Abstract
Background
Some polyphenols induce apoptosis and inhibit angiogenesis. Consumption of black tea, rich in polyphenols, has been found to reduce ovarian cancer risk. Theaflavin (TF1), theaflavin-3-gallate (TF2a), theaflavin-3′-gallate (TF2b) and theaflavin-3, 3′-digallate (TF3) are four main theaflavin derivatives found in black tea.
Materials and Methods
Cell proliferation assay, Hoechst 33342 staining assay, Caspase-Glo Assay, western blot, human umbilical vein endothelial cell tube formation assay and vascular endothelial growth factor (VEGF) enzyme-linked immunosorbent assay were performed.
Results
All four theaflavin derivatives reduced viability of ovarian cancer cells at lower concentrations than with normal ovarian cells. TF1 mainly mediated apoptosis via the intrinsic pathway, while the others via the intrinsic and extrinsic pathways. TF1 inhibited tube formation via reducing VEGF secretion in a hypoxia-inducible factor 1α-independent manner, while the others in a HIF1α-dependent way.
Conclusion
All four theaflavin derivatives inhibited ovarian cancer cells. Some of the effects and mechanisms of TF1 are different from those of the other three theaflavin derivatives.
Keywords: Theaflavins, ovarian cancer, apoptosis, angiogenesis
Ovarian cancer is one of the most widespread and lethal types of gynecological cancer (1). According to cancer statistics, the number of new ovarian cancer cases and deaths in the United States in 2015 are estimated at 21,290 and 14,180, respectively (2). Due to resistance to current first-line chemotherapy and lack of efficient diagnosis at early stages, the prognosis of ovarian cancer is poor, with an approximate 30% 5-year survival rate (3). Recurrence is another challenge. A large proportion of patients will have a relapse of disease, and unfortunately, recurrence is typically less responsive to current chemotherapeutic strategies (1). Thus, it is urgent that novel cancer therapeutic agents to treat ovarian cancer are found.
Apoptosis is an ordered and orchestrated cellular death process that occurs under physiological and pathological conditions (4). Dysregulation of apoptosis is found in a wide spectrum of human diseases, including cancer. Cancer cells can evade apoptosis and continue to proliferate. Therefore, apoptosis is regarded as a vital therapeutic target in cancer treatment. Triggering apoptosis is a mechanism shared by most chemotherapeutic agents.
Angiogenesis is necessary for tumor maintenance and development. Tumor vasculature usually has poor blood flow and high vascular permeability, which may lead to decreased efficacy of cytotoxic chemotherapy and an increased potential for metastasis (5). Angiogenesis inhibitors, which interfere with blood vessel formation, have been used alone or combined with standard chemotherapy for certain cancer treatments. It has been reported that continuing bevacizumab, an angiogenesis inhibitor, as maintenance therapy after carboplatin and paclitaxel chemotherapy prolongs the median progression-free survival of patients with advanced epithelial ovarian cancer (6).
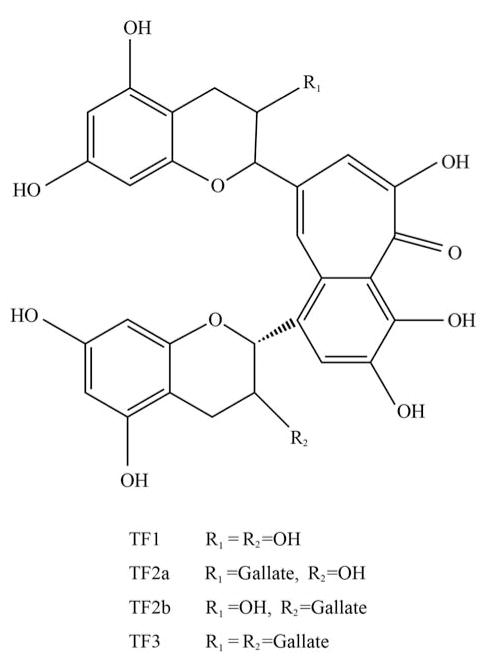
Many natural health products that inhibit angiogenesis also display other anticancer activities (7). Camellia sinensis is a herb traditionally used for anticancer treatment and has antiangiogenic activity (7). Tea, one of the most popular beverages in the world, is made from the leaves of Camellia sinensis. Tea can be classified into three main types based on its degree of fermentation: green tea (unfermented), oolong tea (semi-fermented) and black tea (fully fermented) (8). Approximately 80% of the world’s produced and consumed tea is black tea. Recent studies indicated that black tea consumption is associated with a linear decline in ovarian cancer risk (9). Theaflavins, which account for 2–6% of the solids in brewed black tea, belong to unique black tea polyphenols (10). Theaflavin (TF1), theaflavin-3-gallate (TF2a), theaflavin-3′-gallate (TF2b) and theaflavin-3, 3′-digallate (TF3) are four main theaflavins identified in black tea (Figure 1). Black tea polyphenol mixtures have been demonstrated to have anticancer activities (11). However, there exist few reports examining the inhibitory effect and mechanism of theaflavin derivative monomers on ovarian cancer.
