Abstract
Data from 10 sites of the NICHD/NIDCD Collaborative Programs of Excellence in Autism were combined to study the distribution of head circumference and relationship to demographic and clinical variables. Three hundred thirty-eight probands with autism-spectrum disorder (ASD) including 208 probands with autism were studied along with 147 parents, 149 siblings, and typically developing controls. ASDs were diagnosed, and head circumference and clinical variables measured in a standardized manner across all sites. All subjects with autism met ADI-R, ADOS-G, DSM-IV, and ICD-10 criteria. The results show the distribution of standardized head circumference in autism is normal in shape, and the mean, variance, and rate of macrocephaly but not microcephaly are increased. Head circumference tends to be large relative to height in autism. No site, gender, age, SES, verbal, or non-verbal IQ effects were present in the autism sample. In addition to autism itself, standardized height and average parental head circumference were the most important factors predicting head circumference in individuals with autism. Mean standardized head circumference and rates of macrocephaly were similar in probands with autism and their parents. Increased head circumference was associated with a higher (more severe) ADI-R social algorithm score. Macrocephaly is associated with delayed onset of language. Although mean head circumference and rates of macrocephaly are increased in autism, a high degree of variability is present, underscoring the complex clinical heterogeneity of the disorder. The wide distribution of head circumference in autism has major implications for genetic, neuroimaging, and other neurobiological research.
Keywords: autism, autism-spectrum disorder, head circumference, macrocephaly, height
INTRODUCTION
An increased rate of macrocephaly is a consistent and replicated biological finding in autism. Studies of head circumference in persons with autism have shown that macrocephaly occurs more frequently than expected in children and adults, singletons and twins, and in community, clinic, and epidemiologic samples [Bolton et al., 1994; Bailey et al., 1995; Davidovitch et al., 1996; Woodhouse et al., 1996; Lainhart et al., 1997; Stevenson et al., 1997; Fombonne et al., 1999; Fidler et al., 2000; Miles et al., 2000; Aylward et al., 2002; Gillberg and De Souza, 2002; Courchesne et al., 2003; Deutsch and Joseph, 2003; Dementieva et al., 2005]. These studies show, on average, a rate of macrocephaly of 20% [Fombonne et al., 1999]. Despite awareness of the connection between macrocephaly and autism, many basic questions about the relationship between head size and autism remain unanswered.
Macrocephaly in individuals with autism appears to be due to abnormal enlargement of the brain at some time during post-natal development. The evidence for this conclusion comes from the relationship of head circumference to brain volume and from MRI and post-mortem studies. During early childhood, head circumference and brain volume increase at similar rates [Lainhart et al., 2005]. The correlation between head circumference and total brain volume in young children is high, 0.88 [Hazlett et al., 2005]. Brain volume plateaus at about 13 years of age, but head circumference continues to increase until age 18. The different growth trajectories during adolescence and young adulthood result in a smaller, but still substantial, correlation of 0.67 between head circumference and brain volume [Piven et al., 1996]. Later in adulthood, when brain volume is decreasing, head circumference and total intracranial volume remain stable, as indices of maximal brain volume during development [Hardan et al., 2001; Aylward et al., 2002]. Neuroimaging studies of autism have found increased mean total brain volume in children by 2–4 years of age [Courchesne et al., 2001; Sparks et al., 2002; Hazlett et al., 2005]. Post-mortem studies show increased brain weight in children with autism and frank megalencephaly in some cases [Bailey et al., 1998; Kemper and Bauman, 1998]. Causes of macrocephaly other than increased brain volume are rarely found in persons with idiopathic autism [Lainhart et al., 1997; Stevenson et al., 1997; Bailey et al., 1998; Bigler et al., 2003].
