Abstract
The neural underpinnings of delayed automatic postural responses in people with multiple sclerosis (PwMS) are unclear. We assessed whether white matter pathways of two supraspinal regions (the cortical proprioceptive Broadman’s Area-3; and the balance/locomotor-related pedunculopontine nucleus) were related to delayed postural muscle response latencies in response to external perturbations. 19 PwMS (48.8±11.4 years; EDSS=3.5 (range: 2–4)) and 12 healthy adults (51.7±12.2 years) underwent 20 discrete, backward translations of a support surface. Onset latency of agonist (medial-gastrocnemius) and antagonist (tibialis anterior) muscles were assessed. Diffusion tensor imaging assessed white-matter integrity (i.e. radial diffusivity) of cortical proprioceptive and balance/locomotor-related tracts. Latency of the tibialis anterior, but not medial gastrocnemius was larger in PwMS than control subjects (p=0.012 and 0.071, respectively). Radial diffusivity of balance/locomotor tracts was higher (worse) in PwMS than control subjects (p=0.004), and was significantly correlated with tibialis (p=0.002), but not gastrocnemius (p=0.06) onset latency. Diffusivity of cortical proprioceptive tracts were not correlated with muscle onset. Lesions in supraspinal structures including the pedunculopontine nucleus balance/locomotor network may contribute to delayed onset of postural muscle activity in PwMS, contributing to balance deficits in PwMS.
Keywords: Multiple Sclerosis, Radial Diffusivity, postural responses, muscle onset latency, pedunculopontine nucleus
INTRODUCTION
Automatic postural responses (APRs) to external perturbations such as a slip or trip are critical for fall prevention [1]. APRs are significantly delayed in people with multiple sclerosis (PwMS)[2, 3], and are related to falls [4]. The cause of delayed postural responses in PwMS are poorly understood because the neural circuitry responsible for postural responses is unclear [5].
Spinal conduction of ascending somatosensory information is correlated to APR latency, suggesting disrupted and distorted electrical signaling from muscles contributes to delayed postural responses [2]. Recent work shows that structural connectivity of cortical proprioceptive networks (i.e. white matter emanating from Broadman’s Area-3; BA3) is related to postural control deficits in PwMS such as increased postural sway [6]. Furthermore, brainstem regions including the pedunculopontine nucleus (PPN) and pontomedulary reticular formation are critical for APRs [7, 8], and likely comprise components of a supraspinal balance/locomotion network [9]. While PwMS show pathology within these brainstem regions [10], it remains unknown whether changes in supraspinal structures contribute to altered APRs in this population.
Therefore, our goal was to assess the relationship between APRs and structural connectivity of two supraspinal networks (cortical proprioceptive network, and PPN-balance/locomotion network) in PwMS.
METHODS
Participants
These data are a subset of those reported in [6] on whom we also collected APR latencies. Complete datasets of 19 PwMS (age [mean±SD]: 48.8±11.4 years; 17 female; Expanded Disability Status Scale (EDSS): 3.5, range: 2–4; Mini Balance Evaluation Systems Test (MiniBEST): 22.0±4.7) were analyzed. Fourteen PwMS were relapsing-remitting, 2 secondary-progressive, 2 primary-progressive and 1 was progressive-relapsing. Twelve healthy adults (age: 51.7±12.2 years; 9 female, MiniBEST: 25.4±1.8) were selected as age and sex-matched controls.
Procedures
Postural responses
We assessed postural responses for 20 backward support-surface translations. For purposes unrelated to the current analysis, the 20 perturbations consisted of 4 blocks of 5 trials. Each block consisted the same velocity (15 cm/s), but different amplitude: 3.6 cm, 6.4 cm, 8.0 cm, and 12 cm. Perturbations were delivered in this small-to-large order. This perturbation delivery did not significantly affect muscle-onset latency, as average latency across amplitude blocks was similar (p>0.05) for both groups. Surface electromyography (EMG) was collected bilaterally from the tibialis anterior (TA) and medial gastrocnemius (MG) to assess muscle-onset latency.