Figure 1.
Chemical structures of the four main theaflavin derivatives.
In this study, we measured the antiproliferative effect of the four main theaflavins (TF1, TF2a, TF2b, and TF3) of black tea on two ovarian cancer cell lines OVCAR-3 and A2780/CP70, and one normal ovarian cell line IOSE 364. Apoptotic rate, caspase activity, tube formation by human umbilical vein endothelial cells (HUVECs) and vascular endothelial growth factor (VEGF) secretion of theaflavin derivative-treated OVCAR-3 and A2780/CP70 cells were examined. To better understand the underlying mechanisms for observed effects, western blot assays were carried out to evaluate the expression of apoptotic-related and angiogenic-related proteins.
Materials and Methods
Materials
Human ovarian carcinoma cell lines (OVCAR-3 and A2780/CP70) and human immortalized ovarian surface epithelial cells (IOSE 364), were kind gifts from Dr. Bing-Hua Jiang at Thomas Jefferson University (Philadelphia, PA, USA) and Dr. Auersperg at University of British Columbia (Vancouver, BC, Canada), respectively. Cells were cultured in RPMI-1640 medium (Sigma, St. Louis, MO, USA) incorporating 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY, USA). HUVECs, purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), were maintained in F-12K medium (ATCC) supplemented with 10% FBS (Invitrogen) and Endothelial Cell Growth Kit-VEGF (ATCC). Cells were grown at 37°C in a humidified incubator containing 5% CO2.
Reagents
TF1, TF2a, TF2b, and TF3 monomers were isolated and purified using a previously established method (12). Bisbenzimide H 33342 trihydrochloride (Hoechst 33342) was purchased from Sigma. Caspase-Glo® 3/7 Assay Systems, Caspase-Glo® 8 Assay Systems and Caspase-Glo® 9 Assay Systems were purchased from Promega (Madison, WI, USA). Human VEGF Duo-set enzyme-linked immunosorbent assay (ELISA) kit was purchased from R&D (Minneapolis, MN, USA). Antibodies against BCL2-associated X protein (BAX), death receptor 5 (DR5), Fas-associated death domain (FADD) and hypoxia-inducible factor 1α (HIF1α) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against BCL2-like 1 isoform 1 (BCL-xL) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell proliferation assay
Cells were seeded into 96-well plates at a density of 2×104 cells per well in medium with 10% FBS. After overnight growth, cells were treated with different concentrations of TF1, TF2a, TF2b, TF3 (5, 10, 20, 40 μM) or dimethyl sulfoxide (DMSO) (as vehicle) for 24 h. Cell viability was measured using CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega), according to the manufacturer’s instructions. Cell viability was expressed as a percentage compared to that of control cells (vehicle treatment).
Hoechst 33342 staining assay
Cells were seeded into 96-well plates at a density of 2×104 cells per well and incubated overnight. Cells were treated with different concentrations of TF1, TF2a, TF2b, TF3 (20, 40 μM) or DMSO (as vehicle) for 24 h. Then cells were stained with 10 μg/ml Hoechst 33342 in phosphate-buffered saline (PBS) for 10 min in the dark at 37°C. Apoptosis was examined under a fluorescence microscope (ZEISS). Cells with condensed, fragmented nuclei were regarded as apoptotic cells. Apoptotic rate (%)=number of apoptotic cells/number of total cells ×100%.
Caspase activity assay
Cells were seeded into 96-well plates at a density of 2×104 cells per well in medium with 10% FBS. After overnight growth, cells were treated with DMSO (as vehicle), TF1, TF2a, TF2b or TF3 for 24 h. Caspase-3/7, -8 and -9 activities were detected using Caspase-Glo 3/7, Caspase-Glo 8 or Caspase-Glo 9 Assay kit (Promega), respectively. Caspase activities were expressed as a percentage compared to that of control cells (vehicle treatment).
HUVEC tube formation assay
Growth Factor-Reduced Matrigel (50 μl; BD Biosciences, San Jose, CA, USA) was added into each well of a 96-well plate and polymerized for 30 min at 37°C. HUVECs (1.5×104/well) in 100 μl conditioned medium (90 μl F12-K medium+10 μl cell culture supernatant of vehicle- or theaflavin derivative-treated cancer cells) were seeded into each Growth Factor-Reduced Matriel-coated well, incubated at 37°C in 5% CO2 for 6 h, and then photographed using an Olympus CK2 Inverted Microscope (Olympus Optical Co., Tokyo, Japan). Tube length was quantified using NIH ImageJ software (NIH, Bethesda, MD, USA). Tube length was expressed as a percentage compared to that of the control group.