It is not yet known if macrocephaly identifies a neurobiological subtype of autism. The results of some, but not all, studies suggest that macrocephaly is the “tip of the iceberg” of a more general tendency toward increased head and brain size [Lainhart et al., 1997; Fombonne et al., 1999; Miles et al., 2000]. The results of studies are mixed about age, gender, and IQ effects on the rate of macrocephaly and the relationship of macrocephaly to the core diagnostic features of autism [Lainhart et al., 1997; Fombonne et al., 1999; Miles et al., 2000; Deutsch and Joseph, 2003]. Macrocephaly does not appear to be part of general body overgrowth in most cases, but the relationship between height and head circumference in autism is not well understood [Campbell et al., 1980; Lainhart et al., 1997; Miles et al., 2000]. Reported rates of microcephaly in autism vary from 2.2% to 15% [Lainhart et al., 1997; Fombonne et al., 1999; Miles et al., 2000; Deutsch and Joseph, 2003]. Little is known about the relationship between parent and proband head size [Stevenson et al., 1997; Fidler et al., 2000; Miles et al., 2000]. Samples studied to date have usually been small in size from an anthropometric perspective, diagnostic measures have not been standardized across studies, and some studies have included individuals with autism accompanied by medical disorders.
In the present report, we use data from the NICHD/ NIDCD Collaborative Programs of Excellence in Autism (CPEA) network to examine several important questions about head circumference in autism. The resulting sample is one of the largest samples studied to date. We hypothesize that (1) the tendency toward increased head size in autism is a generalized phenomenon affecting the population of individuals with idiopathic autism-spectrum disorders (ASD), (2) variation in standardized head circumference in autism is related to height, IQ, SES, and parental head circumference but not age, similar to typically developing children, and (3) macrocephaly identifies a unique subgroup of autism. To better understand head size in autism families and the relationship between macrocephaly and ASDs, we also examine data from subjects with PDD-NOS and affected and unaffected siblings.
MATERIALS AND METHODS
Sample
Data were collected between 1999 and 2004 on 420 ASD subjects and 78 unaffected siblings. Of the ASD subjects, 338 were probands (index cases of ASD in their families) including 208 autism probands, 71 were affected siblings, and 11 were more distant affected relatives (not included in this article). Seventy-three percent of the ASD subjects were drawn from four sites (Utah, Pittsburgh, Boston, and Seattle). Subjects with ASD were compared to a reference sample and to typically developing control subjects. We included a typically developing control group so we could test for mild secular and other effects on head circumference between our cohort and the reference sample cohort. From a pool of 152 typically developing subjects, 88% of whom were from two sites (Pittsburgh and Utah), 70 were pairwise-matched to autism probands. Typically developing and autism subjects were matched to within 1 year on age (except for adults) and 10 points on performance IQ by an investigator who was blind to head circumference and height results.
Eighty percent of the ASD subjects were ascertained from community sources, 14% from outpatient clinics, and in 6% the source was unspecified. All typically developing subjects were community ascertained. Both ASD and typically developing subjects were unselected for head size and height. The CPEA sites did not have enough non-autistic intellectually handicapped individuals to include a comparison group with mental retardation.
The diagnosis of ASD was idiopathic in all cases. Individuals with non-psychiatric medical causes of autism were excluded by history, physical examination, karyotype, and fragile-X testing. The physical exam included a dysmorphology examination by a dysmorphologist or other trained clinician in the majority of cases. The 420 individuals with ASDs in the total CPEA sample included some affected-sibling pairs. Data on affected-sibling pairs are not independent because significant proportions of the variances in head circumference and height are attributable to familial factors, at least in typically developing individuals. Only unrelated affected individuals (i.e., probands) are included in the main analyses of this study. Sibling data are reported separately. We define and refer to the autism “proband” as the index case of autism in a family, and the ASD “proband” as the index case of any ASD (autism, Asperger, or PDD-NOS) in a family.
Diagnosis
Autism-spectrum subjects were classified into four categories according to CPEA diagnostic criteria, for standardized diagnostic classification across the 10 CPEA sites. The CPEA criteria are based on the Autism Diagnostic Interview-Revised (ADI-R) [Lord et al., 1994], Autism Diagnostic Observation Schedule [Lord et al., 2000], age, and IQ. In order to meet CPEA diagnostic criteria for an ASD, subjects also have to meet DSM-IV and ICD-10 criteria for the disorder.
