Abstract
Vulnerability to drug related cues is one of the leading causes for continued use and relapse among substance dependent individuals. Using drugs in the face of cues may be associated with dysfunction in at least two frontal-striatal neural circuits: (1) elevated activity in medial and ventral areas that govern limbic arousal (including the medial prefrontal cortex (MPFC) and ventral striatum) or (2) depressed activity in dorsal and lateral areas that govern cognitive control (including the dorsolateral prefrontal cortex (DLPFC) and dorsal striatum). Transcranial magnetic stimulation (TMS) is emerging as a promising new tool for the attenuation of craving among multiple substance dependent populations. To date however, nearly all repetitive TMS studies in addiction have focused on amplifying activity in frontal-striatal circuits that govern cognitive control. This manuscript reviews recent work using TMS as a tool to decrease craving for multiple substances and provides a theoretical model for how clinical researchers might approach target and frequency selection for TMS of addiction. To buttress this model, preliminary data from a single-blind, sham-controlled, crossover study of 11 cocaine-dependent individuals is also presented. These results suggest that attenuating MPFC activity through theta burst stimulation decreases activity in the striatum and anterior insula. It is also more likely to attenuate craving than sham TMS. Hence, while many TMS studies are focused on applying LTP-like stimulation to the DLPFC, the MPFC might be a new, efficacious, and treatable target for craving in cocaine dependent individuals.
Keywords: Brain stimulation, Functional MRI, Orbitofrontal cortex, BA 10, Caudate
1. Frontal-striatal circuits involved in addiction
Chronic cocaine use is among the most difficult substance-use disorders to treat. Nearly 1 in every 7 people seeking treatment for drug abuse is dependent upon cocaine (Abuse N.I.O.D, 2010) and short-term cocaine relapse rates can reach 75% (Sinha, 2011). There are no FDA-approved pharmacotherapy approaches for cocaine dependence and traditional behavioral treatment strategies often have limited success in cocaine dependent populations. This chronic cycle of use, abstinence, and relapse is likely due to factors that involve limbic and executive circuits in the brain, including vulnerability to salient cues and loss of cognitive control (Back et al., 2010; Poling et al., 2007).
1.1. Anatomical connectivity between the frontal cortex and the striatum
In healthy individuals, limbic drive and executive control are modulated by at least two frontal-striatal neural circuits in the brain—the limbic circuit, which includes projections from the medial prefrontal cortex (MPFC) to the ventral striatum, and the executive control circuit, which includes projections from the dorsolateral prefrontal cortex (DLPFC) to the dorsal striatum (Alexander et al., 1986) (Fig. 1, left). Among treatment-seeking cocaine users, vulnerability to drug related cues could theoretically be due to: (1) elevated functional activity within limbic neural circuitry (including the MPFC and ventral striatum) in the presence of a salient cue (Ersche et al., 2012; Moeller et al., 2010; Moreno-Lopez et al., 2012) or (2) depressed activity in executive control circuitry (including the DLPFC and dorsal striatum) (Goldstein et al., 2004; Kubler et al., 2005; Moeller et al., 2010) which is likely required to resist the limbic drive for the drug. These frontal-striatal connections represent the first stage of the frontal-striatal-thalamic loops which were classically characterized based on anatomical connectivity between the frontal cortex, striatum, pallidum and thalamus (Alexander et al., 1986, 1989). Through advances in imaging technology in the past 20 years, these circuits have been further refined (Haber 2003; Lehéricy et al., 2004) and interpreted in relationship to their role in psychiatric disease (Haber and Rauch 2010).
Fig. 1.
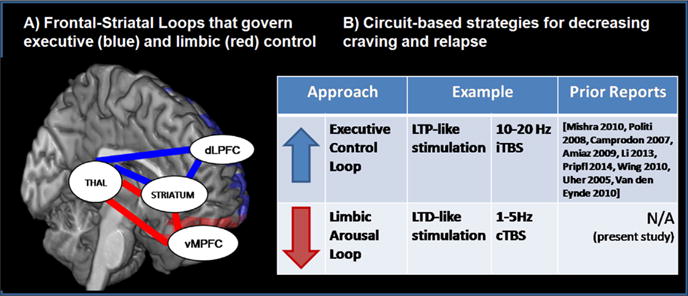
Frontal-striatal circuits that contribute to vulnerability to cues and brain stimulation strategies to modulate these circuits.
The framework for using TMS as an innovative treatment option for addiction presented in this review will capitalize on the anatomical connectivity between the frontal cortex and striatum. Complementing this anatomical connectivity however, are models of functional connectivity in limbic and executive control circuits. The development of functional MRI acquisition and analysis techniques over the past 20 years has led to a rich, emerging literature on intrinsic networks of functional connectivity. These functional connectivity models typically measure temporally correlated changes in BOLD signal in disparate areas of the brain while an individual is resting. Unlike anatomical connectivity studies, functional connectivity studies are typically not constrained by neural architecture. That said, it is appealing to see that these ‘anatomically agnostic’ functional connectivity models have isolated intrinsic networks which are similar to the anatomically defined limbic and executive frontal-striatal-thalamic loops (e.g. default mode network, salience network, and the executive control network) (Seeley et al. 2007). When developing TMS as a tool for addiction however, we have chosen to focus on the anatomical connectivity between frontal and striatal areas. This is because TMS induces a change in BOLD signal in the area immediately under the TMS coil as well as areas monosynaptically connected (Bohning et al., 1999; Thickbroom, 2007). Consequently, any causal effect of TMS on subcortical structures with traditional figure-of-eight coils currently requires anatomical connectivity between the cortical region stimulated and the subcortical target.