ELISA
Cells were seeded into 96-well plates at a density of 2×104 per well, incubated overnight and treated with DMSO (as vehicle), TF1, TF2a, TF2b or TF3 (10, 20 μM) for 24 h. Cell culture supernatants were then collected. The concentration of human VEGF protein was determined by a human VEGF Duo-set ELISA kit (R&D) according to the manufacturer’s instructions (range: 31.20–2000 pg/ml).
Western blot
Cells were treated for 24 h with DMSO (as vehicle), TF1, TF2a, TF2b or TF3 (20 μM) in 60 mm dishes, and then lysed in mammalian protein extraction reagent supplemented with Halt™ Protease and Phosphatase Inhibitor Single-Use Cocktail (LifeTechnologies, Grand Island, NY, USA). The concentration of protein was measured using a BCA Protein Assay Kit (Thermo, Waltham, MA, USA). Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membrane was blocked with 5% non-fat milk in Tris-buffer saline containing 0.1% Tween-20 for 1 h at room temperature and incubated with the indicated primary antibodies overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibody for 2 h at 37°C. Detection was performed by SuperSignal West Dura Extended Duration Substrate (Life technologies) and ChemiDoc™ MP System (Bio-Rad, Hercules, CA, USA). Protein bands were quantified with NIH ImageJ software (NIH), normalized by corresponding GAPDH for analysis.
Statistical analysis
All data are expressed as the mean±standard error of mean (SEM) from at least three independent experiments. The results were analyzed with SPSS Version 18.0 for Windows (SPSS, Chicago, IL, USA) using one- and two-way analysis of variance (ANOVA) followed by Dunnett’s test to test differences between two groups. p-Values of less than 0.05 were considered statistically significant.
Results and Discussion
Cancer is a complex disease, and despite the improvement of current therapies, the prognosis of many cancers remains poor. Treatment failure commonly occurs as cancer develops resistance through multiple mechanisms and finds alternate ways to grow. Multitargeted therapy has emerged as a new paradigm for anticancer treatment (13). Angiogenesis and avoiding apoptosis are vital for cancer development. There is increasing evidence revealing that a combination of conventional cytotoxic chemotherapy with angiogenesis inhibitors is more effective than conventional chemotherapy or antiangiogenic therapy alone (14, 15). Some natural compounds possess both apoptosis-inducing and antiangiogenic activities (13, 16–18). For example, green tea extract played a dual role in reducing angiogenesis and increasing apoptosis in patients with breast cancer (19). As the polymerized and oxidized products of green tea polyphenols, black tea polyphenols shared similar physiological activities. It has been reported that black tea polyphenols have antiangiogenic and proapoptosis potential (20, 21). However, it is not clear which components of black tea are the most effective compounds as potential cancer chemotherapeutic agents. In this article, we hypothesized that TF1, TF2a, TF2b and TF3, the four most abundant theaflavin derivatives in black tea, had inhibitory effects on the ovarian cancer cell lines OVCAR-3 and A2780/CP70 via apoptotic and antiangiogenic mechanisms.
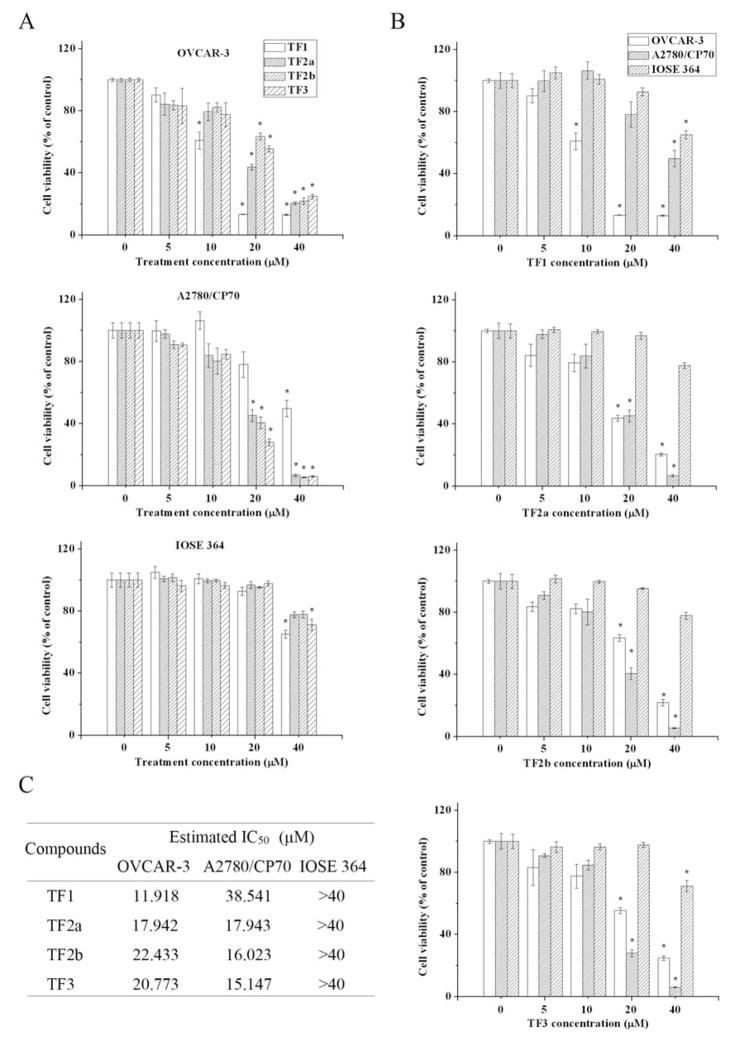
