Abstract
Context
Human and animal studies have suggested an underlying inflammatory mechanism for a variety of neuropsychiatric disorders, including schizophrenia. To date, most available reports focused on adult patients.
Objective
We wished to test the hypothesis that the first psychotic episode in youth is associated with inflammation.
Patients
We studied patients admitted to a pediatric inpatient psychiatric unit. Patients (n=80) had new-onset psychosis diagnosed using DSM-IV TR criteria for Psychosis NOS, Schizophreniform Disorder or Schizoaffective Disorder. Patients were matched for age, race and gender with inpatient controls without psychosis within the same unit (n=66). We also compared these values to normal pediatric hematologic values. To study the role of inflammation in youth with psychosis, we collected serum samples of 28 children presenting with first-episode psychosis and compared their serum cytokine and S100B levels to eight healthy controls.
Main Outcome Measures
In this study, we measured serum markers of systemic inflammation.
Results
Leukocyte counts revealed a statistically significant increase in absolute monocytes compared to patients without psychosis (0.61±0.282 k/ml vs. 0.496±0.14 k/ml; p<0.01) and lymphocytes (2.51±0.84 k/ml vs. 2.24±0.72 k/ml; p<0.05) in patients with psychosis. All other hematologic values were similar between the groups. In addition, psychosis was characterized by increased serum levels of S100B, a peripheral marker of blood-brain barrier (BBB) damage. Several inflammatory mediators (e.g., TNF-α, IL-1β, IL-6, IL-5, IL-10, and IFN-γ) were elevated in children with psychosis.
Conclusions
These results strongly support a link between systemic inflammation, blood-brain barrier disruption and first-episode psychosis in pediatric patients.
Keywords: Pediatric, Blood-Brain Barrier, Markers, Psychosis, Schizophrenia
Introduction
Schizophrenia occurs in approximately 1% of the population (1). Clinical onset typically occurs in late adolescence or early adulthood. Psychosis is characterized by hallucinations, delusions, disorganized thoughts, aggression and agitation. Although a combination of environmental and genetic features are necessary for the emergence of schizophrenia, its etiology remains poorly understood. Predisposing factors include heritability, obstetric complications, prenatal viral exposure and neurodevelopmental abnormalities. A relationship between inflammation and schizophrenia was initially proposed by Kraepelin in 1896 (2). Since that time, multiple studies have researched an infectious cause of schizophrenia in adults. These studies have implicated viruses (3–5), bacteria (6) and parasites (7, 8), while other studies explored a possible role for a sterile inflammatory response (9). Though numerous studies have linked congenital, neonatal and other infections to schizophrenia, the role of infection remains unproven. Bruce and Peebles described increased leukocyte levels during acute psychosis of patients with schizophrenia (10). Inflammatory markers were also altered in acute, chronic and residual stages of schizophrenia (11).
Several animal models have also explored the role of inflammation during pregnancy in the first and second trimester in schizophrenia (12–17). Meyer et al. described how maternal cytokines released in response to infection act as a crucial link for the development of schizophrenia, regardless of the initiating pathogen. Brain specimens were collected from offspring of mice after immune challenge at mid-pregnancy. Hippocampal reelin expression was decreased, while apoptosis was enhanced after immune activation. This group concluded that cytokine-related inflammatory processes in prenatal life are potential causal agents of postnatal brain pathology. The authors concluded that neurodevelopmental disturbances rather than neurodegeneration are central to the etiopathogenesis of schizophrenia (12). In another study, the same group concluded that the prenatal Poly I: C (Polyinosinic: polycytidylic acid) treatment represents one of the most powerful environmental-developmental models of schizophrenia available to date. This model unveils the neuropsychoimmunological mechanisms implicated in the developmental etiology of schizophrenia (12–15). Weiner et al. replicated the Poly I: C model to demonstrate ventricular enlargement and reduced hippocampus volume in Poly I: C rats compared with saline-injected animals. Clozapine, the antipsychotic drug with the most pronounced anti-inflammatory effects (18), given during pubertal day 34–47 prevents the emergence of both the cognitive and the structural abnormalities of schizophrenia (14, 16). Smith and Patterson demonstrated that IL-6 (Interleukin 6) is critical for the development of schizophrenia (13, 17).
However, a specific etiologic mechanism linking inflammation to adult-onset psychosis remains undetermined. Also, studies in pediatric populations of patients with psychosis are lacking. If inflammation is involved in early-onset psychosis, how do systemic events translate to altered brain function? Insights from epilepsy suggest a role for the blood-brain barrier. The blood-brain barrier (BBB) maintains CNS homeostasis by separating the brain and systemic blood circulation. The BBB is composed of endothelial cells, astroglia, pericytes, perivascular macrophages and a basal lamina (19, 20). Linked by tight junctions, these cells protect the brain from various blood-borne elements by obstructing paracellular transport of hydrophilic molecules. While transport is contingent on selective carrier mechanisms, diffusion across the endothelial cells is dependent on lipid solubility, molecular weight and charge or ionization of the compound (20). Dysfunction of the BBB has been established in many neurological diseases including multiple sclerosis, ischemia, epilepsy and psychiatric disorders (20, 21). Though this association is clear, whether BBB impairment is causative or a sequelae of these diseases remains uncertain (19, 21, 22). Confirmation of blood-brain barrier dysfunction or cellular damage occurs when an elevation of S100B, a calcium binding protein predominately in the brain, is found in the blood and cerebrospinal fluid (23–27). Multiple studies report that patients with schizophrenia have increased S100B during acute psychosis (28–34).
Clinical and experimental data have shown a direct link between cerebrovascular integrity failure and seizures where peripheral inflammation leading to blood-brain barrier disruption is a downstream event of leukocyte activation (20, 22, 25–27, 35, 36). In psychiatry, studies have consistently demonstrated increased monocytes in the blood of new-onset and chronic adults with schizophrenia. Recently, a report showed activated monocytes and inflammatory markers in the sons and daughters of patients with schizophrenia that later developed the disease (37–39).
Given the available information from other neurological diseases and adult patients with schizophrenia (11, 40, 41) and considering the virtual absolute lack of data from young or pediatric patients, we tested in children and adolescents the following hypotheses: 1) inflammatory mechanisms involving leukocytes are detectable in first-episode psychosis; and, 2) BBB leakage is present in patients diagnosed with first-psychotic episode.
Methods
A retrospective chart review identified patients that were admitted to the Child and Adolescent In-Patient Psychiatry Unit. An IRB-approved database was established to collect variables from the electronic medical charts and allows for statistical analysis. Complete blood count values measured by the Cleveland Clinic Department of Pathology were examined.
Inclusion criteria for patients with and without psychosis consisted of admittance to the inpatient unit at age >18 years. Psychosis was diagnosed by consensus of two child psychiatrists using the DSM-IV-TR criteria for first-episode psychosis: Psychosis NOS, Schizophreniform, or Schizoaffective Disorder was an inclusion criterion for the group with psychosis. Patient exclusion criteria included those older than 18 years, psychosis secondary to a known medical condition, moderate to severe mental retardation (IQ less than 70), autism, non-verbal agitation, schizophrenia diagnosed more than six months prior to admission, patients taking antibiotics or with fever on admission, patients on lithium or those diagnosed with bipolar disorder. Of the charts reviewed, 80 patients were admitted for new-onset psychosis. All patients with psychosis had at least one of these criteria: hallucinations, delusions or peculiar fantasies. These patients were then matched against a selected group of 66 patients without psychosis matched by age, gender, and race, history of substance abuse, abuse history, legal history, BMI and medications. We determined the extent and pattern of substance abuse in order to control for this variable in both groups. Thus, this variable was not significantly different between the two groups (with and without psychosis). In addition, we performed a study to determine if substance abuse (alcohol, opiates, cocaine, and amphetamines) has any effect on S100B levels. We found no correlation between levels of S100B and use of recreational drugs or substance abuse (data not shown).