MRI assessment
Participants underwent MRI neuroimaging, described in detail elsewhere [6], via a 3.0 T Siemens Magnetom Tim Trio scanner. A whole-brain echoplanar imaging sequence was used (TR=9,100 ms, TE=88 ms, field of view=240 mm2, b-value=1,000 s/mm2, isotropic voxel dimensions=2 mm3); images were sensitized for diffusion along 90 different directions with a b-value of 1000 s/mm2. For every 36 diffusion-weighted images, a non-diffusion weighted image (b=0 s/mm2) was acquired (three total).
Cortical Proprioceptive Pathway
The cortical proprioceptive tract ascends the dorsal column, synapses in the ipsilateral gracile nucleus, and then follows the medial lemniscus to the contralateral ventral-posterolateral nucleus of the thalamus and terminates in the primary somatosensory cortex [11].
PPN Pathway
The PPN is located in the mesencephalic reticular formation and has ascending supratentorial axonal projections reaching numerous targets including the internal globus pallidus, thalamus, and sensorimotor cortices, along with descending projections to the cerebellum and spinal cord.
Based upon previous work [6,12], all regions of interest (ROIs) were created in MNI 1 mm space and affine-transformed into each participant’s native diffusion space to carry out tractography.
Data analyses
Muscle onset latency
Muscle onset after perturbation was identified via a semi-automated program (Matlab, Natick, NJ), which determined the first instance of either the left or right leg in which muscle activity increased above 2 standard deviations of standing EMG and remained above for >25 ms. This was completed separately for TA and MG muscles. If onset-latency was larger than 300 ms or less than 50 ms, data from this trial were discarded, as these represent highly atypical (>300 ms) or non-physiological (<50 ms) postural responses.
Diffusion Tensor Imaging Analysis
Diffusion data were processed using tools implemented in FMRIB Software Library (FSL; Version 5.0) using standard processing techniques [6, 12]. Briefly, data were corrected for eddy current distortions and motion artifacts, averaged to improve signal-to-noise ratio [13] and subsequently skull-stripped. Probabilistic fiber tracking (using FDT 1.0 [14]) was initiated from every voxel within the binarized seed ROI in each participant’s native diffusion space. Streamline samples (25,000) were sent out from each voxel, with a step length of 0.5 mm and a curvature threshold of 0.2. For group analyses, the probabilistic connectivity distribution maps from individual participants were thresholded at 50% (thus selecting all connections where >12,500 of 25,000 samples passed [12, 15]. Radial diffusivity, an indirect neural marker of myelination [16], was calculated for each participant by taking the mean of the second and third eigenvalues– (λ2+λ3)/2. Lower radial diffusivity is interpreted as better white matter tract microstructure. After probabilistic fiber tracking, radial diffusivity for each tract was averaged across hemispheres.
Statistical analyses
Independent sample t-tests compared muscle-onset latency of TA and MG as well as radial diffusivity of PPN and BA-3 tracts across groups. Spearman’s rho (r[s]) correlation statistics were used to correlate EMG and radial diffusivity to protect against outliers given the relatively small sample size. Data are reported as mean and standard deviation. Significance level (alpha) was set to 0.05.
RESULTS
Muscle onset latencies
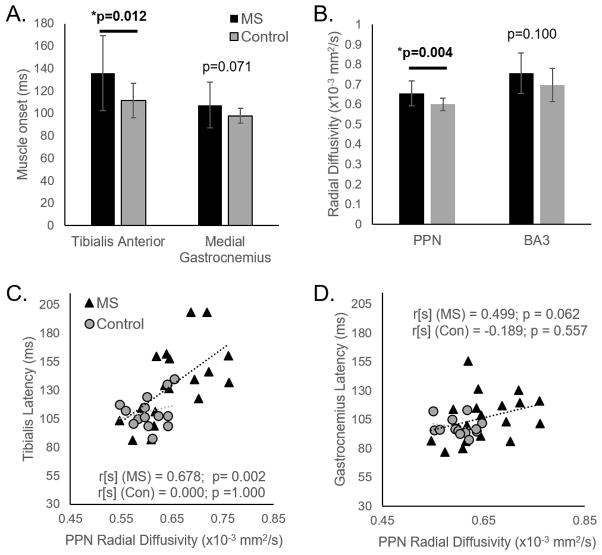
Latency of the TA (antagonist) was later in PwMS compared to healthy adults (MS:136.0±33.3 ms; Control:111.5±15.5 ms; p=0.012). Latency of the MG (agonist) was not different between groups (MS:107.3±20.4 ms; Control:97.7±6.5 ms; p=0.071; Figure 1A).