Our data demonstrate that all four theaflavin derivatives reduced the proliferation of OVCAR-3 and A2780/CP70 cells. The antiproliferative activities of theaflavin derivatives were cell-specific (Figure 2A) and derivative-specific (Figure 2B). For OVCAR-3 cells, the half-maximal inhibitory concentration (IC50) of TF1 was lower than that of TF1, TF2a and TF2b (Figure 2C). For A2780/CP70 cells, the IC50 of TF1 was highest among the four main theaflavin derivatives (Figure 2C). To test whether theaflavin derivatives had an adverse effect on normal ovarian cells, the cytotoxic effect of theaflavin derivatives on IOSE 364 cells was measured. None of the theaflavin derivatives decreased the viability of IOSE 364 cells at concentrations less than 30 μM (Figure 2A). When treated with the same concentration of specific theaflavin derivatives, the viability of IOSE 364 cells was much higher than that of OVCAR-3 and A2780/CP70 cells (Figure 2B). These results suggest that the four theaflavin derivatives preferentially inhibited ovarian cancer cells, with little cytotoxicity on normal ovarian cells. Similar characteristics were found in a former study, where theaflavin mixtures were proven to inhibit the growth of SV40-transformed WI38 human cells (WI38VA) and Caco-2 colon cancer cells but had little effect on the growth of their normal counterparts (22).
Figure 2.
Cytotoxic effects of the four main theaflavin derivatives on ovarian cancer cells and normal ovarian cells compared according to cell type (A) and theaflavin derivative (B). C: The estimated half-maximal inhibitory concentration (IC50) of the four main theaflavin derivatives against ovarian cancer cells and normal ovarian cells. *p<0.05 compared to the control group.
Apoptosis is a form of cell death in which a programmed sequence of events leads to elimination of cells without releasing harmful substances into the surrounding environment. Inappropriate apoptosis causes many diseases. Defective or inefficient apoptosis is an acquired hallmark of cancer cells (23). Induction of apoptosis is an important strategy for cancer treatment. The intrinsic (mitochondria-mediated) and extrinsic (receptor-mediated) pathways are two major apoptotic pathways. The intrinsic pathway is initiated by stress signals through release of apoptogenic factors, such as cytochrome c, from the mitochondrial intermembrane space into the cytosol, triggering caspase-3 activation through formation of the cytochrome c/apoptotic peptidase activating factor 1 (APAF1)/caspase-9-containing apoptosome complex (24). The BCL2 family plays a fundamental role in the regulation of the intrinsic pathway by controlling the mitochondrial membrane permeability and the release of cytochrome c. BCL2 proteins can be either proapoptotic (for example, BAX) or antiapoptotic (for example, BCL-xL). The ratio of pro- to antiapoptotic BCL2 family proteins determines the cell’s fate. The extrinsic pathway is stimulated by DRs (for example, DR5) binding to corresponding ligands. This results in receptor aggregation and recruitment of the adaptor molecule FADD and caspase-8 (24). Upon recruitment, caspase-8 becomes activated and initiates apoptosis by direct cleavage of downstream effector caspases (for example, caspase-3 and caspase-7) (24). Previous studies demonstrated theaflavins caused a change in the ratio of pro- and antiapoptotic proteins, leading to apoptosis of prostate cancer cells (25). In addition, theaflavins targeted the FAS/caspase-8 and protein kinase B (AKT)/phosphor-BCL2-associated death promoter (pBAD) pathways to induce apoptosis of human breast cancer cells (26).