Complete Blood Counts with differential values were examined for each patient with psychosis and without psychosis. WBC, neutrophil, lymphocyte, monocyte, eosinophil and basophil values—both absolute and percentage—were compared to a reference range provided by the Cleveland Clinic Department of Laboratory Medicine (42).
For analysis of a panel of inflammatory cytokines that included IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-a, C-Reactive Protein (CRP), TNF-β, and IL-5. The analysis was performed using a multiplex protein array technique described as the Q-Plex™ Human Cytokine Array. This is a fully quantitative ELISA-based test where up to 16 distinct capture antibodies can be absorbed to each well of a 96-well plate.
To assess the role of inflammation with more specific markers, a group of pediatric patients (n=28) with a diagnosis of first-episode psychosis were recruited using the same inclusion and exclusion criteria used in the inpatient sample. Patients were recruited after the first initial consultation for psychosis to the outpatient child psychiatry clinic. Data obtained from this group were compared to gender- and age-matched healthy controls (n=8). During this outpatient visit, a medical history and physical examination were performed. Blood samples were collected for chemistry and hematology analysis.
Serum levels were assessed after collection via antecubital venipuncture between 9 a.m. and 11 a.m. Blood was then centrifuged at 1,000 g for 10 minutes. Serum separated and stored at −80°C until assayed. Levels of PIMs were measured with commercial quantitative, high-sensitivity, enzyme-linked immunoassay (ELISA) kits according to the manufacturer’s instructions as described (www.quansysbio.com/ELISA/index.html). The Q-Plex™ Human Cytokine -Screen (16-plex) is a fully quantitative ELISA-based chemiluminescent assay allowing the concurrent measurement of 16 biomarkers. Extensive data and literature are available at Quansybio’s web site. Since the assay was not performed in-house, variability and consistency of results were not determined by us. We relied on extensive published work listed in Quansybio’s web site for quality control. Data in Figure 4 are presented as Log10 of the values to accommodate all data in a single plot for easy readout and comparison. A Bonferroni correction was used to account for multiple tests. The detection levels for each analyte were not investigated since all samples had a measurable amount of cytokines.
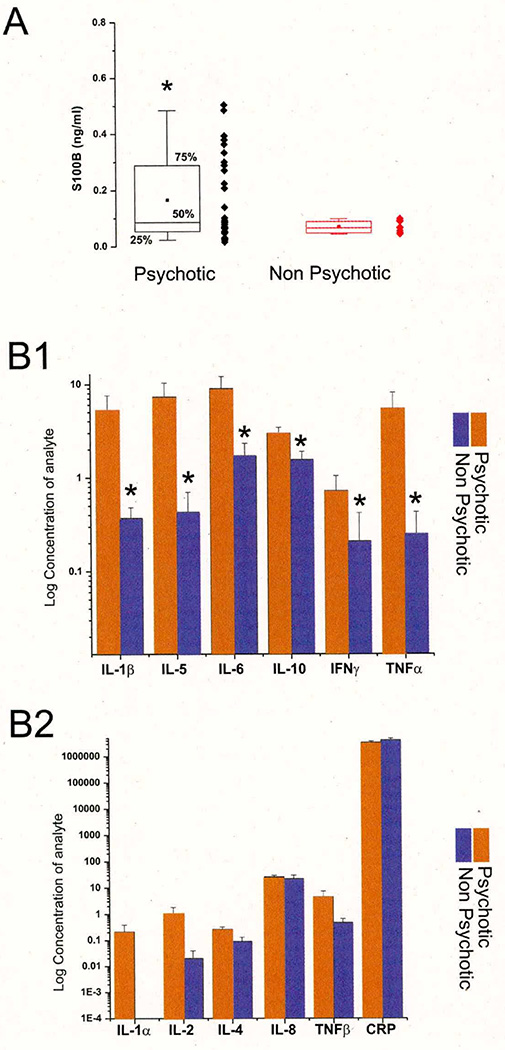
Figure 4. BBB Leakage in Patients Affected by First-Episode Psychosis.
A: Note the statistically significant increase in S100B in psychotic vs. non-psychotic patients. S100B was measured at the time of diagnosis (and admission) in both cases. Note that most of psychotic S100B serum values were above the 0.12 ng/ml threshold, which is commonly employed as a means to discriminate between blood-brain barrier disruption and integrity. In contrast, non-psychotic children had S100B values below threshold. B1: Increase in inflammatory mediators in serum of psychotic patients. Note that not all measured markers were elevated (see B2). * indicates p<0.05.
The medical records further revealed that 12 of the patients with psychosis had also been admitted to the Cleveland Clinic for reasons other than psychosis. These patients’ leukocyte counts were again documented during this second admission when patient was not psychotic. Leukocyte counts were compared between the two admissions.
Statistical Analysis
All statistical analyses were performed using JMP 9.0 Pro. A Student’s t-test was run to compare the two patient groups: with psychosis and without psychosis. The only statistically significant difference was found in absolute lymphocytes (T=2.1016, p=.0373*), absolute monocytes (T=3.1442, p=.002**) and S100B/cytokines (see Figure 4). In both of these cases, patients from the psychotic group had significantly elevated levels. These findings were confirmed when examining the data from patients who were admitted immediately after a psychotic episode and then subsequently for an outpatient, non-psychiatric exam. Using a matched pairs t-test comparing values from the two visits we found a significantly elevated level of absolute monocytes (T=−2.8407, p=.0161*) and a trend toward statistically elevated absolute lymphocytes values (T=−2.0365, p=.0665) when comparing data from the psychotic admission and the subsequent outpatient visit.
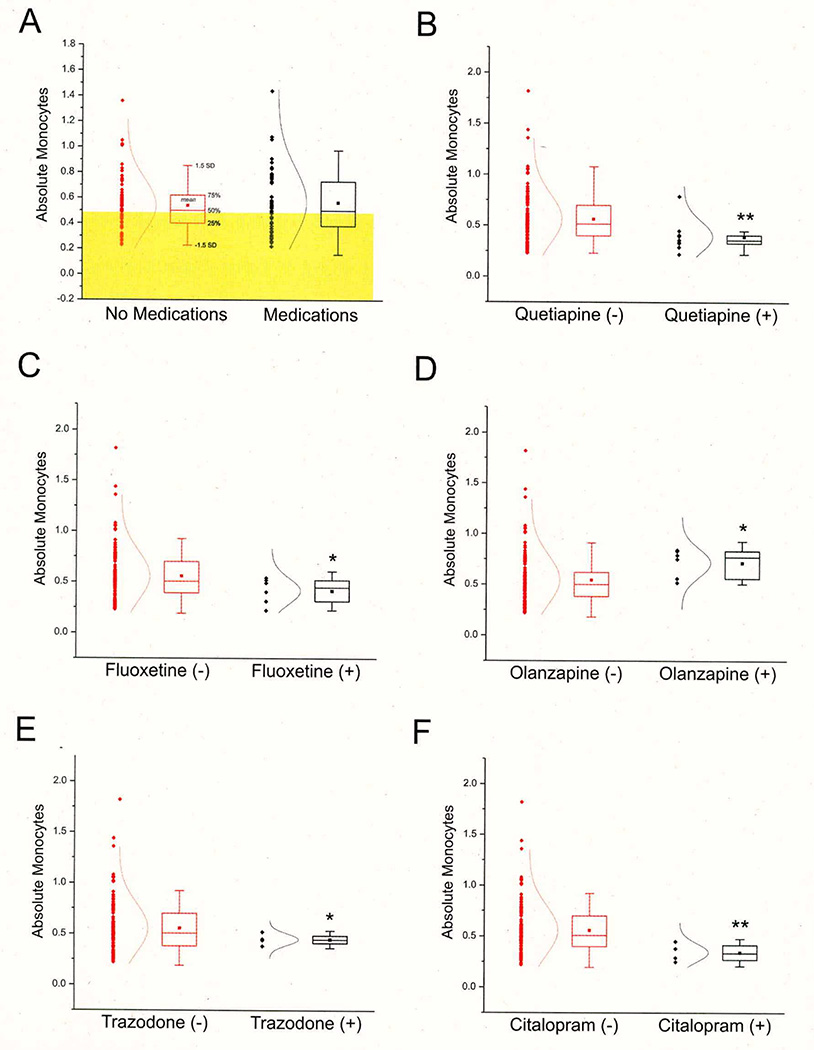
To measure the effect of drugs on absolute monocytes, again a Student’s t-test was used. We found no significant effect from medication in general (T=.6172, p=.5381) but a few specific therapeutic drugs had significant effects: quetiapine (T=−3.2088, p=.0071**), fluoxetine (T=−2.5889, p=.0375*), olanzapine (T=2.7922, p=.0295*), trazodone (T=−3.0607, p=.0189*) and citalopram (T=−4.3741, p=.0099**). However, in all cases except for olanzapine, the effects were in the opposite direction from what would be expected if drug treatment was the cause for increased monocyte levels. In other words, the effects of the drugs, if any, consisted of a decrease in leukocytes. While these findings were statistically significant, we acknowledge that the small sample size in each treatment group warrants further investigations.