The ADI-R is an investigator-based parent interview about the individual’s early childhood and current social and communication development and stereotyped, repetitive behaviors and interests. The ADI-R has good reliability and validity [Lord et al., 1994]. The ADOS-G is a semi-structured interactive observation session that involves play and activities for young children and non-verbal individuals, and activities and an interview for older and verbal subjects. Individuals are tested with one of four different modules appropriate for their age and verbal ability.
Autism-spectrum disorders (ASD) were diagnosed in a hierarchal fashion (Table I). First, subjects were considered for a diagnosis of autism. Subjects who did not meet criteria for autism were considered for a possible diagnosis of Asperger syndrome, and barring that, PDD-NOS. A final classification of “broad Autism-Spectrum Disorder” (broad ASD) took into consideration the fact that all of the data necessary for a specific ASD diagnosis might not be available for all subjects. Subjects classified as having broad ASD included many individuals who may have met criteria for autism, Asperger, or PDD-NOS if additional data were available. Subjects with ASDs were sub-classified according to whether or not they had a regression in development during early childhood and were functionally verbal at the time of evaluation. Regression was defined as loss of language skills that the subject had and then lost for at least 3 months before the 5th birthday. Non-verbal functioning was defined as no functional use of language involving at least three word phrases. These characteristics were assessed by specific questions in the ADI-R.
TABLE I.
Method of Diagnosis of Autism-Spectrum Disorders
| Autism? No→ | Asperger? No→ | PDD-NOS? No→ | Broad ASD? | |
|---|---|---|---|---|
| CA | ≥36 monthsa | ≥60 monthsa | Any | Any |
| MA | ≥18 months | Any | Any | |
| Verbal IQ | Any | ≥70 | Any | Any |
| Delay lang onset | ± | No | ± | ± |
| Onset | <36 months | |||
| Algorithm scores | ||||
| ADI-social | + | + | +b | ±c |
| ADI-Comm | + | − | +b | ±c |
| ADI-SRIB | + | +Within 1pt | ±c | |
| ADOS-Comb | + | + | + | ±c |
CA, chronological age; MA, mental age (CA × verbal IQ); Delay lang onset: no single words by 24 months or phrases by 33 months of age; ADI, Autism Diagnostic Interview-Revised [Lord et al., 1994]; ADOS: Autism Diagnostic Observation Schedule-Generic [Lord et al., 2000]; Comm, communication; SRIB, stereotyped repetitive interests and behaviors; ADOS-Comb, ADOS combined social + communication score met cutoff for ASD.
These age cutoffs were chosen for the integrity of CPEA cross-network studies. They are not required for DSM-IV diagnoses of autistic disorder or Asperger syndrome.
Met cutoff for autism in the social domain plus within two points of the cutoff in the communication domain or met cutoff in the communication domain and within two points of cutoff in the social domain.
Met ADI-R cutoff for autism in the social domain and at least one other domain or met ADOS cutoff for combined social + communication score.
Typically developing subjects had no history of learning, developmental, cognitive, neurological, or neuropsychiatric problems. All of them served as normal controls for a variety of neuropsychological, imaging, and developmental studies and had extensive testing to confirm that they were typically developing.
Measures
IQ
The majority of autism and control subjects had the WISC-III or WAIS-III [Wechsler, 1991, 1997]. Sixteen percent of the autism subjects had the Differential Abilities Scale [Elliott, 1990]. Some ASD subjects were tested with the Leiter International Performance scales [Roid and Miller, 1997] or the Mullen Tests of Early Learning [Mullen, 1995]. The remaining subjects were tested with other IQ tests. The results of all of these tests were used to estimate verbal and performance IQ, using a consistent method recommended by our expert in psychometrics [S. Ozonoff].