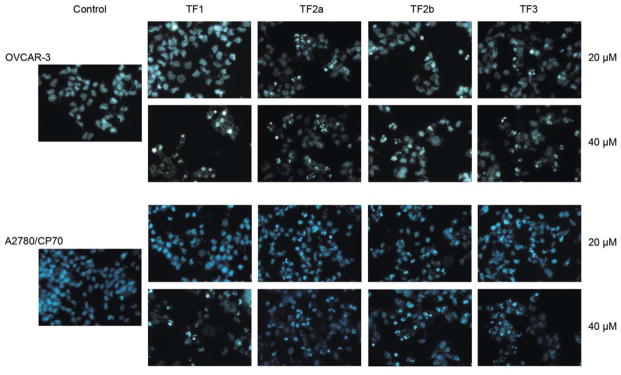
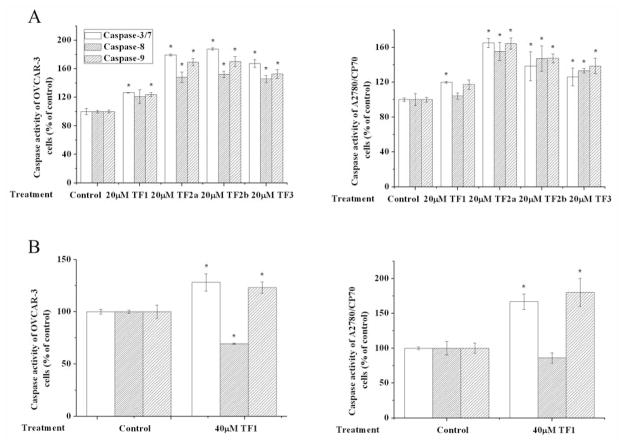
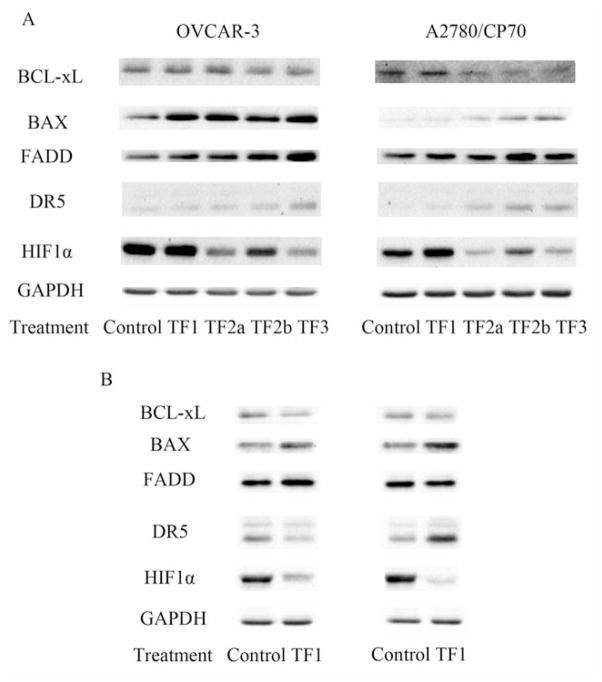
In the present study, all four theaflavin derivatives induced apoptosis in both OVCAR-3 and A2780/CP70 cells (Figure 3). TF2a, TF2b and TF3 exhibited more potent apoptosis-inducing activity than TF1 in both cells. TF2a, TF2b and TF3 significantly triggered apoptosis at 20 and 40 μM. No obvious morphological changes were observed in 20 μM TF1-treated OVCAR-3 and A2780/CP70 cells. When exposed to 40 μM TF1, nuclear fragmentation was observed in A2780/CP70 cells. For OVCAR-3 cells, 40 μM TF1 led to nuclear condensation rather than nuclear fragmentation (Figure 3). Nuclear condensation happens at an early apoptotic stage, while nuclear fragmentation takes place at a much later time (27). This finding hinted that TF1 took longer to mediate apoptosis of OVCAR-3 cells than did the other three theaflavin derivatives. The result of the caspase activity assay was in accordance with that of the Hoechst 33342 staining assay. The caspase-3/7, -8 and -9 activities of 20 μM TF2a, TF2b or TF3-treated OVCAR-3 and A2780/CP70 cells were significantly increased compared to that of the control group (Figure 4A). These results suggest that these three theaflavin derivatives might trigger apoptosis through intrinsic and extrinsic apoptotic pathways. Despite the fact that 20 μM TF1 failed to cause apoptosis, it did increase caspase-3/7 activity in both OVCAR-3 and A2780/CP70 cells, and increase caspase-9 activity in OVCAR-3 cells (Figure 4A). This observation can be explained by the fact that molecular changes occur before morphological changes. TF1 at 40 μM enhanced caspase-3/7 and caspase-9 activity in OVCAR-3 and A2780/CP70 cells, revealing TF1 mediated apoptosis via the intrinsic pathway (Figure 4B). Western blot analysis (Figure 5) demonstrated that all four theaflavin derivatives increased the proportion of pro-/antiapoptotic BCL2 family proteins to activate the intrinsic pathway in OVCAR-3 and A2780/CP70 cells. TF2a up-regulated the expression of FADD in OVCAR-3 cells and DR5 in A2780/CP70 cells to stimulate the extrinsic pathway. TF2b and TF3 potentiated the expression of FADD and/or DR5 in OVCAR-3 and A2780/CP70 cells to initiate the extrinsic pathway. Although TF1 had no impact on activating caspase-8, it did modify the expression of FADD in OVCAR-3 cells and DR5 in A2780/CP70 cells.