A Fisher’s exact test was used to compare the rates of drug use between groups with and without psychosis. In all cases—except for quetiapine (p=.0227*)—we found no significant difference in drug regimen between patient groups. In other words, patients with or without psychosis were equally likely to being prescribed any given therapy. Please note that the sample size for each given drug was small (see Table 4). Also note that the number of patients on medication was low compared to what one expects in a population of individuals with psychosis. However, this was due to the fact that these patients were diagnosed with first-episode psychosis. In general, there was no significant difference in percent of patients on any medication in the groups with versus without psychosis (p=0.868). Note: a p-value <.05 is considered significant in all cases and is denoted as*; a p-value <.01 is denoted**.
Table 4.
Incidence of Therapeutic Drug Use in the Psychiatry Population Used for this Study Reveals a Few Differences between the Psychotic and Non-Psychotic Groups
| Drug Taken | Psychotic Percent of Total (N=) |
Non-Psychotic Percent of Total (N=) |
P-Value | |
|---|---|---|---|---|
| Atypical Antipsychotic | ||||
| Ziprasidone | 2.50 (2) | 1.52 (1) | 1.0000 | |
| Quetiapine | 11.25 (9) | 1.52 (1) | 0.0227* | |
| Olanzapine | 5.00 (4) | 3.03 (2) | 0.6899 | |
| Risperidone | 11.25 (9) | 18.18 (12) | 0.2474 | |
| Aripiprazole | 5.00 (4) | 4.55 (3) | 1.0000 | |
| Typical Antipsychotic | ||||
| Perphenazine | 1.25 (1) | 0 (0) | 1.0000 | |
| Pimozide | 0 (0) | 1.52 (1) | 0.4521 | |
| Fluphenazine | 3.75 (3) | 0 (0) | 0.2518 | |
| Bupropion | 5.00 (4) | 1.52 (1) | 0.3781 | |
| Thiothixene | 0 (0) | 1.52 (1) | 0.4521 | |
| Haloperidol | 0 (0) | 1.52 (1) | 0.4521 | |
| Antidepressant | ||||
| Mirtazapine | 0 (0) | 1.52 (1) | 0.4521 | |
| Venlafaxine | 2.50 (2) | 3.03 (2) | 1.0000 | |
| Trazodone | 1.25 (1) | 4.55 (3) | 0.3283 | |
| Atomoxetine | 5.00 (4) | 3.03 (2) | 0.6899 | |
| SSRIs | ||||
| Citalopram | 3.75 (3) | 1.52 (1) | 0.6268 | |
| Sertraline | 11.25 (9) | 3.03 (2) | 0.1118 | |
| Fluoxetine | 1.25 (1) | 7.58 (5) | 0.0913 | |
| Paroxetine | 0 (0) | 1.52 (1) | 0.4521 | |
| Escitalopram | 1.25 (1) | 1.52 (1) | 1.0000 | |
| Anticonvulsants | ||||
| Topiramate | 1.25 (1) | 0 (0) | 1.0000 | |
| Clonazepam | 3.75 (3) | 0 (0) | 0.2518 | |
| Lamotrigine | 1.25 (1) | 0 (0) | 1.0000 | |
| Oxcarbazepine | 5.00 (4) | 3.03 (2) | 0.6899 | |
| Valproate | 7.50 (6) | 6.06 (4) | 1.0000 | |
| Stimulants | ||||
| Methlyphenidate | 6.25 (5) | 9.09 (6) | 0.5453 | |
| Methylphenidate | 2.50 (2) | 3.03 (2) | 1.0000 | |
| Amphetamine | 10.00 (8) | 16.67 (11) | 0.3233 | |
| Other Medications | ||||
| Clonidine | 2.50 (2) | 4.55 (3) | 0.6584 | |
| Benztropine | 3.75 (3) | 3.03 (2) | 1.0000 | |
| Eszopiclone | 1.25 (1) | 0 (0) | 1.0000 | |
| Digoxin | 1.25 (1) | 0 (0) | 1.0000 | |
| Buspirone | 0 (0) | 1.52 (1) | 0.4521 | |
| Lithium | 0 (0) | 3.03 (2) | 0.2026 | |
p-values <.05.
Bold type highlights statistically significant differences.
Results
Over three years 1,500 children were admitted to our inpatient child and adolescent psychiatry service. Of these, 80 were admitted with a positive diagnosis of psychosis and matched the inclusion criteria of our study. The 66 nonpsychotic patients were admitted for diverse reasons: Attention Deficit Hyperactivity Disorder (n=12), Oppositional Defiant Disorder (n=8), Depression (n=16), Mood Disorder (n=8) or other behavioral disorders (n=22). These patients served as controls without psychosis. Table 1 shows demographic characteristics of these two groups of children. The criteria for admission are presented in Table 2.
Table 1.
Demographic Data for the Population Enrolled in the Study
| Demographic Summary | |||||
|---|---|---|---|---|---|
| Age | N | Mean Age | SD | Min | Max |
| Psychotic | 80 | 13.6 | 2.89 | 6 | 18 |
| Non-Psychotic | 66 | 13.7 | 2.8 | 6 | 18 |
| Gender | Male | Female | |||
| Psychotic | 50 | 30 | |||
| Non-Psychotic | 36 | 30 | |||
| Race | White | Non-Caucasian | |||
| Psychotic | 42 | 38 | |||
| Non-Psychotic | 39 | 27 | |||
Note: Due to study design and matching by race, age and gender, no differences were found between the psychotic and non-psychotic patients.
Table 2.