1.2. Tools available to modulate frontal-striatal circuits in addiction
Our understanding of the neural circuitry that governs drug seeking and cue-induced reinstatement has significantly advanced via developments in optogenetics (Cao et al., 2011; Steinberg and Janak, 2013) and designer receptors exclusively activated by designer drugs (DREADD) (Ferguson and Neumaier, 2012; Aston-Jones and Deisseroth, 2013). With optogenetics, populations of neurons that have been infected with channel rhodopsin (Boyden et al., 2005) or halorhodopsin (Zhang et al., 2007) can be selectively activated or inhibited through exposure to different frequencies of light. In an analogous approach, DREADDs involve the mutation of muscarinic acetylcholine receptors on neurons such that they can be selectively activated or inhibited through the inert ligand clozapine-N-oxide (Rogan and Roth, 2011). Using these techniques a number of studies have demonstrated that it is possible to increase or decrease drug self-administration by amplifying or attenuating activity in subcortical areas (e.g. the nucleus accumbens, ventral tegmental area of the thalamus) (Stuber, 2010; Stuber et al., 2012). Medial prefrontal cortex optogenetic stimulation can directly control habitual responding (Smith et al., 2012). Additionally, prelimbic cortex amplification and inhibition may alter cocaine seeking in a direction specific manner (Chen et al., 2013; Stefanik et al., 2013).
Until recently however, we have not had the ability to selectively modulate limbic or executive control circuits in human clinical research in the manner that optogenetics or designer receptors allow in preclinical research. Deep brain stimulation is becoming more widely used in psychiatric disorders and was being actively pursued as an FDA-approved treatment for depression. In its current form however, deep brain stimulation uses one of several very high frequencies to block neural transmission through a specific brain region, rather than selectively activating or inhibiting neurons in the target area with different frequencies. TMS is the only brain stimulation tool currently available which enables us to selectively activate or inhibit populations of neurons. When stimulation is delivered at particular frequencies this method is known as repetitive TMS (rTMS). It is possible to induce long term potentiation (LTP) of both behavior and neural activity by applying either a single high frequency (e.g. 10 Hz) or an intermittent bursting frequency (intermittent theta burst stimulation (iTBS)) to the cortex. Furthermore it is possible to induce transient long term depression (LTD) of behavior and neural activity by applying either a single low frequency (e.g. 1 Hz) or continuous bursting frequency (continuous theta burst stimulation (cTBS)). Additionally, TMS can alter neural activity in a circuit specific manner. Single pulses to the MPFC/frontal pole are associated with increased BOLD signal (the primary dependent measure of functional MRI) in the ventral striatum (part of the ‘limbic loop’ involved in craving) whereas single pulses to the DLPFC are associated with increased BOLD signal in the dorsal striatum (part of the ‘executive control loop’) (Hanlon et al., 2013). Although a comprehensive review of studies that have demonstrated these principles of TMS is beyond the scope of this manuscript; prior behavioral, electrophysiological, and neuroimaging work in this area is well described and summarized in several review articles (see: Fitzgerald et al., 2006; Thickbroom, 2007, Di Lazzaro et al., 2008, Hoogendam et al. 2010).
2. Developing neural-circuit based rTMS treatment strategies for addiction: fundamental questions
The potential of rTMS as a new tool for modulating craving among substance dependent populations has garnered significant attention from both the National Institute of Health (NIH) and in the literature [see reviews: Bellamoli et al., 2014; Gorelick et al., 2014, Wing et al., 2013; Barr et al., 2011]. As of August 2014, there were 131 NIH funded studies using transcranial magnetic stimulation, 23 of which are sponsored by National Institute of Mental Health and 7 of which are sponsored by National Institute on Drug Addiction (NIDA). Nearly all of the NIDA sponsored grants have been funded in the past 5 years. There were also approximately 16 original research reports and at least four reviews (Bellamoli et al., 2014; Feil and Zangen, 2010; Gorelick et al., 2014; Wing et al., 2013) on the efficacy of repetitive transcranial magnetic stimulation as a tool to decrease craving (Table 1). As this field develops the primary questions will likely be (1) what cortical location should we target in order to maximally effect the circuitry we are interested in changing? and (2) what stimulation frequency should we choose? There will likely not be a single ‘optimal’ protocol for all individuals or all classes of drugs. For example, some individuals may benefit the most of a treatment strategy that amplifies their executive control circuitry (e.g. 10 Hz dorsolateral prefrontal cortex stimulation) while others may benefit the most from a strategy that attenuates limbic circuitry involved in drug craving (e.g. 1 Hz medial prefrontal/frontal pole stimulation). Before moving forward with expensive and slow multisite clinical trials investigating the efficacy of rTMS as a viable treatment tool for addiction however, it is useful to explore these combinations of frequencies and cortical targets to maximize our potential impact on the patients.
Table 1.