Figure 3.
Apoptosis-inducing effect of the four main theaflavin derivatives on OVCAR-3 and A2780/CP70 cells.
Figure 4.
Caspase-3/7, -8, -9 activities of theaflavin derivative-treated OVCAR-3 and A2780/CP70 cells. *p<0.05 compared to the control group.
Figure 5.
The expression of apoptotic-related and angiogenic-related proteins in theaflavin derivative-treated OVCAR-3 and A2780/CP70 cells. Bcl-xL: BCL2-like 1 isoform 1; BAX: BCL2-associated X protein; FADD: Fas-associated death domain; DR5: death receptor 5; HIF1α: hypoxia-inducible factor 1α; GAPDH: glyceraldehydes-3-phosphate dehydrogenase.
Taken together, TF2a, TF2b and TF3 were more effective than TF1 in inducing apoptosis of OVCAR-3 and A2780/CP70 cells. TF2a, TF2b and TF3 mediated apoptosis via both the intrinsic and extrinsic pathways, while TF1 mainly mediated apoptosis via the intrinsic pathway. Considering TF1 had a stronger cytotoxic effect and weaker apoptosis-inducing effect on OVCAR-3 cells than did TF2a and TF2b, we speculate that TF1 might have other mechanisms, such as induction of cell-cycle arrest, to retard cell proliferation. Previous reports have shown that theaflavins can induce G2/M arrest by modulating expression of p21, cdc25C and cyclin B in human prostate cancer cells (28). Further studies are required to verify this assumption.
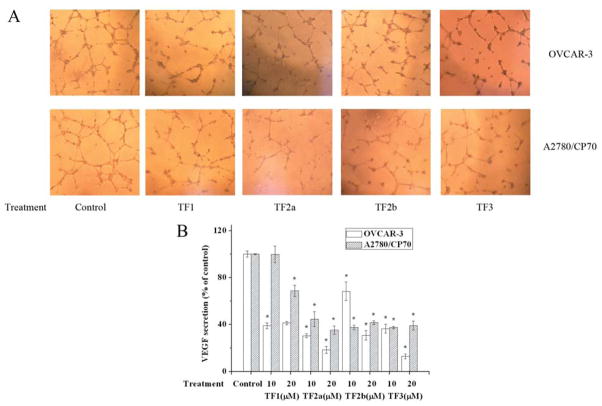
Angiogenesis is necessary for sustained tumor growth and plays a central role in the development and progression of cancer. Ovarian tumors are richly vascularized, and the degree of neovascularization and angiogenesis is associated with poor prognosis (29). Angiogenesis can be regulated by many signaling molecules and growth factors. Among them, VEGF is one of the most crucial factors. VEGF promotes proliferation and migration and inhibits apoptosis of vascular cells (30). The expression of VEGF can be directly regulated by hypoxia-inducible factor 1 (HIF1), a heterodimer consisting of HIF1α and HIF1β (31). Black tea polyphenols have been demonstrated to reverse carcinogen-induced angiogenesis through inhibiting VEGF expression (20, 21). In this study, OVCAR-3 and A2780/CP70 cells were treated with 0, 10 or 20 μM TF1, TF2a, TF2b or TF3 for 24 h. The cell culture supernatant was collected for HUVEC tube-formation assay and VEGF ELISA. All four theaflavin derivatives enlisted an excellent antiangiogenic effect (Figure 6). HUVECs exposed to the presence of culture supernatant from vehicle-treated cancer cells formed complex and well-organized capillary structures (Figure 6A). While HUVECs exposed to culture supernatant from theaflavin derivative-treated cancer cells formed leaky and less organized structures (Figure 6A). VEGF ELISA confirmed reduction of VEGF in culture supernatant from theaflavin derivative-treated cancer cells was responsible for this observation (Figure 6B). Most theaflavin derivative treatments caused over a 50% decrease of VEGF secretion in both ovarian cancer cell lines. Western blot analysis revealed TF2a, TF2b and TF3 lowered HIF1α expression (Figure 5A). These results suggest that TF2a, TF2b and TF3 reduced VEGF expression in an HIF1α-dependent way, while TF1 reduced it in an HIF1α-independent way.