Summary of Criteria for Inclusion and Exclusion for this Study
| Inclusion Criteria for Study |
| • Admission to the Cleveland Clinic Child and Adolescent Psychiatric Inpatient Unit |
| • Age 18 years or under |
| • Presence of first psychotic episode (for the psychotic group only) |
| Exclusion Criteria for Study |
| • Age over 18 years |
| • Psychosis secondary to a medical condition |
| • Moderate or severe mental retardation |
| • Autism |
| • Non-verbal patient |
| • Schizophrenia diagnosed more than six months prior to admission |
| • Known infection of inflammatory process |
| • Presence of a first-psychotic episode (for the non-psychotic group only) |
| Additional Restrictions |
• To be defined as “psychotic,” patients must exhibit at least one of the following:
|
| • Patients must have Complete Blood Counts and Auto-Differential Values available for time of admission |
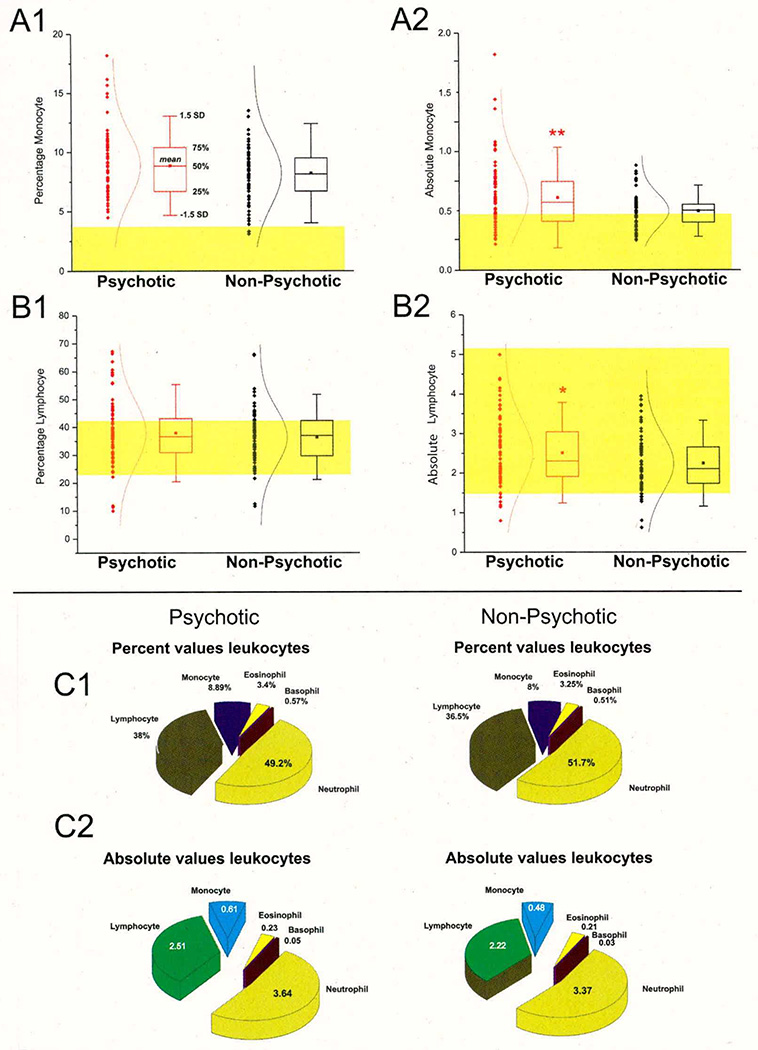
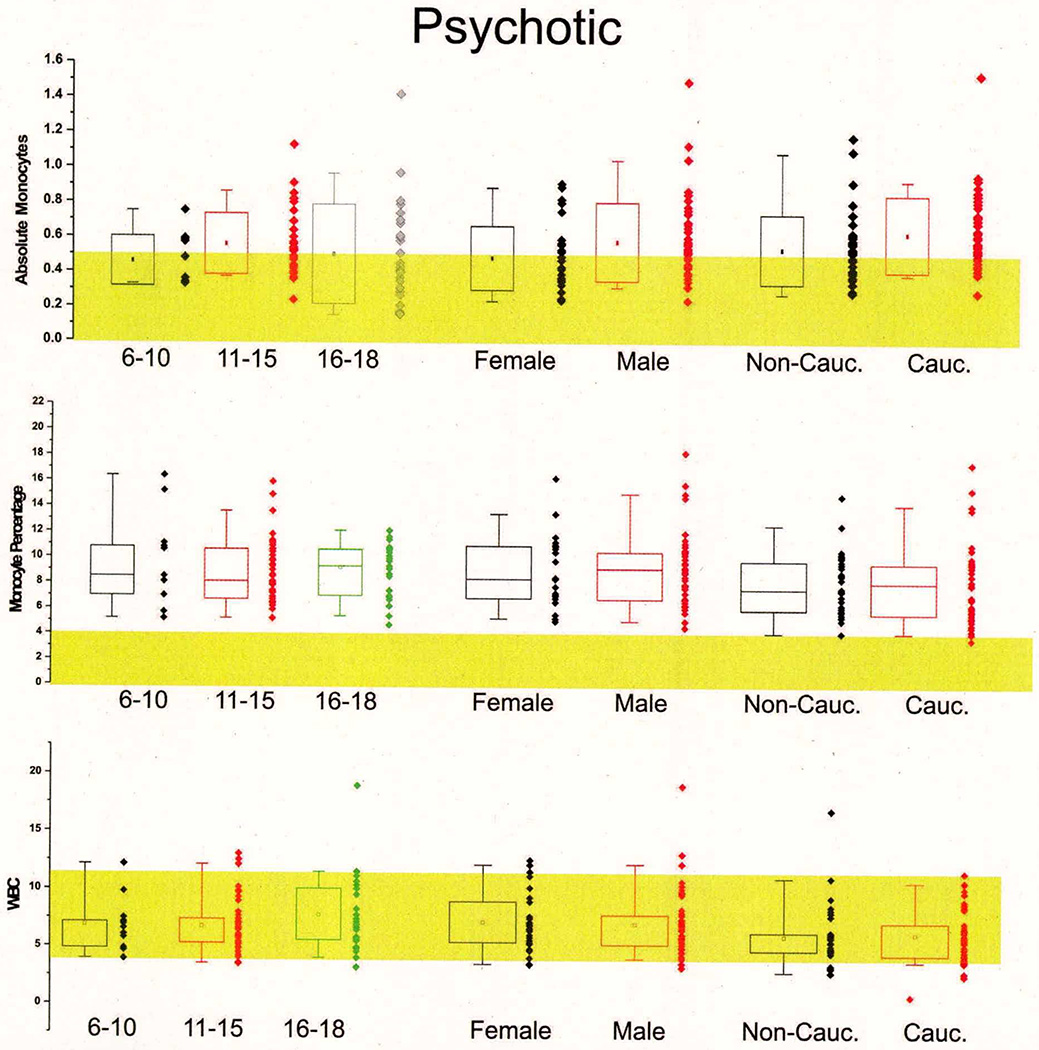
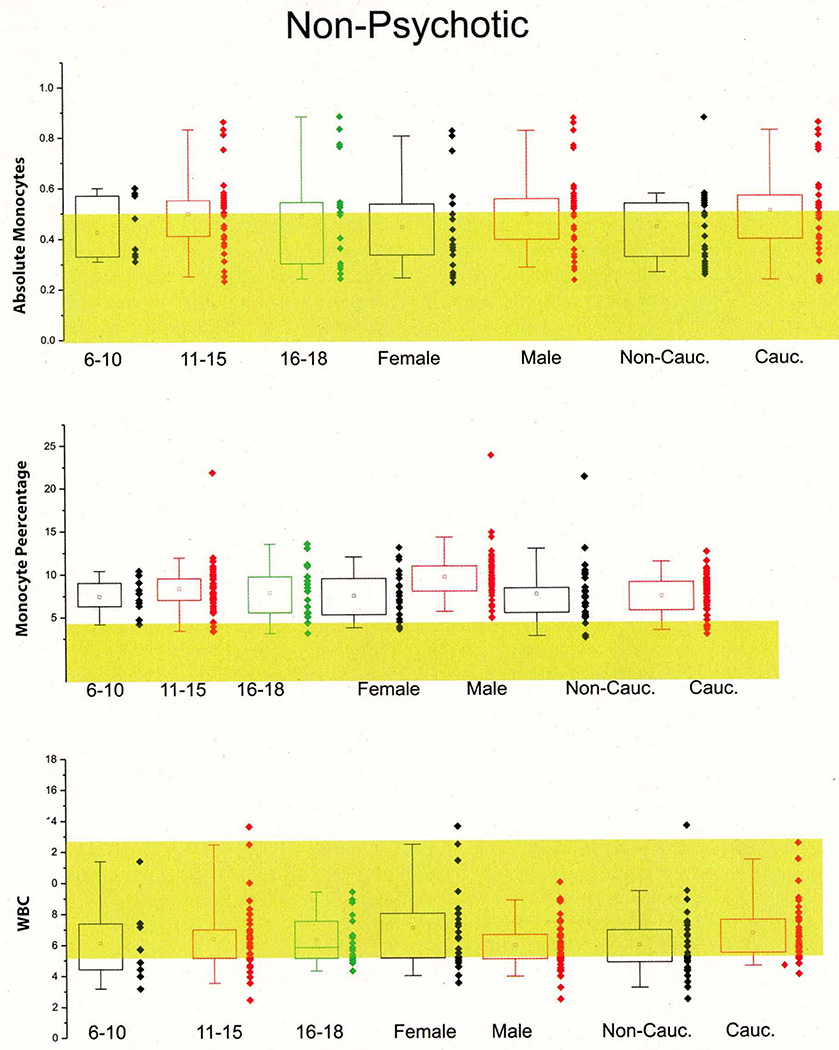
When comparing the hematologic values—summarized in Table 3 and shown in Figure 1—we found only two statistically significant differences between children who experienced a psychotic episode compared to those who were affected by other psychiatric illnesses, namely the elevated absolute lymphocyte and monocyte counts in children with psychosis (see Figure 1 [A, B]). The percent values of monocyte and lymphocytes, however, were nearly identical between cohorts. The complete breakdown of percent and absolute values for white blood cells in the two groups is shown in Table 3. Despite the fact that lymphocyte numbers were within normal range (indicated by the yellow shaded area), most of the children enrolled in this study had elevated monocyte counts (normal range is indicated again by the yellow shaded area) regardless of their psychotic or non-psychotic admission.