Studies that have used repetitive TMS as a tool to change craving in patients with substance use disorders.
| First author | Year | Drug of abuse | Sample (real TMS) |
Site of TMS | Frequency | Sessions (real TMS) |
Active sham controla | Effect on craving | |
|---|---|---|---|---|---|---|---|---|---|
| De Ridder | 2011 | Alcohol | 1 | ACC | 1–35 Hz | 25 | No | Yes | Down |
| Herremans | 2012 | Alcohol | 15 | R DLPFC | 20 Hz | 1 | Between grps | No | |
| Herremans | 2013 | Alcohol | 29 | R DLPFC | 20 Hz | 2 | Within, active | No | |
| Hoppner | 2011 | Alcohol | 10 | L DLPFC | 20 Hz | 10 | Between grps | No | |
| Mishra | 2010 | Alcohol | 30 | R DLPFC | 10 Hz | 10 | Between grps | Yes | Down |
| Camprodon | 2007 | Cocaine | 6 | R DLPFC | 10 Hz | 1 | Within, 2 sites | Yes | Down |
| 6 | L DLPFC | 10 Hz | 1 | Within, 2 sites | No | ||||
| Hanlon | present data | Cocaine | 11 | L MPFC | cTBS | 1 | Within, active | Yes | Down |
| Politi | 2008 | Cocaine | 36 | L DLPFC | 15 Hz | 10 | No | Yes | Down |
| Li | 2013 | Meth. | 10 | L DLPFC | 1 Hz | 1 | Yes, within | Yes | Up |
| Amiaz | 2009 | Nicotine | 22 | L DLPFC | 10 Hz | 10 | Between grps | Yes | Down |
| Dinur-Klein | 2014 | Nicotine | 115b | LPFC/Insula | 10 Hz | 13 | Within, active | Yes | Down |
| 115b | LPFC/Insula | 1 Hz | Within, active | No | |||||
| Eichhammer | 2003 | Nicotine | 14 | L DLPFC | 20 Hz | 2 | Between grps | No | |
| Li | 2013 | Nicotine | 16 | L DLPFC | 10 Hz | 2 | Within, active | Yes | Down |
| Pripfl | 2014 | Nicotine | 14 | L DLPFC | 10 Hz | 1 | Within, 2 sites | Yes | Down |
| Rose | 2011 | Nicotinec | 15 | SFG | 10 Hz | 1 | Within, 2 sites | Yes | Up |
| Rose | 2011 | Nicotinec | 15 | SFG | 1 Hz | 1 | Within, 2 sites | No | |
| Wing | 2010 | Nicotine | 6 | L&R DLPFC | 20 Hz | 20 | Between grps | Yes | Down |
| Uher | 2005 | Foodd | 13 | L DLPFC | 10 Hz | 1 | Between grps | Yes | Down |
| Van den Eynde | 2010 | Foodd | 17 | L DLPFC | 10 Hz | 1 | Between grps | Yes | Down |
ACC=Anterior cingulate cortex, DLPFC=dorsolateral prefrontal cortex, L=left, R=Right, Meth=methamphetamine, BA=Brodmann 10.
Sham control is typically performed either within subject, crossover design (yes,within) or between groups (grps). Additionally some studies used 2 sites as a within subject control.
115 started, 77 completed the protocol.
This study performed 10 Hz TMS on the superior frontal gyrus (SFG) and found an increase in craving but did not find a decrease in craving with 1 Hz to the SFG.
although ‘food addiction’ is not universally accepted, it likely involves similar neural circuitry. Therefore, to be inclusive these two studies were added to the table.
2.1. Choosing a location
2.1.1. Strategy 1—amplifying executive control circuits
To date the vast majority of rTMS studies in addiction have targeted the same neural region—the DLPFC—a node in the frontal-striatal network that governs executive control (Amiaz et al., 2009; Camprodon et al., 2007; Eichhammer et al., 2003; Herremans et al., 2012, 2013; Hoppner et al., 2011; Li et al., 2013; Mishra et al., 2010; Politi et al., 2008; Pripfl et al., 2014). While many of these studies demonstrated that high frequency (LTP-like) rTMS stimulation to the DLPFC can result in a significant reduction of craving, the neurobiological mechanism through which this happens is not clear. In a comprehensive review of the literature on the efficacy of rTMS as a treatment tool for smoking, Wing et al. (2013) present a model in which the beneficial effects of LTP-like rTMS on the DLPFC are associated with a release of dopamine in the nucleus accumbens. This model is supported by important earlier work from Strafella and colleagues who used positron emission tomography to demonstrate that DLPFC stimulation was associated with a change in dopamine binding in the caudate/dorsal striatum (Strafella et al., 2001).
2.1.2. Strategy 2—attenuating limbic drive circuits
The primary cortical input to the ventral striatum (which includes the caudate and nucleus accumbens); however, is the medial and orbital prefrontal cortex. Given that the nucleus accumbens is one of the primary brain regions involved in craving (Robinson and Berridge, 1993), it seems that targeting the MPFC would be a more direct method to modulate nucleus accumbens activity among substance dependent populations. Recent work by Cho and colleagues demonstrated that LTP-like (10 Hz) rTMS to the MPFC in a group of 11 healthy, non-drug using individuals was associated with a significant decrease in dopamine binding potential in the dorsal striatum, reflecting a release of dopamine in these areas. Unlike many studies in this field which use a figure-of-eight coil (in which the opposing loops in the coil are largely coplanar), these investigators used a double-cone coil which has an angular geometry that likely results in a greater depth of stimulation. Although they did not find a significant change in dopamine binding in the nucleus accumbens in these healthy individuals, LTP-like stimulation of this medial prefrontal-striatal circuit was associated with a shift towards more immediate rewards in a delayed discounting task (Cho et al., 2015).