Figure 6.
The antiangiogenic effect of theaflavin derivatives. A: Tube formation in human umbilical vein endothelial cells induced by medium from theaflavin derivative-treated OVCAR-3 and A2780/CP70 cells. B: Vascular endothelial growth factor secretion of theaflavin derivative-treated OVCAR-3 and A2780/CP70 cells. *p<0.05 compared to the control group.
In general, our research shows that the four main theaflavin derivatives in black tea preferentially inhibited the proliferation of ovarian cancer cells. Inducing apoptosis and reducing tumor angiogenesis are involved in the inhibitory effect of theaflavin derivatives. Among these four theaflavin derivatives, TF2a, TF2b and TF3 showed similarities in their anticancer functions and mechanisms. TF1 seemed to have different anticancer mechanisms. Comparing the chemical structures of the four main theaflavin derivatives, all of them except TF1 contain at least one gallate group at the R1 and/or R2 position (Figure 1). This observation suggests that the gallate group at R1 or R2 position is related to the anticancer activity of theaflavin derivatives. Former studies elucidated that the number and position of the gallate groups had an impact on the antioxidant activities of theaflavin derivatives (32). However, it was not noted whether there was a relationship between the anticancer activity and the number and position of the gallate groups. Further studies are needed to define more clearly the role of gallate in the anticancer properties of theaflavins.
Conclusion
The four main theaflavin derivatives in black tea, TF1, TF2a, TF2b and TF3, had inhibitory effects on OVCAR-3 and A2780/CP70 ovarian cancer cells, but were less cytotoxic to normal ovarian cells. These four theaflavin derivatives effectively induced apoptosis of both ovarian cancer cell lines. TF2a, TF2b and TF3 mediated apoptosis via the intrinsic and extrinsic apoptotic pathways. TF1 predominantly induced apoptosis through the intrinsic apoptotic pathway. Moreover, TF2a, TF2b and TF3 impaired tumor angiogenesis via reducing VEGF secretion of both ovarian cancer cells lines in an HIF1α-dependent way. TF1 reduced VEGF secretion of both ovarian cancer cell lines in an HIF1α-independent way. Our study provides evidence that theaflavin derivatives can be regarded as potent apoptosis-inducing and anti-angiogenic agents. Among the four theaflavin derivatives, TF2a, TF2b and TF3 exhibited similar anticancer activities. TF1 exhibited some differences in its anticancer effect and targets. The underlying mechanisms remain unclear.
Acknowledgments
The Authors acknowledge financial support by NIH grants P20RR016477 from the National Center for Research Resources and P20GM103434 from the National Institute for General Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network of Biomedical Research Excellence. They also acknowledge financial support from Collaborative Innovation Center of Chinese Oolong Tea Industry (Grant No. 2015-75), the National Natural Science Foundation of China (Grant No. 31501474) and the National Natural Science Foundation of Zhejiang Province (Grant No. LY15C200007).
References
- 1.Mizuno T, Suzuki N, Makino H, Furui T, Morii E, Aoki H, Kunisada T, Yano M, Kuji S, Hirashima Y, Arakawa A, Nishio S, Ushijima K, Ito K, Itani Y, Morishige K. Cancer stem-like cells of ovarian clear cell carcinoma are enriched in the aldh-high population associated with an accelerated scavenging system in reactive oxygen species. Gynecol Oncol. 2015;137(2):299–305. doi: 10.1016/j.ygyno.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Chen AY, Huang H, Ye X, Rollyson WD, Perry HE, Brown KC, Rojanasakul Y, Rankin GO, Dasgupta P, Chen YC. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the akt pathway. Int J Oncol. 2015;46(6):2629–2638. doi: 10.3892/ijo.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong RS. Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger RA. Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol Oncol. 2011;121(1):230–238. doi: 10.1016/j.ygyno.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX Gynecologic Oncology G. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 7.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: A potential source for investigational new agents to treat cancer-part 1. Curr Oncol. 2006;13(1):14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preedy VR. Tea in Health and Disease Prevention. Elsevier/Academic Press; London, Waltham, MA: 2013. [Google Scholar]
- 9.Baker JA, Boakye K, McCann SE, Beehler GP, Rodabaugh KJ, Villella JA, Moysich KB. Consumption of black tea or coffee and risk of ovarian cancer. Int J Gynecol Cancer. 2007;17(1):50–54. doi: 10.1111/j.1525-1438.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Sen G, Bera B. Black tea as a part of daily diet: A boon for healthy living. International journal of Tea Science. 2013;9:51–59. [Google Scholar]