Head circumference and height
Maximal occipital-frontal head circumference was measured. Reliability for head circumference measurement was established in the Salt Lake City, Boston, and Pittsburgh sites and across the Salt Lake City and Boston sites. The within-site intra-class correlation coefficients and the across-site intraclass correlation coefficient for head measurements were ≥0.90, including inter-rater and test-retest reliability. In most cases, height was measured using a stadiometer.
Head circumference and height data were converted to standardized z scores using reference data. For example, zHC = (subject’s HC–reference sample mean HC for the subject’s age and sex)/reference sample standard deviation for the subject’s age and sex. zHC and zHgt values more than two standard deviations above or below the reference means were double-checked with the site that provided the data.
Fels head circumference data [Roche et al., 1987] and Center for Disease Control (CDC) [Kuczmarski et al., 2002] height data were the reference data used in this study. The height reference data were collected between 1963 and 1994 from an epidemiologic sample of children and adults pooled across ethnicities and representative of the population in the US. The CDC data include height data from the Fels sample. There are no recent CDC head circumference reference data for individuals older than 3 years of age, the age of most individuals in this study. For this reason, we used head circumference reference data from the Fels study, collected between 1929 and 1975 from Caucasian individuals living in one large region of the US.
In this report, macrocephaly refers to absolute macrocephaly, defined as a head circumference >1.88 standard deviations above the mean for age and sex, which is the same as above the 97th centile. Microcephaly is defined as a head circumference ≤1.88 standard deviations below the mean for age and sex, which is the same as below the 3rd centile. Tall and short stature are defined as heights >1.88 standard deviations above and ≤1.88 standard deviations below the mean for age and sex, respectively.
Statistical Analysis
Because we were not able to establish reliability for head circumference measurement within and across all 10 CPEA sites, and because the head circumference reference data were from Caucasian individuals only, we compared a conservative analysis of the data (Caucasian subjects only, from the Salt Lake City, Boston, and Pittsburgh sites) with a more liberal analysis of the data (all subjects of all races and ethnicities from all sites). There were no significant differences between the two levels of analysis. The results presented are from the combined sample of all subjects from all sites.
Distribution analysis
Tests of normality use the Kolmogorov–Smirnov goodness-of-fit statistic with Lilliefors significance correction and the Shapiro–Wilk statistic. Comingling (admixture) analysis was done for the zHC distribution to test for bimodality. An admixture program was used to determine the likelihood of the data fitting a distribution with a single mean and standard deviation compared to the likelihood of two or more means and standard deviations. The comparison of these nested likelihood tests results in a chi-square statistic with three degrees of freedom.
Univariate analysis
We used t-tests and one-way ANOVA, and correlation analysis so our results would be comparable to the results of published head circumference studies and because it is controversial what factors should be corrected for in autism head circumference and brain volume analyses. A number of case-control comparisons were specified before examination of the data. For the most conservative presentation of levels of significance, however, the P-values for both planned and exploratory analyses were corrected for multiple comparisons using the false discovery rate (FDR) method of Benjamini and Hochberg [1995, 2000]. The corrected P-values are shown. Pearson correlations were used to study the relationship between head circumference and other quantitative variables.
Analysis after adjustment for covariates
Because a number of the variables relevant to head circumference and height were correlated with one another, the data were also analyzed with multiple regression methods to examine for independent effects.