Given that craving for cocaine is associated with an increase in dopamine in the striatum, it is reasonable to pursue an LTD-like rTMS strategy over the MPFC to attenuate activity in this neural circuit. Prior data from our laboratory demonstrates that in healthy, non-drug using individuals, it is possible to induce a significant rise in BOLD signal in the ventral striatum with a single pulse of transcranial magnetic stimulation (TMS) to the MPFC (Hanlon et al., 2013). Decreasing the sensitivity of this circuit through rTMS may be a valuable treatment strategy.Given that MPFC stimulation has not been widely pursued however, and is subjectively more painful than DLPFC rTMS it will be critical to determine whether stimulating at this location is tolerable inn substance dependent populations, especially prescription opiate dependent individuals who may have lower pain thresholds than other individuals. To date there are no published studies of rTMS as a treatment for craving in opiate dependent individuals.
2.2. Choosing a frequency
2.2.1. Strategy 1—single frequency stimulation
One of the central principles of TMS is that low frequency stimulation decreases neural firing, while high frequency stimulation increases neural firing. This can be achieved through either single frequency stimulation protocols or bursting protocols, such as theta burst stimulation. Low frequencies (typically ~1Hz) induce an LTD-like effect on cortical excitability, whereas high frequencies (typically 10–20 Hz) produce an LTP-like effect on cortical excitability (see: Fitzgerald et al., 2006). To date the vast majority of rTMS studies have applied a single frequency of stimulation (e.g. 1 Hz, 10 Hz or 20 Hz) to a single cortical region. A very common design in TMS literature is to compare the efficacy of real rTMS versus an active sham TMS treatment on various dependent measures (e.g. behavioral measures such as mood, pain, craving, and movement, and neurophysiological measures including motor evoked potentials, cortical field potentials, regional glucose or dopamine uptake, and resting or task induced BOLD signals). In substance dependence literature all of the presently published studies (Table 1) have used a single frequency of stimulation. The primary dependent measure is typically subjective craving ratings, but a growing number of these studies are using electrophysiological and neuroimaging metrics as dependent measures as well. The effects of a single session of TMS typically do not last more than 1 h. Studies in depression and pain literature suggest that multiple treatments of a single frequency may produce lasting changes in behavioral and neuroimaging metrics, but the sustainability of clinically relevant effects in substance dependent populations (e.g. craving, consumption) has not yet been well addressed.
2.2.2. Strategy 2—bursting pattern stimulation (e.g. theta burst)
An LTD or LTP-like effect can also be achieved through a bursting frequency, such as theta burst (Di Lazzaro et al., 2005; Huang et al., 2005). In preclinical literature theta burst stimulation is a well-known form of electrical stimulation which can induce long term potentiation (LTP) or depression (LTD) of synaptic activity in a given brain region (Bear and Malenka, 1994; Malenka and Bear, 2004). Human theta burst stimulation protocols use rTMS to induce similar forms of LTP and LTD by using intermittent or continuous bursts respectively (Di Lazzaro et al., 2005; Huang et al., 2005). With continuous theta burst stimulation (cTBS), bursts of 3 pulses at 50 Hz are applied at 5 Hz at an amplitude that is typically determined by the active motor threshold. When performed over the primary motor cortex, a lower amplitude of cTBS for 40 s leads to an attenuation of motor evoked potentials that is comparable to a higher amplitude of 1 Hz single frequency stimulation for 20 min (Huang et al., 2005). To date there are no published studies that have applied theta burst stimulation to substance dependent populations in an effort to attenuate their craving or to enhance their control over craving. Given the neurobiological basis for choosing this stimulation protocol, and the growing use of this TMS technique in other patient populations, we recently performed a single-blind, sham-controlled crossover study testing the efficacy of cTBS over the MPFC as a tool to modulate craving and limbic circuit activity in cocaine users. This was achieved using interleaved transcranial magnetic stimulation and functional MRI before and after a single session of real versus sham cTBS. We tested the hypothesis that real cTBS would significantly decrease TMS-induced BOLD signal in the area directly under the coil (MPFC) as well as the monosynaptic targets of the ventral striatum.
3. Medial prefrontal cortex theta burst stimulation in cocaine dependent individuals: preliminary data
3.1. Overall protocol design
This single-blind, sham-controlled pilot study involved one screening visit and two scanning/stimulation visits (occurring within 7–14 days of each other). At each scanning/stimulation visit functional MRI data was acquired before and after exposure to a session of real or sham theta burst stimulation (TBS). Continuous TBS was applied over the left MPFC (landmark based on EEG 10–20 system: FP1). Self-reported craving was measured before and after the cTBS session and the fMRI sessions. We tested the hypotheses that continuous TBS over the medial PFC would induce a long-term depression of stimulus evoked brain activity in the medial PFC and ventral striatum using interleaved TMS/BOLD imaging. [Due to space constraints, greater detail about the participants, assessments, interleaved TMS/BOLD imaging, and rTMS sessions is in the Supplementary material].
3.2. Protocol design and methods (see Supplementary material for more detail)
Briefly, non-treatment seeking chronic cocaine users (n=11) were recruited from the Charleston, SC area. Participants signed informed consent documents approved by the Medical University of South Carolina Institutional Review Board. They then completed a series of assessments related to protocol safety, mental status and drug use (See Supplementary Material, Table 2). A multidrug urine panel (Quikvue 6-panel urine drug screen (UDS), Quidel, San Diego, CA) was given to all participants at the screening and scanning/stimulation visits. Participants were required to have a negative UDS for cocaine, methamphetamine, opiates, and benzodiazipines. One individual had a positive UDS for tetrahydrocannabidiol at both scanning/stimulation visits. The experimental procedure at the scanning/stimulation visits included (Fig. 2A): (1) behavioral assessments and UDS, (2) Pretreatment interleaved TMS/BOLD imaging (EEG 10–20 system: FP1), (3) real/sham rTMS treatment at FP1, (4) Posttreatment interleaved TMS/BOLD. Self-reported craving was collected immediately before and after cTBS treatment (scale: 0–10, 10=most imaginable)
Table 2.