Table 3.
Hematologic Values for Psychotic and Non-Psychotic Children
| WBC (K/µL) |
RBC (M/µL) |
HGB (g/dL) |
HCT % |
Platelet (K/µL) |
MCV (fL) |
MCHC % |
MCH (pg) |
MPV (fL) |
RDW- CV % |
Neutrophil % |
ABS Neutro (k/µL) |
Lympho- cyte % |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
MEAN (Psychotic N=80) |
7.044 | 4.790 | 14.059 | 40.441 | 280.601 | 83.285 | 33.731 | 28.270 | 13.970 | 13.225 | 49.163 | 3.636 | 37.996 |
|
STD DEV (Psychotic) |
2.672 | 0.486 | 3.779 | 3.992 | 74.335 | 10.679 | 1.155 | 2.843 | 31.921 | 1.081 | 12.909 | 2.182 | 11.627 |
|
MEAN (Non- Psychotic N=66) |
6.363 | 4.824 | 13.856 | 40.785 | 270.106 | 83.994 | 33.964 | 28.692 | 10.343 | 13.017 | 51.488 | 3.373 | 36.442 |
|
STD DEV (Non- Psychotic) |
1.955 | 0.456 | 1.203 | 3.187 | 64.424 | 8.830 | 1.078 | 3.052 | 0.955 | 0.927 | 11.458 | 1.649 | 10.200 |
| p-value | 0.0782 | 0.6637 | 0.6518 | 0.564 | 0.3624 | 0.6613 | 0.2114 | 0.3922 | 0.3159 | 0.2121 | 0.2511 | 0.4301 | 0.3914 |
|
ABS Lympho (K/µL) |
Monocyte % |
ABS Mono (K/µL) |
Eosinophil % |
ABS Eosin (K/µL) |
Basophil % |
ABS Baso (K/µL) |
|||||||
|
MEAN (Psychotic N=80) |
2.515 | 8.871 | 0.610 | 3.385 | 0.226 | 0.571 | 0.047 | ||||||
|
STD DEV (Psychotic) |
0.846 | 2.791 | 0.282 | 2.066 | 0.157 | 0.405 | 0.102 | ||||||
|
MEAN (Non- Psychotic N=66) |
2.240 | 8.218 | 0.496 | 3.340 | 0.218 | 0.505 | 0.030 | ||||||
|
STD DEV (Non- Psychotic) |
0.724 | 2.801 | 0.143 | 2.568 | 0.186 | 0.386 | 0.025 | ||||||
| p-value | 0.0373* | 0.1621 | 0.0021** | 0.9078 | 0.7911 | 0.3143 | 0.1694 | ||||||
p-values <.05;
p -values <.01.
See text for details. Normal ranges were obtained from the Cleveland Clinic Department of Pathology.
Figure 1. First-Episode Psychosis is Associated with Abnormal White Blood Cell Counts.
A1–A2: Significantly increased absolute monocyte (T=2.1016, p=.0373*) values were found in children with first-episode psychosis compared to non-psychotic children who were matched by gender, ethnicity and age. The shaded areas show the normal range as reported by the Cleveland Clinic Department of Pathology and Laboratory medicine. Note that in contrast to absolute monocyte counts, the percent monocyte values were within normal range and did not differ between the two groups. Similar findings are reported for lymphocytes (B1–B2). Thus, absolute lymphocytes were increased in the psychotic group while percent lymphocytes were unchanged (T=3.1442, p=.0021**). C1–C2: the pie charts depict the overall impact of changes in monocytes and lymphocytes on white blood cell counts. Note that overall the observed changes were minimal and circumscribed to percent monocytes and lymphocytes. The numbers next to the pie graph represent the percent or absolute values of each group. Note: * indicates p<0.05 and **indicates p<0.01.
One of the potential problems with pediatric studies is that hematologic or other values sharply depend on age. We measured hematologic values in psychiatric patients and compared these to normal values. A slight age-dependent correlation was found for absolute monocytes in both the groups with psychosis and without psychosis, but these differences did not reach a statistical significance (see Supplemental Figures 5 and 6). Similarly, gender and ethnic differences were not significant. Together these results suggest that monocyte and lymphocyte counts are altered in psychotic patients for reasons unrelated to patient selection.
As expected, most of the patients in both groups were receiving pharmacological therapy to address their psychiatric illness or other health-related problems (see Table 4 and Figure 2). Prescription medication use was not a predictor of psychotic versus non-psychotic status (see Figure 2). Similarly, individual medications had little if any effect on hematologic variables with the exception of fluoxetine, quetiapine, olanzapine, trazodone and citalopram. As noted above, the small sample size of the “+-drug” groups makes a definitive conclusion impossible without further confirmation. Figure 2 shows the effects of these medications on absolute monocyte counts. The effects were in the opposite direction from what would be expected if drug treatment was the cause for increased monocyte levels, with the exception of olanzapine. We found no statistical link between tobacco, alcohol, or illicit drug use and blood cell counts in patients with or without psychosis (not shown).
Figure 2. Absolute Monocyte Counts are not, in General, Affected by Drug Treatment.
A: Shows the lack of overall impact of drug therapy on monocytes (T=.6172, p=.5381). B–F: Examples of drug treatment impact on leukocyte counts are shown. See also Table 4. * indicates p<0.05 and ** indicates p<0.01.