RESULTS
Sample Characteristics
Table II shows the characteristics of the autism probands, all the ASD probands (including autism, PDD-NOS, Asperger, and “broad” ASD), the head circumference reference sample, and the matched autism probands and typically developing controls. Compared to the population of individuals with idiopathic autism [Fombonne et al., 2001; Chakrabarti and Fombonne, 2005], the autism sample had a higher proportion of males (85.6% vs. 80%; z = 2.0, P = 0.023), a lower rate of mental retardation (30.8% vs. 66.7%, z = 10.8, P < 0.0001), and a higher proportion of subjects from SES I or II families (professional or semi-professional; 72.8% vs. 58.6%, z = 4.20, P < 0.0001). The predominance of males and cognitively high-functioning subjects was the result of some of the CPEA sites focusing on samples more homogeneous than the population of individuals with autism. The proportion of autism subjects from families of SES level I and II was higher than the 65% rate in the reference sample (z = 2.36, P = 0.009) but was similar to other non-epidemiologic community-ascertained samples (78%) [Roche et al., 1987; Lainhart et al., 1997]. The matched autism and typical control samples did not significantly differ in age, non-verbal IQ, or standardized height. Verbal IQ and SES differed in these groups and were considered in the analyses (t = 2.65, P = 0.009; t = 4.20, P < 0.0001, respectively).
TABLE II.
Demographic and Clinical Characteristics of Autism-Spectrum Disorder Probands, the Reference Sample, and Matched Autism and Typically Developing Subjects
| Matched samples |
|||||
|---|---|---|---|---|---|
| Characteristic | Autism probands | All ASD Probands | Reference samplea | Autism | Typicals |
| N | 208 | 338 | 70 | 70 | |
| Mean age (years) (sd) | 9.7 (5.4) | 10.8 (7.5) | 12.4 (4.6) | 12.3 (4.1) | |
| Range | 3–47 | 2–48.5 | 1–18 | 7–30 | 7–26.5 |
| Caucasian | 90.9% | 89.9h | 100% | 92.9% | 95.7% |
| Male:female ratio | 5.9:1 | 5.1:1 | 9:1 | 6:1 | |
| Male | 85.6% | 83.7% | 91.4% | 85.7% | |
| Multiplex | 25.0% | 27.2% | 14.3% | ||
| Socioeconomic status | |||||
| Mean (sd) | 1.56 (0.81)o | 2.04 (0.98)s | |||
| SES I & II | 72.8%b | 70.2%i | 65.0% | 80% | 63.7% |
| SES III | 25.3% | 26.2% | 25.0% | 20% | 32.1% |
| SES IV & V | 1.9% | 3.6% | 8.0% | 0 | 3.6% |
| Mean NVIQ (sd) | 82.5c (23.2) | 82.4j (25.0) | 102.8 (13.4) | 106.2 (12.6) | |
| Range | 24–126 | 12–137 | 67–126 | 75–128 | |
| NVIQ<70 | 30.8% | 28.1% | 1.4% | 0 | |
| Mean VIQ (sd) | 75.2d (26.3) | 79.6k (27.7) | 95.2 (23.9) | 108.9 (12.7) | |
| Range | 13–141 | 8–153 | 48–141 | 71–141 | |
| Early regression (%) | 23.4%e | 25.8%l | 17.8%p | ||
| Non-verbal | 15.3%f | 22.0%m | 3%q | 0 | |
| Seizure disorder | 15.7%g | 14.9%n | 12.9%r | 0 | |
ASD, autism-spectrum disorder;
N = 162;
N = 195;
N = 193;
N = 141;
N = 203;
N = 121;
N = 329;
N = 248;
N = 313;
N = 298;
N = 194;
N = 286;
N = 155;
N = 55;
N = 28;
N = 65;
N = 31;
N = 55.
Site Effects
Mean standardized head circumference (zHC) did not significantly differ across the 10 network sites (F = 0.692, df = 8, P = 0.70).