Demographic and drug use variables.
| Total sample | 11 |
| Gender | 9 Males, 2 females |
| Age | 39±8.6 years |
| Ethnicity | 11 African American |
| Cocaine use information: | |
| Preferred drug | 54% crack, 46% powder |
| Age of first cocaine use | 20.9 (±3.3) years |
| Duration of cocaine use | 17.2 (±2.7) years |
| Amount Spent per week | $168.18 (±120) |
| Time since last use (at scan) | 2.4 (±1.06) days |
| Other drug use: | |
| Nicotine smokers | 11 |
| Nicotine severity (Fagerstrom) | 3.1 (±2.4) |
| Marijuana smokers | 2 of 11 |
| Positive UDS marijuana | 1 of 11 |
| Alcohol use (AUDIT) | 7.8 (±5.8) |
| Depressive symptoms (BDI) | 7.72 (±6.8) |
| Anxiety state (Spielberger) | 37.45 (±14.0) |
| Anxiety trait | 39.2 (±11.4) |
Fig. 2.
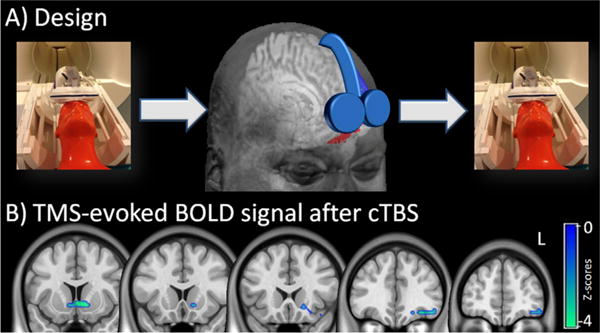
The effects of FP1 continuous theta burst stimulation (cTBS) on TMS-evoked brain activity. In this pilot study, interleaved TMS/BOLD imaging was used to measure TMS-evoked BOLD signal immediately before and after cocaine users were given a dose of cTBS to the left frontal pole (A). The TMS coil was placed over FP1 (EEG:10–20 system) for both the Interleaved TMS/BOLD scan (left & right panel) and the cTBS session (center panel). The red area represents the region of interest to which the coil is targeted (AAL: left superior and middle orbital prefrontal cortex inferior to the anterior commisure). Real cTBS (LTD-like) led to a significant decrease in BOLD signal in the left orbital/medial prefrontal cortex and ventral striatum (SPM8, p<0.05 Family Wise Error correction, negative Z-scores shown). The cTBS protocol was 2 trains of 1800 pulses, 110% RMT, 60 s intertrain interval., intensity ramped from 80–110% over first 30 s. L=left hemisphere.
3.2.1. Interleaved TMS/BOLD protocol
Resting motor threshold (RMT) was determined in the MRI scanning room. The coil was positioned over FP1 based on the EEG 10–20 system (Okamoto et al., 2004). Following high resolution anatomical image acquisition (Siemens 3 T TIM trio, TR=1900ms, TE=4ms, voxel dimensions 1.0×1.0×1.0 mm3,160 slices), participants received interleaved TMS/BOLD imaging with the coil on FP1 (20 pulses, 110% RMT, interpulse interval=10.18 s; flip=90, TR=2.5s, TE=0.023s, FOV=192 mm, voxel size= 3×3×3).
3.2.2. Continuous theta burst protocol
Within 5 min of the first TMS/BOLD acquisition, participants walked to an adjacent room for the cTBS procedure. Two trains of either real or sham LTD-like cTBS were applied over FP1 (1 train: 120 s, 3 pulse bursts presented at 5 Hz, 15 pulses/sec, 1800 pulses/train, 60 s intertrain interval; 110% RMT, MagPro) using a figure-of-8 coil (Coil Cool-B65 A/P). The amplifier output was escalated (over 30 s) from 80% to 110% RMT to enhance tolerability. Real and sham stimulation were well tolerated. Subjective reports indicated that the painfulness of the treatment subsided after the first 15–30 s, consistent with prior studies showing an endogenous opiate effect of prefrontal rTMS (Taylor et al., 2012, 2013). At the conclusion of each visit the participants filled out a form indicating their confidence (scale 1–10) on whether they received sham or real treatment. Pooled accuracy was 47.6% suggesting that individuals were not aware of the treatment being received. A timer was started after the final cTBS pulse and the participants were led back to the scanner to begin the second TMS/BOLD procedure. The average time between conclusion of cTBS and initiation of TMS/BOLD was: sham: 6:17±1:03 (range: 4:50–8:31), real cTBS: 7:00±1:23 (range 4:50–7:56), p=0.23).
3.2.3. Image analysis
Functional image preprocessing and within subjects fixed-effects modeling was performed in SPM8 (see Supplementary material for details). The evoked BOLD signal after real and sham TBS was compared to the evoked signal before real and sham TBS using paired t-tests. Statistical maps were created for both the within treatment (real or sham) and between treatment contrasts. A Monte Carlo simulation (p<0.01, 3dClustSim, see Supplementary material) was employed for all statistical contrasts to generate a Family Wise Error correction equivalent to α<0.05.