Figure 1.
Means and standard deviations of (A) Muscle onset latency after perturbations in the tibialis anterior (TA) and medial gastrocnemius (MG) and (B) radial diffusivity of pedunculopontine nucleus (PPN) and Broadman’s Area-3 (BA3) regions are shown. Lower panels show the correlation between PPN radial diffusivity and muscle onset latency after perturbations in the (C) tibialis, and (D) gastrocnemius.
Radial diffusivity
White matter fiber tract integrity (radial diffusivity) of BA-3 cortical proprioceptive tracts was not different between groups (p=0.100). However, PPN-balance/locomotor tract radial diffusivity was larger (worse) in PwMS compared to healthy adults (p=0.004; Figure 1B).
Correlation analyses
In PwMS, diffusivity of PPN-balance/locomotor tracts was significantly correlated with muscle onset latency. This relationship was stronger in the antagonistic TA (r[s]=0.68, p=0.002), than the agonist MG (r[s]=0.45, p=0.062; Table 1, Figure 1C&D). BA3-cortical proprioceptive tracts were not significantly correlated to agonist or antagonist muscle onset (Table 1).
Table 1.
Spearman’s Rho r[s] correlation statistics and p-values relating muscle onset latency and radial diffusivity in PwMS and healthy controls
| PPN (r[s]; p) | BA3 (r[s]; p) | |
|---|---|---|
| PwMS- TA | 0.678; 0.002 | 0.284; 0.254 |
| PwMS- MG | 0.449; 0.062 | 0.230; 0.344 |
| Control- TA | 0.000; 1.000 | 0.322; 0.308 |
| Control- MG | −0.189; 0.557 | 0.488; 0.145 |
Abbreviations: TA: Tibialis Anterior; MG: Medial Gastrocnemius; PPN: Pedunculopontine Nucleus; BA3: Broadman’s Area-3; PwMS: people with multiple sclerosis
DISCUSSION
Structural integrity of the PPN-balance/locomotion network was significantly related to delayed postural muscle latencies in response to external perturbations. This suggests the PPN-balance/locomotion network plays a role in postural response dysfunction in PwMS.
Brainstem structures are critical for APRs. Human [7] and animal [8] models suggest that basal ganglia-cortical loops may pre-select APRs, which are then stored-in and released-from brainstem structures (for reviews see [5, 17]). The current study extends these findings, showing that dysfunction of brainstem white matter tracts play a specific role in delayed postural responses in PwMS.
Cortical proprioceptive connectivity is correlated with postural sway [6]; however was not significantly correlated to postural response latency in the current study. Previous work suggests that short-latency, feet-in-place postural responses may rely more heavily on subcortical brainstem regions, whereas longer latency, stepping responses involve more cortical structures [5]. Indeed, in the current study feet-in-place postural responses were correlated to white matter tracts emanating from brainstem regions, but not from cortical proprioceptive regions.
Unlike previous investigations showing significantly delayed MG onset in PwMS with respect to healthy adults [2], we observed only a trend (p=0.071) in this direction. This could relate to the relatively mild MS population in our analysis. Similarly, we did not observe significantly worse diffusivity of cortical proprioceptive tracts in PwMS compared to healthy adults. The subset of participants in the current study had less severe balance dysfunction than the sample reported in [6] (mean MiniBEST score 22.0 vs. 20.4). Given the relationship between balance dysfunction and cortical proprioceptive tract integrity [6], the better balance scores in the current dataset could explain the similar cortical proprioceptive diffusivity across groups.