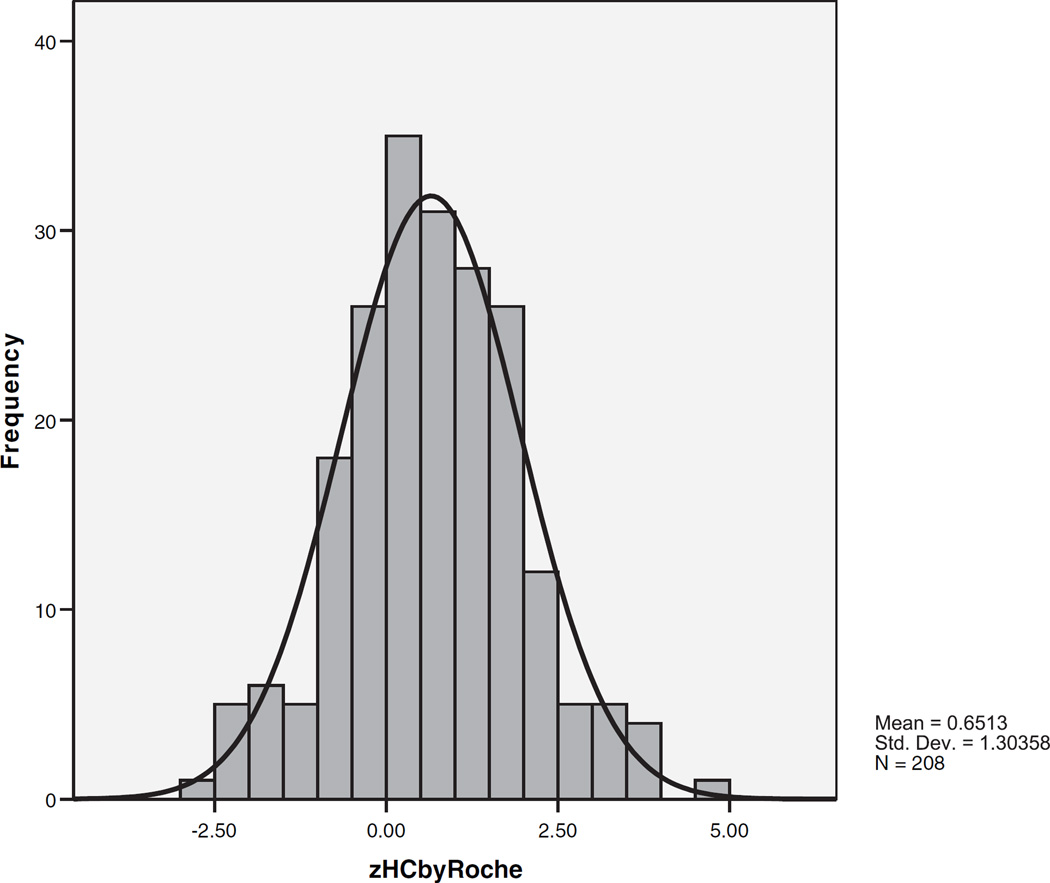
The Distribution of Standardized Head Circumference in Autism
Figure 1 shows the distribution and Table III shows the values of standardized head circumference in the autism probands. The distribution is unimodal and fits a normal distribution. The mean is shifted to the right and the variance is increased relative to reference data parameters (t = 7.21, P < 0.0004; skewness = 0.122, standard error = 0.169, kurtosis = 0.261; Kolmogorov–Smirnov statistic = 0.040, P = 0.200; Shapiro–Wilk statistic = 0.993, P = 0.484). A single distribution with one mean fit the data better than two or three distributions with differing means (chi-square = 1.22, df = 3, not significant). All of the above results were similar when all probands with ASD (n = 338) were included in the distribution analyses.
Fig. 1.
The distribution of standardized head circumference in autism probands.
TABLE III.