3.3. Results
3.3.1. Cue reactivity
Self-reported craving on a scale of 0–10 was sampled 3 times before treatment (baseline, after cue exposure, after interleaved TMS) and 3 times after treatment (immediately after treatment baseline, after interleaved TMS, after cue exposure). The difference in craving before and after treatment was compiled for each individual and classified as an increase, decrease, or no change. Chi square test demonstrated that changes in self-reported craving following sham stimulation were no different than chance [χ2=2.354, p=0.31; increase craving: 3, decrease craving: 6, no change in craving: 2]. Changes in craving following real cTBS were different from chance [χ2=5.64; p=0.05] and significantly different than sham stimulation [χ2=19.14, p<0.0001; increase craving: 0, decrease craving: 6, no change in craving: 5] (Fig. S1A). The significant differences in the distribution were driven by increase in craving following sham cTBS. There was no significant difference in the mean scores between these groups however (Fig. S1B).
3.3.2. TMS-evoked BOLD signal before and after real and sham theta burst stimulation
Following real TBS treatment cocaine users had significantly lower TMS-evoked activity in the cortical areas in the vicinity of the coil as well as in the projection regions of the ventral striatum including the nucleus accumbens. (p<0.05, Family Wise Error corrected clusters, k=96) (Table 3A, Fig. S2). [Contrast: before cTBS- after cTBS, real only] (Fig. 2B). There was also significantly higher activity in the right precuneus and angular gyrus, nodes in the central executive network (Chen et al., 2013) (Table 3B). A direct comparison between real and sham cTBS [(real after cTBS<before cTBS)-(sham after cTBS- before cTBS)] demonstrated that real stimulation led to a significantly greater reduction in the insula, middle temporal gyrus, thalamus, and caudate (Table 3C). Sham alone resulted in a significant reduction in the cingulate, paracingulate, and the right inferior frontal gyrus (p<0.01, clusters corrected for family wise error, k=95).
Table 3.
The effect of medial prefrontal cortex cTBS treatment (LTD-like) on the BOLD response to medial prefrontal cortex stimulation (MPFC). The statistical contrasts in the table include: (A) real TMS: after treatment<before treatment, (B) real TMS: after treatment>before treatment, (C) [(real TMS: after<before)- (Sham TMS: after<before)].
| Cluster Statistics
|
MNI coordinates
|
Regions (including Harvard-Oxford Labels) | ||||
|---|---|---|---|---|---|---|
| size | p(unc) | Z | x | y | z | |
| (A) Real cTBS: Areas with lower BOLD signal after MPFC cTBS | ||||||
| 124 | 0.001 | 3.97 | 0 | 8 | −5 | Medial prefrontal cortex, cingulate, subcallosal (14%) |
| 3.76 | −9 | 8 | −8 | Left nucleus accumbens (93%) | ||
| 3.55 | −30 | 32 | −17 | Left orbital frontal cortex (58%) | ||
| (B) Real cTBS: Areas with higher BOLD signal after MPFC cTBS | ||||||
| 190 | 0 | 3.88 | 21 | −52 | 37 | Precuneus |
| 3.77 | 12 | −49 | 52 | Precuneus (52%) | ||
| 3.74 | 33 | −43 | 52 | Superior parietal (46%) | ||
| 97 | 0.002 | 3.22 | 57 | 2 | 28 | Right dorsolateral Prefrontal, Precentral gyrus (42%) |
| 3.18 | 33 | 17 | 13 | Frontal operculum (49%) | ||
| 3.07 | 48 | 8 | 16 | Right inferiorfrontal gyrus (27%) | ||
| (C) Real cTBS versus sham cTBS: areas with lower BOLD signal after cTBS | ||||||
| 128 | 0.001 | 4.07 | −57 | −46 | −5 | Left insula, middle temporal gyrus (31%) |
| 3.97 | −66 | −52 | 1 | Left middle temporal gyrus (62%) | ||
| 97 | 0.014 | 3.44 | −15 | −19 | 13 | Left thalamus, caudate |
3.3.3. Individual variability and relationship to drug use variables and craving
A post-hoc region of interest analysis was performed to determine whether there was a relationship between BOLD signal change in the nucleus accumbens and demographic, drug use, or brain stimulation parameter values. This was done by extracting the primary eigenvector from the cluster that was significantly reduced following real cTBS treatment. The eigenvalues from that region (a representation of normalized BOLD signal change to TMS pulses) were extracted for each participant and correlated with multiple characteristics of the participants. There was positive correlation between change in striatal BOLD signal and the amplitude of the cTBS treatment, suggesting that there may be a dose dependent effect of cTBS on striatal function (R2=0.487, df=9, p<0.01) (Fig. 3). There was no significant relationship amplitude of TMS and change in self-reported craving.
Fig. 3.
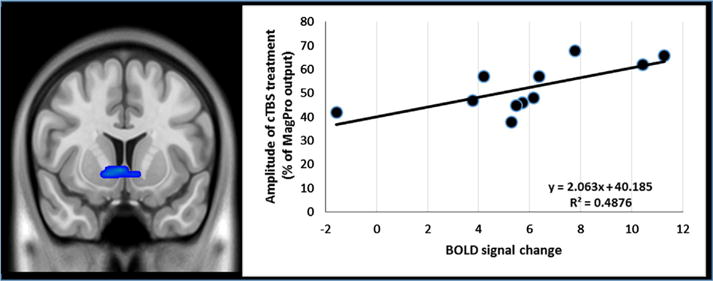
The relationship between change in BOLD signal in the ventral striatum with the amplitude of cTBS treatment. Individual variability in the change in ventral striatum BOLD signal was extracted via the eigenvalues from the functionally defined region of interest (Table 3). This was significantly correlated with the amplitude of the cTBS treatment (110% resting motor threshold) suggesting a dose-response effect on neural circuitry. Individual variability in BOLD signal was not correlated with other drug use variables.