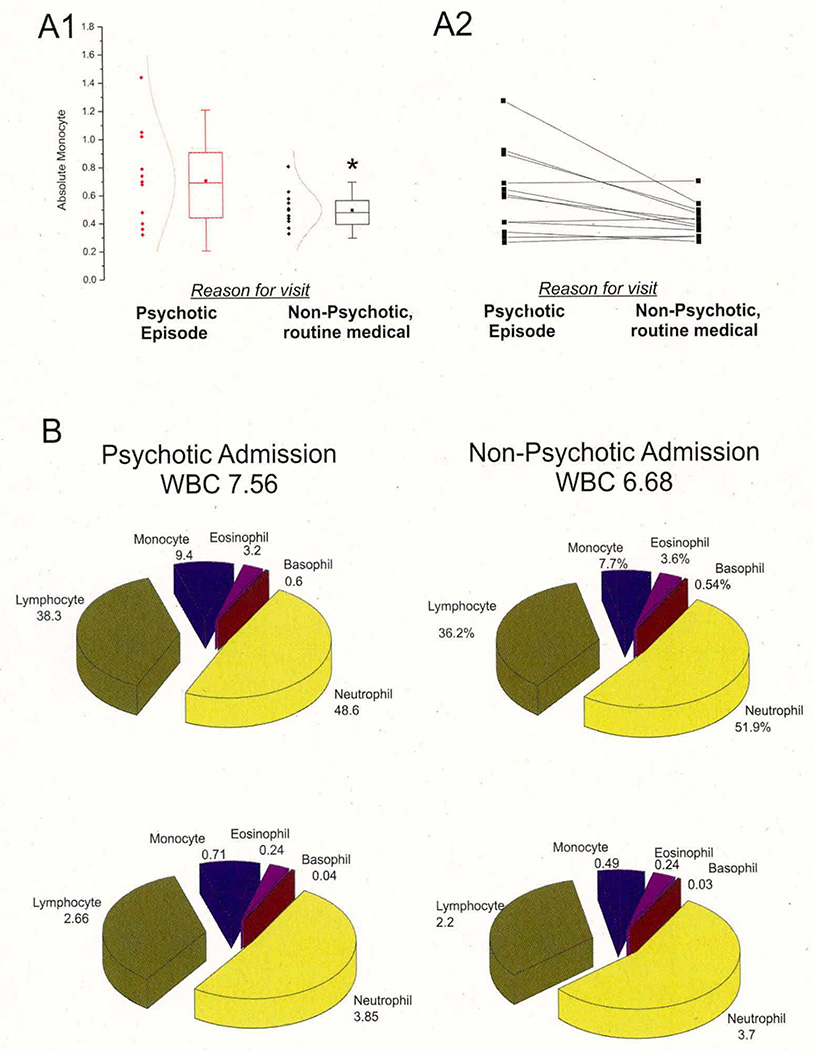
We investigated the correlation between monocytosis and multiple admissions during a three-year period in the same group of 80 patients with psychosis (see Figure 3). Patients who were diagnosed with a first-episode psychosis and who were enrolled in Stage 2 of our study were asked for an additional blood sample at the time of a routine visit to the Cleveland Clinic for a checkup or follow-up. At this time, patients were not suffering from any of the psychotic symptoms used to diagnose disease and were free from psychiatric illness. Comparing the first and second test results revealed a statistically elevated monocyte count when a diagnosis of psychosis was made (n=12). Significantly lower levels of monocytes were detected when the same patients were admitted to the hospital for reasons other than psychosis. Therapeutic drugs did not influence monocytosis in multiple admission patients from either group.
Figure 3. Correlation between White Blood Cell Counts and Psychotic Symptoms in Multiple Admission Patients.
A1–A2: Average and individual values of monocyte counts in a group of patients (n=12) admitted with a diagnosis of psychosis or when visiting the outpatient clinic for other non-psychiatric exams. The psychotic admission always preceded the post-discharge visit, which was performed on an outpatient basis for routine tests or checkup. The left panel illustrates the spread of data (shown as black or red diamonds, psychotic admission and routine admission respectively); the comparison of means and normal fit of data points is also shown. The right panel refers to individual values in the same patients to emphasize that changes in monocytes occurred in a large percentage of patients. The overall percent hematologic values for these patients are reported in B. * indicates p<0.05.
In summary, our results have so far shown that monocytosis and perhaps increased lymphocyte counts are associated with first-episode psychosis. Once population variables were ruled out and the data confirmed by analyzing hematologic values within the same population during multiple admissions, we wished to further test the hypothesis that pro-inflammatory changes were responsible for the observed differences. Specifically, we wished to test the hypothesis that the downstream effect of monocytosis was damage to the endothelial cells constituting the BBB.
To further test the role of pro-inflammatory changes in patients with psychosis, we hypothesized that monocytosis might damage the endothelial cells of the BBB. Serum samples of 28 children with psychosis and 8 without psychosis were tested for the serum marker of BBB integrity, S100B (24, 25, 43–45). In children diagnosed with a psychotic episode, S100B levels were significantly elevated compared to age- and gender-matched subjects without psychosis (see Figure 4 [A]). Note that all subjects without psychosis had S100B values below the 0.12 ng/ml threshold, which is considered the ceiling of the normal range (24, 43, 46). Most children with psychosis had serum S100B levels above this value. BBB permeability measured by serum S100B is a powerful but non-specific tool. In fact, elevated levels of S100B do not give insight into the mechanism of BBB disruption. We wished to further investigate the serum factor(s) that may be causally related to trans-endothelial leakage of S100B and analyzed the serum samples for cytokines and mediators believed to play a significant role in inflammation (see Figure 4 [B1–B2]). Of the total panel, only IL-1β, TNF-α, IL-5, IL-6, IL-10, IFN-γ were found to be elevated.
Discussion
There are two primary findings in this study. The first is that the initial episode of psychosis in children is associated with changes in immune cells consistent with sterile inflammation. This is due to the fact that none of the patients were diagnosed with ongoing or recent infection, nor were signs or symptoms of infectious disease (e.g., fever) present at the time of testing or shortly thereafter in the case of inpatients. Although both lymphocyte and monocyte absolute levels were increased in children with psychosis, this was not simply a consequence of hospitalization or psychiatric illness since these values were significantly less elevated in other psychiatric inpatients without psychosis. The second important outcome is a link between psychiatric illness, inflammation (cytokine levels), and BBB disruption, demonstrated by elevation in S100B, a peripheral marker of BBB function.
A crucial finding in this study is that first-episode psychosis in children is associated with changes in immune cells demonstrating psychosis-associated leukocyte changes. This study was performed in a large pediatric population and compared with a true “control” group consisting of pediatric patients with non-psychotic illness. Although both lymphocyte and monocyte absolute levels were increased in children with psychosis compared to their inpatient counterparts without psychosis, the observed anomaly in leukocyte counts was not a consequence of hospitalization or psychiatric illness alone. In non-psychotic pediatric psychiatric inpatients these values were slightly elevated and yet statistically lower than in pediatric patients with psychosis. Inflammation has also been reported in patients with mood disorders, as we can see in our group, but certainly the levels are much higher in the group of patients with psychosis.
The results show an increased cell-mediated inflammatory response in patients with psychosis; specifically, absolute cell counts were altered, demonstrating that the correlation is somewhat related to active, acquired immunity. The immune system recognizes foreign antigens and differentiates between self and non-self. Psychiatric symptoms, especially psychosis and mood symptoms (32–34, 39), have been described both during inflammation and with autoimmune disorders involving the CNS. These observations suggest that inflammation may be an important pathogenetic mechanism underlying schizophrenia (12, 47–53). Anti-inflammatory therapy seems to be promising, particularly in early stages of disease (54).
Similar findings were obtained from adult patients suffering from chronic disease (47, 48, 51, 53, 55, 56). We do not believe chronicity was a factor in our sample since our patients’ sera were analyzed immediately after a first observed and reported psychotic episode (see Figure 1). However, we acknowledge that illness may develop slowly over a longer period and due to etiologic factors other than those described herein. Importantly, none of our patients had an identifiable infectious disease or fever. These data support the hypothesis that sterile inflammation is one of the etiological variables of psychosis and also rule out the possibility that only prolonged disease contributed to the observed changes. This is further supported by the fact that a sub-sample of our patients was studied during a psychotic episode and at other time(s) when psychosis was not present. Our data on multiple admissions (see Figure 3) show an association between symptoms of psychosis and inflammation, the latter not being detected when the same patients were not affected by psychosis and admitted to the hospital for another medical reason such as orthopedics, GI symptoms, or headaches.
An alternative explanation for increased absolute monocyte counts is autoimmune disease. None of our patients were diagnosed with autoimmune disorders, but the presence of an autoantigen as etiological trigger cannot be at this stage ruled out. It is well known that autoimmune disorders commonly lead to psychiatric or neurologic symptoms. In the case of seizure disorders, the autoantigen can be an ion channel or receptor crucial for normal brain function. Our interpretation of data in psychosis shares a surprising overlap with the recently proposed inflammatory mechanisms of acute seizures (20, 25–27, 35, 36). Monocyte and lymphocyte activations are involved in the regulation of BBB permeability, and both cell types are activated in neurological diseases. In addition, it is well known that BBB dysfunction is a hallmark of many neurological diseases. For example, as mentioned above and discussed elsewhere: 1) acute psychosis and seizures are associated with an increased serum level of the blood-brain barrier integrity marker S100B (27, 36, 47); 2) anti-inflammatory treatments are surprisingly efficacious even when the trigger pathology is not of an infectious origin (27); and, 3) brain pathology reveals ongoing CNS inflammation in spite of the absence of etiologically defined pathogens. If diverse diseases share common features, why does BBB damage lead to different symptoms (e.g., seizures or psychosis, or seizures and psychosis)? A possible explanation is that both epilepsy and schizophrenia are diseases with a complex, multifactorial etiology where predisposition (e.g., pruning abnormalities or malformation of cortical development) plays an essential role. This suggests a scenario where a “disease-primed” brain does not produce clinically relevant symptoms until the BBB is breached in the malformed region or near disease-prone neuronal circuits. This was repeatedly demonstrated in models of focal seizure disorders (e.g., methylazoxymethanol- or thalidomide-treated animals [57, 58]) or pilocarpine-treated animals or patients (26, 27, 36) where leakage of the BBB and hyperexcitability are commonly observed in the region of neuronal malformation or gliotic CNS region. Breakdown of BBB or “latency states” in the development of neurotransmitters may explain late onset of psychotic symptoms.