Mean Standardized Head Circumference (zHC), Rates of Macrocephaly and Microcephaly, Standardized Height (zHgt), and the Discrepancy Between zHC and zHgt in Autism-Spectrum Disorder and Typically Developing Subjects
| Subjects | n | zHC mean (sd) | Microcephalya | Macrocephaly (%)b | zHgt mean (sd) | zHC-zHgtc mean (sd) |
|---|---|---|---|---|---|---|
| Autism probands | 208 | 0.651 (1.30) | 3.8% | 17.3 | −0.085 (1.22) | 0.708 (1.26)d |
| Males | 178 | 0.707 (1.25) | 4.5% | 18.0 | −0.055 (1.18) | 0.708 (1.23)e |
| Females | 30 | 0.320 (1.55) | 0 | 13.3 | −0.339 (1.47) | 0.714 (1.55)f |
| 3–5 years | 31 | 0.628 (1.30) | 0 | 16.1 | 0.356 (.82) | 0.479 (1.18)g |
| 6–11 years | 130 | 0.630 (1.38) | 6.2% | 17.8 | −0.176 (1.32) | 0.677 (1.32)h |
| 12–18 years | 39 | 0.650 (1.15) | 0 | 15.4 | −0.181 (1.11) | 0.912 (1.14)i |
| 19+ years | 8 | 1.02 (0.88) | 0 | 25.0 | 0.152 (1.14) | 0.873 (1.40) |
| PIQ<70 | 60 | 0.492 (1.5) | 5.0% | 18.3 | −0.487 (1.32) | 0.763 (1.41)j |
| PIQ ≥ 70 | 135 | 0.743 (1.18) | 3.7% | 16.3 | 0.045 (1.17) | 0.730 (1.16)k |
| Simplex | 156 | 0.688 (1.29) | 4.5% | 17.3 | −0.065 (1.25) | 0.764 (1.21)l |
| Multiplex | 52 | 0.539 (1.35) | 1.9% | 17.3 | −0.248 (.91) | 0.272 (1.57)m |
| All ASD probands | 338 | 0.741 (1.32) | 3.3% | 17.5 | −0.029 (1.19) | 0.799 (1.30)n |
| Reference sample | 0 (1.0) | 3.0% | 3.0 | 0 (1.0) | 0 (1.0) | |
| Matched samples | ||||||
| Autism | 70 | 0.957 (1.14) | 2.9% | 18.6 | 0.134 (1.08) | 0.809 (1.02)o |
| Typical controls | 70 | 0.264 (0.92) | 1.4% | 4.3 | 0.223 (1.20) | 0.047 (1.15)p |
zHC = standardized head circumference.
Microcephaly: defined as head circumference, standardized for age and sex (zHC), 1.88 or more standard deviations below the reference sample mean (i.e., below the 3rd centile).
Macrocephaly: defined as head circumference, standardized for age and sex (zHC), greater than 1.88 standard deviations above the reference sample mean (i.e., above the 97th centile).
Mean zHC–zHgt is the mean of the difference between standardized head circumference and standardized height which was calculated for each subject (it differs slightly from the difference between mean zHC and mean zHgt of the groups).
N = 150.;
N = 134;
N = 16;
N = 21;
N = 90;
N = 31;
N = 36;
N = 102;
N = 133;
N = 17;
N = 224;
N = 54;
N = 59.
The mean zHC of typically developing controls was greater than the reference data mean, suggesting some small secular or other sampling effect on our samples compared to the reference sample (t = 2.39, P = 0.02). Despite this effect, mean zHC of the matched autism probands was significantly greater than the typical controls when subjects with macrocephaly and microcephaly were included and when they were excluded (included: t = 3.93, P < 0.004; excluded: t = 3.57, P = 0.004).
The rate of absolute macrocephaly (i.e., zHC more than 1.88 standard deviations above the mean, regardless of height) was increased in the autism probands (autism 17.3% vs. reference 3%, z = 12.12, P < 0.0004; matched autism 18.6% vs. typically developing 4.3%, z = 5.00, P < 0.0004). The rate of relative macrocephaly (i.e., zHC two or more standard deviations greater than standardized height) was also increased in the autism subjects (autism 16.7% vs. reference 2.5%, z = 11.18, P < 0. 0004; matched autism 29.8% vs. typicals 6.7%, z = 3.34, P = 0.0015). Absolute or relative macrocephaly was present in 22.6% of the autism proband sample. The rate of microcephaly was low in the autism sample (3.8%) and similar to the reference rate (autism 3.8%, reference 3%, z = 0.66, ns).