- 11.Konarikova K, Jezovicova M, Kerestes J, Gbelcova H, Durackova Z, Zitnanova I. Anticancer effect of black tea extract in human cancer cell lines. Springerplus. 2015;4:127. doi: 10.1186/s40064-015-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu YY, Tang AB, Watanabe N. The theaflavin monomers inhibit the cancer cells growth in vitro. Acta Biochim Biophys Sin (Shanghai) 2004;36(7):508–512. doi: 10.1093/abbs/36.7.508. [DOI] [PubMed] [Google Scholar]
- 13.Khan M, Maryam A, Qazi JI, Ma T. Targeting apoptosis and multiple signaling pathways with icariside ii in cancer cells. Int J Biol Sci. 2015;11(9):1100–1112. doi: 10.7150/ijbs.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu TG, Huang Y, Cui DD, Huang XB, Mao SH, Ji LL, Song HB, Yi C. Inhibitory effect of ginsenoside rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9:250. doi: 10.1186/1471-2407-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7(12):3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuete V, Eichhorn T, Wiench B, Krusche B, Efferth T. Cytotoxicity, anti-angiogenic, apoptotic effects and transcript profiling of a naturally occurring naphthyl butenone, guieranone a. Cell Div. 2012;7(1):16. doi: 10.1186/1747-1028-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Chen LJ, Liu L, Chen X, Chen PL, Yang G, Hou WL, Tang MH, Zhang F, Wang XH, Zhao X, Wei YQ. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp Mol Med. 2008;40(6):617–628. doi: 10.3858/emm.2008.40.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong SW, Jung KH, Lee HS, Choi MJ, Zheng HM, Son MK, Lee GY, Hong SS. Apoptotic and anti-angiogenic effects of Pulsatilla koreana extract on hepatocellular carcinoma. Int J Oncol. 2012;40(2):452–460. doi: 10.3892/ijo.2011.1204. [DOI] [PubMed] [Google Scholar]
- 19.Safia M, AHA, IHAMS, AAZA, El-AllA Effect of green tea extract on the apoptotic and anti-angiogenic phenomena in peripheral blood of breast cancer patients. Egyptian J Exp Biol (Zool) 2010;6(1):117–127. [Google Scholar]
- 20.Letchoumy PV, Mohan KV, Prathiba D, Hara Y, Nagini S. Comparative evaluation of antiproliferative, antiangiogenic and apoptosis inducing potential of black tea polyphenols in the hamster buccal pouch carcinogenesis model. J Carcinog. 2007;6:19. doi: 10.1186/1477-3163-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugan RS, Mohan KV, Uchida K, Hara Y, Prathiba D, Nagini S. Modulatory effects of black tea polyphenols on oxidant-antioxidant profile and expression of proliferation, apoptosis, and angiogenesis-associated proteins in the rat forestomach carcinogenesis model. J Gastroenterol. 2007;42(5):352–361. doi: 10.1007/s00535-007-2018-z. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Ho CT, Ghai G, Chen KY. Differential effects of theaflavin monogallates on cell growth, apoptosis, and COX-2 gene expression in cancerous versus normal cells. Cancer Res. 2000;60(22):6465–6471. [PubMed] [Google Scholar]
- 23.Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807(6):735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 25.Kalra N, Seth K, Prasad S, Singh M, Pant AB, Shukla Y. Theaflavins induced apoptosis of LNCAP cells is mediated through induction of p53, down-regulation of NF-kappa B and mitogen-activated protein kinases pathways. Life Sci. 2007;80(23):2137–2146. doi: 10.1016/j.lfs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Lahiry L, Saha B, Chakraborty J, Adhikary A, Mohanty S, Hossain DM, Banerjee S, Das K, Sa G, Das T. Theaflavins target FAS/caspase-8 and AKT/pBAD pathways to induce apoptosis in p53-mutated human breast cancer cells. Carcinogenesis. 2010;31(2):259–268. doi: 10.1093/carcin/bgp240. [DOI] [PubMed] [Google Scholar]
- 27.Collins JA, Schandi CA, Young KK, Vesely J, Willingham MC. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45(7):923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- 28.Prasad S, Kaur J, Roy P, Kalra N, Shukla Y. Theaflavins induce G2/M arrest by modulating expression of P21WAF1/CIP1, CDC25C and cyclin B in human prostate carcinoma PC-3 cells. Life Sci. 2007;81(17–18):1323–1331. doi: 10.1016/j.lfs.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, Chen YC. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J Funct Foods. 2015;15:464–475. doi: 10.1016/j.jff.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase AKT. Circ Res. 2000;86(8):892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 31.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YY, Li W, Xu Y, Jin EH, Tu YY. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J Zhejiang Univ Sci B. 2011;12(9):744–751. doi: 10.1631/jzus.B1100041. [DOI] [PMC free article] [PubMed] [Google Scholar]