4. Summary, implications, and future directions
One of the largest causes of relapse to drug use in treatment seeking individuals is cue-induced craving. The vulnerability to drug-related cues in substance dependent populations is likely due to disrupted activity in multiple frontal-striatal circuits that guide executive control and limbic arousal. The ability to modulate craving in a circuit-specific manner through non-invasive brain stimulation techniques, such as repetitive TMS, would be a powerful new tool to use as an adjuvant to behavioral treatment in addiction, especially for cocaine use in which there is currently no FDA-approved pharmacotherapy. While a growing number of original research reports have demonstrated that LTP-like rTMS over the DLPFC (a node in the executive control network), can decrease craving to multiple classes of drugs, to date there have been no reports that have investigated the efficacy of LTD-like rTMS over the MPFC (a node in the limbic network) on craving. The intent of this manuscript is to provide a theoretical model for how we might approach target and frequency selection for transcranial magnetic stimulation (TMS) of addiction, and present original data that suggests attenuating MPFC activity through an LTD-like stimulation protocol—cTBS—is tolerable, feasible, and can directly decrease craving and decrease activity in a circuit specific manner to the orbitofrontal cortex and ventral striatum, including the nucleus accumbens.
As the substance dependence field moves forward with pursuing rTMS as a potential treatment tool for substance dependent populations there are a few primary things to consider: 1) what is the best target, 2) what is the best frequency to use, 3) which patients are likely to benefit the most. The answers to these questions seem very time sensitive as large scale multicenter clinical trials for smoking addiction have already begun (Clinical Trials number: NCT02126124), and companies are already receiving consumer electronics (CE) marking approval to pursue rTMS as a treatment for smoking cessation (Brainsway, Jerusalem, Israel). The biggest trial to date was a cohort study of 115 smokers randomized to receive high frequency, low frequency, or sham TMS over 13 sessions. Using a unique H-coil design (which likely induces changes in a wider, deeper distribution of cortical areas including the anterior insula and lateral prefrontal cortex), this team demonstrated that 10 Hz (LTP-like) rTMS decreased cigarette consumption and improved abstinence rates for 6 months following the intervention (Dinur-Klein et al., 2014). These data are very promising and buttressed by other evidence that rTMS to the DLPFC can modulate craving (see Table 1), that the anatomical integrity (Naqvi et al., 2007) and functional connectivity (Moran Santa Maria et al., 2014) of the insula is important for craving, and that the insula can be modulated by TMS (Gratton et al., 2013). Unfortunately, because of the geometry of the H-coil, it is currently not possible to put it inside of the MRI scanner and map the distribution of its effects on regional BOLD signal. Although this might not be necessary in order to observe a clinically relevant effect, when designing and optimizing TMS treatments in the future a solid foundation in anatomical connectivity may allow us to harness and translate the wealth of preclinical knowledge into efficient, evidence based treatments for cocaine users.
In this manuscript we present the first study to date which examines the effect of an LTD-like rTMS protocol (cTBS) on craving and the frontal-striatal circuitry involved in craving. We demonstrate that, relative to sham stimulation, a single session of cTBS can decrease craving and that cTBS over the MPFC leads to a decrease of activity in both the prefrontal cortex and the nucleus accumbens. Although this is a relatively small pilot study, it suggests that this may be a promising new target to modulate frontal-strital circuitry involved in craving among cocaine dependent individuals. By coupling LTP-like or LTD-like rTMS with interleaved TMS/BOLD imaging, as was done in the present study, it is possible to directly investigate the causal effects of rTMS on frontal-striatal circuit activity. A recent study by Chen et al., 2013 demonstrated that it was possible to directly modulate the lateral prefrontal aspects of the central executive network and the medial prefrontal aspects of the salience network by applying an LTD-like rTMS protocol (1 Hz) in healthy controls. In a similar study by this group, they demonstrated that a 5 weeks course of LTP-like stimulation (10 Hz) to the DLPFC of 17 patients with major depressive disorder normalized previously high connectivity within the default mode network (which includes the MPFC). Additionally they demonstrated that activity in the DLPFC was inversely related to activity in the MPFC (Liston et al., 2014). In the addiction literature, unfortunately, none of the studies that have demonstrated decrease craving from LTP-like rTMS over the DLPFC have acquired neuroimaging data. Based on these prior studies however, it is reasonable to assume that 10 Hz stimulation over the DLPFC is decreasing craving by attenuating activity in the MPFC and other overlapping areas of the limbic and default mode networks.
Interleaved TMS/BOLD imaging before and after TMS treatment is a powerful approach to investigate the causal effects of a TMS treatment paradigm on brain activity in the area in the vicinity of the coil as well as afferent projections in the striatum. Although functional connectivity between the MPFC and striatum was not directly quantified in this manuscript interleaved TMS/BOLD imaging is a powerful tool which can be used in future studies to probe cortical connectivity in a controlled, causal manner. Through the use of this tool, the preliminary results presented in this manuscript demonstrate that 3600 pulses of cTBS at 110% of resting motor threshold decrease activity in the medial and orbital PFC in the vicinity of the TMS coil as well as in the projection regions of the striatum. It is not clear yet however if this attenuation of baseline frontal-striatal activity generalizes to lower frontal and striatal activity in the presence of drug related cues (which typically induce elevated BOLD signal in these regions). Future studies are necessary in order to evaluate the generalizability of these findings to cue-associated craving.
