the locomotor stimulant effects of cocaine following a 10 day drug washout. Given the current paucity of studies on the combined effects of various synthetic cathinones, and despite the prevalence of polysubstance use by recreational drug users, this study represents an initial step toward developing a model to explore the behavioral effects of drug mixtures. Finally, considered together with recent findings that 4-MMC and MDPV have different sites of action, but both influence monoaminergic functioning, further investigations of these and other synthetic cathinone mixtures using a variety of behavioral procedures may be warranted.
Highlights.
Locomotor activity sensitized to MDPV and to low dose mixtures of 4-MMC and MDPV.
Cross-sensitization to cocaine was evident following exposure to MDPV and 4-MMC.
Studies on the abuse liability of synthetic cathinone mixtures may be warranted.
Acknowledgments
Role of Funding Source
The senior author’s (Lisa E. Baker) research program is currently funded by the National Institutes of Health (R15 DA038295). The National Institute on Drug Abuse drug control supply program provided all drugs used in this study.
The authors acknowledge Edward J. Kohler, Cameron R. Kraepel, Kelly N. Psotka, and Alysha Yavornitsky for their assistance with conducting some of the behavioral experiments as part of an undergraduate research practicum.
All experiments conducted comply with current laws of the United States of America.
Footnotes
Contributors
All authors contributed to the study planning and design. Authors Michael D. Berquist, Haily K. Traxler, and Alyssa M. Mahler conducted the behavioral experiments, with the assistance of students listed in acknowledgements. Lisa E. Baker prepared all test compound solutions. Michael D. Berquist conducted statistical analysis of the data. Lisa E. Baker created the graphic displays of the data. All authors contributed to the manuscript preparation and have approved the final manuscript for submission to Drug and Alcohol Dependence.
Conflict of Interest
All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methyenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, II, Peet MM, Baker LE. Behavioral sensitization following concurrent exposure to mephedrone and D-amphetamine in female mice. Behav Pharmacol. 2015;26:180–183. doi: 10.1097/FBP.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013a;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts”, produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 2013b;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Agency (DEA) Federal Register. Vol. 76. U.S. Government Printing Office; Washington, DC: 2011. Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cathinones Into Schedule I; p. 174. [Google Scholar]
- Drug Enforcement Agency (DEA) Federal Register. Vol. 81. U.S. Government Printing Office; Washington, DC: 2016. Schedules of Controlled Substances: Placement of 10 Synthetic Cathinones Into Schedule I; p. 43. [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013a;133:746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, Rawls SM. Mephedrone interactions with cocaine: prior exposure to the ‘bath salt’ constituent enhances cocaine-induced locomotor activation in rats. Behav Pharmacol. 2013b;24:684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs. 2014;4:369–378. doi: 10.1080/02791072.2014.962717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in the nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu Y, Reitz AB, Liu-Chen L, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. 2015;3:365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RTR, Jayson R, Korsah C, Pillidge KE, Wigmore PM, Ebling FJP, Green AR, Fone KCF, King MV. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2013;23:1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DAWN Report. “Bath Salts” Were Involved In Over 20,000 Drug-Related Emergency Department Visits In 2011. [Accessed 5/31/14];The DAWN Report Data Spotlight. 2013 www.samhsa.gov/data/spotlight/spot117-bath-salts-2013.pdf.
- Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202:e62–e63. doi: 10.1016/j.forsciint.2010.07.014. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) World Drug Report 2014. United Nations publication; 2014. [Accessed 1/22/16]. Sales No. E.14.XI.7. http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2010;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, Dargan PI. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol. 2010a;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2010b;28:280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]