While our data were obtained from a large number of patients, the possibility exists that these findings are due to uncontrolled confounding variables. It is well documented that elevated levels of cortisol induce an elevated total white blood cell count (59). An additional concern is the effect of elevated cortisol levels during acute psychotic states on the hematological parameters we found to be elevated. Acutely psychotic patients may have elevated cortisol levels in response to the acute stress occurring during a psychotic episode. Unfortunately, the serum cortisol values for our patients were not available, as they are considered outside of the standard of care for children admitted to the inpatient Child and Adolescent Psychiatry Unit. However, patients with psychosis within our study demonstrated no statistically significant increase in granulocytes in comparison to patients without psychosis. Additionally, it is unlikely that cortisol-induced demargination accounted for only elevations of monocytes and not other cell types.
If inflammation is a major contributor to pediatric schizophrenia, then one expects that anti-inflammatory therapy may be beneficial. Several investigators have shown that a variety of anti-inflammatory approaches have beneficial effects (60–73). These include non-steroidal anti-inflammatory drugs, immunosuppressants and anti-IL1B receptor antibodies (60–73). Not surprisingly, several inflammatory mediators were found elevated in adult patients with schizophrenia (40, 47, 48, 53, 56, 68, 74–76), and now supported in a pediatric cohort. Consistent with these findings is the fact that some antipsychotic medications have immunomodulatory effects (76). This may also be true of the selective serotonin reuptake inhibitors. Future studies—including anti-inflammatory medication for youth in pre-psychotic stages—seem promising.
Schizophrenia, a relatively common psychiatric syndrome, affects virtually all brain functions yet has eluded explanation for more than 100 years. Abnormalities of neurons and synapses have been the main focus of attention. Our results strongly support the “inflammatory theory” of schizophrenia formulated over 100 years ago (10) and recently reviewed (6, 11, 47, 74, 75). Our findings support recent studies using anti-inflammatory medications that have been proven effective for patients in the early stages of psychosis (first-psychotic episode and pre-psychotic stages) (61, 72, 73, 77, 78). If our hypothesis is correct, aggressive and rapid management of early symptoms with a targeted anti-inflammatory therapy in addition to antipsychotic medication may be able to have an impact in the progression of the disease, especially if the symptoms are targeted in early stages (i.e., first-episode psychosis in youth) (75).
Supplementary Material
Figure 5. Age, Ethnicity and Gender do not Affect Absolute Monocyte and White Blood Cell Counts in Psychotic Patients.
Figure 6. Age, Ethnicity and Gender do not Affect Absolute Monocyte and White Blood Cell Counts in Non-Psychotic Patients.
Clinical Implications.
There are two primary findings in this study. The first is that the initial episode of psychosis in children is associated with changes in immune cells consistent with sterile inflammation. This is due to the fact that none of the patients were diagnosed with ongoing or recent infection, nor were signs or symptoms of infectious disease (e.g., fever) present at time of testing or shortly thereafter in the case of inpatients. Although both lymphocyte and monocyte absolute levels were increased in children with psychosis, this was not simply a consequence of hospitalization or psychiatric illness since these values were significantly less elevated in other psychiatric inpatients without psychosis. The second important outcome is a link between psychiatric illness, inflammation (cytokine levels), and blood-brain barrier (BBB) disruption, demonstrated by elevation in S100B, a peripheral marker of BBB function.
Acknowledgments
Supported by NIH R01NS43284, R41MH093302, R21NS077236, R42MH093302 and R21HD057256 awarded to DJ. Erin Carlton was supported by Flocel, Inc., Cleveland, OH.
References
- 1.Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53(11):1022–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- 2.Kraepelin E. Dementia praecox and paraphrenia. Edinburgh: Livingston; 1919. [Google Scholar]
- 3.Torrey EF, Leweke MF, Schwarz MJ, Mueller N, Bachmann S, Schroeder J, et al. Cytomegalovirus and schizophrenia. CNS Drugs. 2006;20(11):879–885. doi: 10.2165/00023210-200620110-00001. [DOI] [PubMed] [Google Scholar]
- 4.Torrey EF, Yolken RH. The schizophrenia-rheumatoid arthritis connection: infectious, immune, or both? Brain Behav Immun. 2001;15(4):401–410. doi: 10.1006/brbi.2001.0649. [DOI] [PubMed] [Google Scholar]
- 5.Rothschild DM, O’Grady M, Wecker L. Neonatal cytomegalovirus exposure decreases prepulse inhibition in adult rats: implications for schizophrenia. J Neurosci Res. 1999;57(4):429–434. [PubMed] [Google Scholar]
- 6.Fellerhoff B, Laumbacher B, Mueller N, Gu S, Wank R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry. 2007;12(3):264–272. doi: 10.1038/sj.mp.4001925. [DOI] [PubMed] [Google Scholar]
- 7.Ledgerwood LG, Ewald PW, Cochran GM. Genes, germs, and schizophrenia: an evolutionary perspective. Perspect Biol Med. 2003;46(3):317–348. doi: 10.1353/pbm.2003.0038. [DOI] [PubMed] [Google Scholar]
- 8.Yolken RH, Bachmann S, Ruslanova I, Lillehoj E, Ford G, Torrey EF, et al. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis. 2001;32(5):842–844. doi: 10.1086/319221. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurological and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71(4):444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 10.Bruce LC. Clinical and experimental observations in katatonia. Journal of Mental Science. 1903;49:614–628. [Google Scholar]
- 11.Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29(6):913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318(5850):576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 14.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12(1):115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 15.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39(3):311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim HW, Cheon Y, Modi H, Rapoport S, Rao J. Effects of chronic clozapine administration on markers of arachidonic acid cascade and synaptic integrity in rat brain. Psychopharmacology (Berl) 2012;222(4):663–674. doi: 10.1007/s00213-012-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant GA, Janigro D. The blood-brain barrier. In: Winn HR, editor. Youmans Neurological Surgery. 4th. Philadelphia (PA): Saunders; 2004. pp. 153–174. [Google Scholar]
- 20.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 22.Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl 1):26–34. doi: 10.1111/j.1528-1167.2012.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, et al. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940(1–2):102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- 24.Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci. 2003;21(3–4):109–121. [PMC free article] [PubMed] [Google Scholar]
- 25.Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48(4):732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33(2):171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchi N, Granata T, Freri E, Ciusani E, Ragona F, Puvenna V, et al. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS ONE. 2011;6(3):e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesmann M, Wandinger KP, Missler U, Eckhoff D, Rothermundt M, Arolt V, et al. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol Psychiatry. 1999;45(11):1508–1511. doi: 10.1016/s0006-3223(98)00217-0. [DOI] [PubMed] [Google Scholar]
- 29.Lara DR, Gama CS, Belmonte-de-Abreu P, Portela LV, Goncalves CA, Fonseca M, et al. Increased serum S100B protein in schizophrenia: a study in medication-free patients. J Psychiatr Res. 2001;35(1):11–14. doi: 10.1016/s0022-3956(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 30.Wiesmann M, Missler U, Gottmann D, Gehring S. Plasma S-100b protein concentration in healthy adults is age- and sex-independent. Clin Chem. 1998;44(5):1056–1058. [PubMed] [Google Scholar]