The primary limitations of this study include the fact that it is a relatively small sample of cocaine dependent individuals, it was single-blind, and only contained one session of rTMS (rather than multiple sessions likely required to produce a lasting effect on craving). The effects of a single session of cTBS likely do not last more than 1 h (Di Lazzaro et al., 2005; Huang et al., 2005, 2009) and consequently for this to be a viable treatment adjuvant for patients, multiple sessions will likely be required in order to achieve a sustainable effect. In the FDA-approved treatment protocol for depression for example, several weeks of 10 Hz TMS sessions are given in order to achieve a clinical effect that lasts for several months beyond the treatment itself. It is not known whether this long term potentiation would be faster or slower with a bursting protocol (as in theta burst stimulation) rather than a single frequency. Additionally, although we aimed to recruit a diverse group of individuals, this small sample lacked the racial and gender diversity that is required to generalize future studies to the larger population of cocaine/crack cocaine users.
Selective modulation of frontal-striatal circuits involved in limbic and executive control may be an innovative and useful treatment strategy to prevent cue-associated relapse in substance-dependent individuals. Repetitive TMS is an FDA approved treatment for depression and is growing in clinical use and acceptance, with >700 machines in the US and emerging insurance reimbursement. As the field of addiction moves forward with pursuing repetitive TMS as a new tool to modulate craving and the frontal-striatal circuits that contribute to chronic use and relapse, it will be important to consider the optimal site, frequency, and patient population to target. The data from the present study demonstrate that while most of the efforts for rTMS in addiction have been focused on increasing activity in the DLPFC, decreasing activity in the MPFC and ventral striatum may also be a feasible and fruitful target to consider. It seems plausible that either increasing neural firing in the executive control circuit (perhaps via high frequency TMS in the DLPFC), or decreasing firing in the limbic circuit in the presence of cues (perhaps via low frequency TMS in the MPFC) may be valuable strategies for decreasing vulnerability to drug-related cues among our patients. Before moving forward with slow and expensive clinical trials however, it is important to have a comprehensive understanding of limbic and executive circuit functioning in a diverse cross section of substance dependent individuals. With this knowledge we will be able to develop circuit-specific treatment strategies for these populations.
Supplementary Material
Acknowledgments
Financial disclosures
This effort was funded directly by NIH Grants K01DA027756 (Hanlon), R01DA036617 (Hanlon), P50 DA015369 (Kalivas), P50 AA010761 (Becker). Additional assistance was given by the South Carolina Translational Research Institute UL1 TR000062 and R25 DA033680. The authors would also like to acknowledge the Neuroscience Institute at the Medical University of South Carolina, the Neurobiology of Addiction Research Center, the Center for Biomedical Imaging, Truman R. Brown, Jayce Doose, James Purl, Melanie Canterberry, and Kathleen Brady, all of whom provided intellectual support and resources for this endeavor.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.brainres.2015.02.053.
Footnotes
This article is part of a Special Issue entitled SI:Addiction circuits.
References
- Abuse NIOD. Research Reports. 2010. Cocaine: Abuse and Addiction. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander Garrett E, Crutcher Michael D, DeLong Mahlon R. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1989;85:119–146. [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Deisseroth K. Recent advances in optogenetics and pharmacogenetics. Brain Res. 2013;1511:1–5. doi: 10.1016/j.brainres.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23(5):454–466. doi: 10.3109/09540261.2011.618827. http://dx.doi.org/10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol. 2014;2014815215 doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, Teneback C, Vincent DJ, George MS. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cao ZF, Burdakov D, Sarnyai Z. Optogenetics: potentials for addiction research. Addict Biol. 2011;16:519–531. doi: 10.1111/j.1369-1600.2011.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cho SS, Koshimori Y, Aminian K, Obeso I, Rusjan P, Lang AE, Strafella AP. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40(3):546–553. doi: 10.1038/npp.2014.211. http://dx.doi.org/10.1038/npp.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1(4):345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64:951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2012;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Neumaier JF. Grateful DREADDs: engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology. 2012;37:296–297. doi: 10.1038/npp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. http://dx.doi.org/10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, D’Esposito M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:124. doi: 10.3389/fnsys.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber Suzanne N. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Rauch SL. Neurocircuitry: a window into the networks underlying neuropsychiatric disease. Neuropsychopharmacology. 2010;35(1):1–3. doi: 10.1038/npp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Canterberry M, Taylor JJ, Devries W, Li X, Brown TR, George MS. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One. 2013;8:e67917. doi: 10.1371/journal.pone.0067917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120:209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C. Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. 2013;48:552–557. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hoppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry. 2011;12(Suppl 1):S57–S62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W, Classen J, Peterchev AV, Zangen A, Ugawa Y. Consensus: new methodologies for brain stimulation. Brain Stimul. 2009;2:2–13. doi: 10.1016/j.brs.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, De Moortele V, Francois C, Thivard L, Poupon C, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55(4):522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, Brady KT, George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73:714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M, Megan Hartwell, Karen J, Hanlon Colleen A, Canterberry Melanie, Lematty Todd, Owens Max, Brady Kathleen T, George Mark S. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2014 doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abus. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(46):18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Janak PH. Establishing causality for dopamine in neural function and behavior with optogenetics. Brain Res. 2013;1511:46–64. doi: 10.1016/j.brainres.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber G. Dissecting the neural circuitry of addiction and psychiatric disease with optogenetics. Neuropsychopharmacology. 2010;35:341–342. doi: 10.1038/npp.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71(12):1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain. 2012;153:1219–1225. doi: 10.1016/j.pain.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, George MS. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology. 2013;38:1189–1197. doi: 10.1038/npp.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