Interestingly, the correlation between PPN diffusivity and postural response latency was stronger with the antagonist TA than the agonist MG. The non-significance (p=0.06) of the correlation between PPN diffusivity and MG latency may be due to the lack of dysfunction and relatively small variance in MG onset-latency in the MS group. Alternatively, the more pronounced relationship between antagonist (TA) latency and PPN connectivity may relate to alterations in PPN-cerebellar connectivity. The cerebellum is critical for coordinating and timing antagonistic EMG bursts after perturbations and for ballistic, voluntary movements (for review, see [18]). Given the dense connectivity between the PPN and the cerebellum, alterations in PPN-cerebellar connectivity may have driven the close relationship between PPN diffusivity and antagonistic muscle latency.
A limitation of this report is the delivery of multiple-amplitude perturbations. Although no differences were observed across amplitude blocks for either group, this delivery method may have incorporated variability into latency outcomes, reducing our ability to detect MG latency differences across groups.
CONCLUSION
This study shows that supraspinal connectivity is related to delayed postural responses in PwMS, demonstrating the importance of supraspinal regions in automatic responses in this population. However, additional research is necessary to fully understand the neural underpinnings of postural response dysfunction in PwMS.
Highlights.
Supraspinal causes of delayed automatic postural responses in people with MS are unknown
We correlated supraspinal structural connectivity to postural response latency
Pedunculopontine nucleus structural connectivity was worse in people with MS
Pedunculopontine nucleus connectivity was correlated to tibialis onset latency
Altered supraspinal connectivity may contribute to delayed postural responses in MS
Acknowledgments
Funding Statement
This work was supported by The United States Department of Veteran’s Affairs Rehabilitation Research and Development Service, grant numbers #I01BX007080 (Career Development Award-1; Peterson) and E10750R (VA Merit Award; Horak), the National Institutes of Health, grant number R01 AG006457 29 (Horak), the Medical Research Foundation of Oregon, and the National MS Society, grant numbers RG-5273 (Fling) FG 2058-A-1 (Gera) and MB-0027 (Horak). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank Heather Schlueter for assisting in participant recruitment, screening and data collection and Ed King for technical assistance. The experiments were conducted in the Balance Disorders Laboratory at the Oregon Health and Science University.
Footnotes
Competing Interests
Dr. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU. No other authors declare any conflict of interest.
Contributor Statement
Drs. Gera, Horak, and Fling contributed to the conception and design of the project. Drs. Gera, and Fling acquired data. All authors contributed to the analysis and interpretation of data. All authors contributed to writing and revising of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- 2.Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosensory & motor research. 2008;25:113–22. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huisinga JM, St George RJ, Spain R, Overs S, Horak FB. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehabil. 2014;95:1390–7. doi: 10.1016/j.apmr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27:526–33. doi: 10.1177/1545968313478486. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fling BW, Dutta GG, Schlueter H, Cameron MH, Horak FB. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front Hum Neurosci. 2014;8:814. doi: 10.3389/fnhum.2014.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, et al. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci. 2014;34:275–81. doi: 10.1523/JNEUROSCI.2948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol. 2009;101:1334–50. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- 9.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28:1483–91. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- 10.Lu Z, Zhang B, Qiu W, Kang Z, Shen L, Long Y, et al. Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PLoS One. 2011;6:e22766. doi: 10.1371/journal.pone.0022766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner M, Jessell . The bodily senses. In: Kandel Eric RJHS, Jessell Thomas M., editors. Principles of neural science. New York: McGraw-Hill; 2000. pp. 446–8. [Google Scholar]
- 12.Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–18. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–21. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex. 2012;22:1564–76. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- 16.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Bolton DA. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci Biobehav Rev. 2015;57:142–55. doi: 10.1016/j.neubiorev.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997;77:517–33. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]