- 31.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60(6):614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- 32.Rothermundt M, Ponath G, Arolt V. S100B in schizophrenic psychosis. Int Rev Neurobiol. 2004;59:445–470. doi: 10.1016/S0074-7742(04)59017-7. [DOI] [PubMed] [Google Scholar]
- 33.Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav Immun. 2001;15(4):319–339. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- 34.Schroeter ML, Abdul-Khaliq H, Fruhauf S, Hohne R, Schick G, Diefenbacher A, et al. Serum S100B is increased during early treatment with antipsychotics and in deficit schizophrenia. Schizophr Res. 2003;62(3):231–236. doi: 10.1016/s0920-9964(02)00383-3. [DOI] [PubMed] [Google Scholar]
- 35.Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14(12):1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchi N, Teng Q, Ghosh C, Fan Q, Nguyen MT, Desai NK, et al. Blood-brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010;1353:176–186. doi: 10.1016/j.brainres.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drexhage RC, Padmos RC, de Wit H, Versnel MA, Hooijkaas H, van der Lely AJ, et al. Patients with schizophrenia show raised serum levels of the pro-immflamatory chemokine CCL2: association with metabolic syndrome in patients? Schizophr Res. 2008;102(1–3):352–355. doi: 10.1016/j.schres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 39.Zorrilla EP, Cannon TD, Gur RE, Kessler J. Leukocytes and organ-nonspecific autoantibodies in schizophrenics and their siblings: markers of vulnerability or disease? Biol Psychiatry. 1996;40(9):825–833. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 40.Yokota O, Terada S, Ishihara T, Nakashima H, Kugo A, Ujike H, et al. Neuronal expression of cyclooxygenase-2, a pro-inflammatory protein, in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):715–721. doi: 10.1016/j.pnpbp.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 41.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49(2):143–155. [PubMed] [Google Scholar]
- 42.Taylor MR, Holland CV, Spencer R, Jackson JF, O’Connor GI, O’Donnell JR. Haematological reference ranges for schoolchildren. Clin Lab Haematol. 1997;19(1):1–15. doi: 10.1046/j.1365-2257.1997.00204.x. [DOI] [PubMed] [Google Scholar]
- 43.Marchi N, Fazio V, Cucullo L, Kight K, Masaryk T, Barnett G, et al. Serum transthyretin monomer as a possible marker of blood-to-CSF barrier disruption. J Neurosci. 2003;23(5):1949–1955. doi: 10.1523/JNEUROSCI.23-05-01949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogelbaum MA, Masaryk T, Mazzone P, Mekhail T, Fazio V, McCartney S, et al. S100beta as a predictor of brain metastases: brain versus cerebrovascular damage. Cancer. 2005;104(4):817–824. doi: 10.1002/cncr.21220. [DOI] [PubMed] [Google Scholar]
- 45.Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38(9):2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- 46.Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clin Chim Acta. 2004;342(1–2):1–12. doi: 10.1016/j.cccn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Rothermundt M, Arolt V. Schizophrenia and immunity. In: Ader R, editor. Psychoneuroimmunology. Boston: Elsevier; 2007. pp. 563–577. [Google Scholar]
- 48.Maino K, Gruber R, Riedel M, Seitz N, Schwarz M, Muller N. T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res. 2007;152(2–3):173–180. doi: 10.1016/j.psychres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Maes M, Delanghe J, Bocchio Chiavetto L, Bignotti S, Tura GB, Pioli R, et al. Haptoglobin polymorphism and schizophrenia: genetic variation on chromosome 16. Psychiatry Res. 2001;104(1):1–9. doi: 10.1016/s0165-1781(01)00298-0. [DOI] [PubMed] [Google Scholar]
- 50.Maes M. Interleukin-2 and schizophrenia. Psychiatry Res. 1998;77(1):63–64. doi: 10.1016/s0165-1781(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 51.Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura G, Pioli R, Boin F, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol. 2000;10(2):119–124. doi: 10.1016/s0924-977x(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 52.Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res. 1997;26(2–3):221–225. doi: 10.1016/s0920-9964(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 53.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 54.Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpiride treatment. Schizophr Res. 2010;121(1–3):118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Maes M. Cytokines in schizophrenia. Biol Psychiatry. 1997;42(4):308–309. doi: 10.1016/S0006-3223(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 56.Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res. 1997;71(1):11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 57.Bassanini S, Hallene K, Battaglia G, Finardi A, Santaguida S, Cipolla M, et al. Early cerebrovascular and parenchymal events following prenatal exposure to the putative neurotoxin methylazoxymethanol. Neurobiol Dis. 2007;26(2):481–495. doi: 10.1016/j.nbd.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hallene KL, Oby E, Lee BJ, Santaguida S, Bassanini S, Cipolla M, et al. Prenatal exposure to thalidomide, altered vasculogenesis, and CNS malformations. Neuroscience. 2006;142(1):267–283. doi: 10.1016/j.neuroscience.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deutsch V, Lerner-Geva L, Reches A, Boyko V, Limor R, Grisaru D. Sustained leukocyte count during rising cortisol level. Acta Haematol. 2007;118:73–76. doi: 10.1159/000103216. [DOI] [PubMed] [Google Scholar]
- 60.Muller N, Riedel M, Schwarz MJ. Psychotropic effects of COX-2 inhibitors—a possible new approach for the treatment of psychiatric disorders. Pharmacopsychiatry. 2004;37(6):266–269. doi: 10.1055/s-2004-832682. [DOI] [PubMed] [Google Scholar]
- 61.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159(6):1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 62.Muller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255(2):149–151. doi: 10.1007/s00406-004-0548-4. [DOI] [PubMed] [Google Scholar]
- 63.Muller N, Strassnig M, Schwarz MJ, Ulmschneider M, Riedel M. COX-2 inhibitors as adjunctive therapy in schizophrenia. Expert Opin Investig Drugs. 2004;13(8):1033–1044. doi: 10.1517/13543784.13.8.1033. [DOI] [PubMed] [Google Scholar]
- 64.Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 65.Bresee CJ, Delrahim K, Maddux RE, Dolnak D, Ahmadpour O, Rapaport MH. The effects of celecoxib augmentation on cytokine levels in schizophrenia. Int J Neuropsychopharmcol. 2006;9(3):343–348. doi: 10.1017/S1461145705005808. [DOI] [PubMed] [Google Scholar]
- 66.Levine J, Susnovski M, Handzel ZT, Leykin I, Shinitzky M. Treatment of schizophrenia with an immunosuppressant. Lancet. 1994;344(8914):59–60. [PubMed] [Google Scholar]
- 67.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90(1–3):179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20(6):532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Kowalski J, Blada P, Kucia K, Madej A, Herman ZS. Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 2001;50(3):169–175. doi: 10.1016/s0920-9964(00)00156-0. [DOI] [PubMed] [Google Scholar]
- 70.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57(12):1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Akhondzadeh S, Safarcherati A, Amini H. Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: a double blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):253–259. doi: 10.1016/j.pnpbp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 73.Laan W, Selten JP, Kahn RS, Huisman AM, Heijnen CJ, Grobbee DE, et al. Acetylsalicylic acid as an adjuvant therapy for schizophrenia. Trials. 2006;7:31. doi: 10.1186/1745-6215-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miuller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007;(72):269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- 75.Muller N, Riedel M, Gruber R, Ackenheil M, Schwarz MJ. The immune system and schizophrenia. An integrative view. Ann N Y Acad Sci. 2000;917:456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 76.Song C, Lin Ah, Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42(2):157–164. doi: 10.1016/s0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- 77.Riedel M, Strassnig M, Schwarz MJ, Muller N. COX-2 inhibitors as adjunctive therapy in schizophrenia: rationale for use and evidence to date. CNS Drugs. 2005;19(10):805–819. doi: 10.2165/00023210-200519100-00001. [DOI] [PubMed] [Google Scholar]
- 78.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64(6):468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.