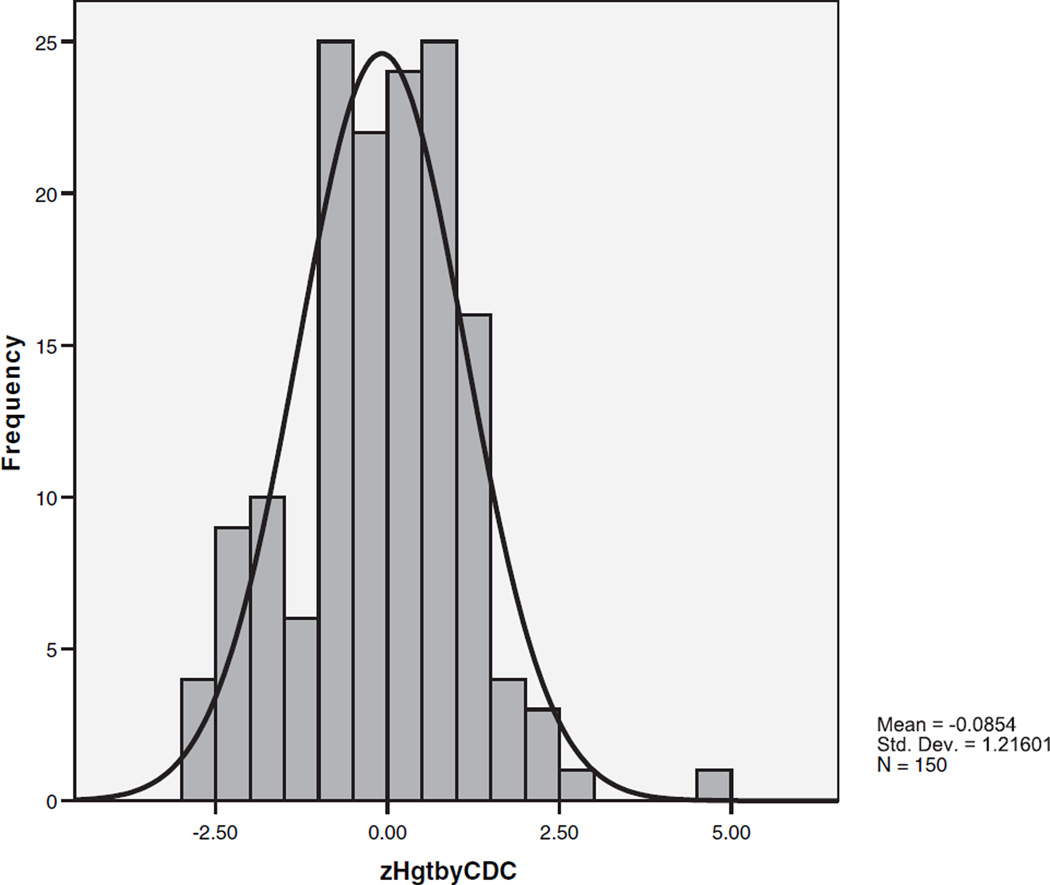
The Distribution of Standardized Height in Autism
Height data, available on 72% of the autism probands and 91% of typically developing subjects, are shown in Table III. Autism probands with and without height data did not differ on SES, severity of core features of autism measured with the total ADI algorithm score, seizures, mean zHC, or rates of macrocephaly and microcephaly. Compared to probands without data, autism probands with height data were older (mean age 10.2 vs. 8.2 years), had higher performance and verbal IQs (85 vs. 77, 80 vs. 64), and more of them were males (89% vs. 76%), multiplex (89% vs. 40%), and Caucasian (95% vs. 81%). These differences occurred because some sites ascertaining younger, lower-functioning children did not initially measure height.
The distribution of standardized height (zHgt) in the autism probands is shown in Figure 2. The distribution has a mean very close to the reference mean of zero and some indication of departure from normality (skewness = 0.125, standard error = 0.198, kurtosis = 0.925; Kolmogorov–Smirnov statistic = 0.053, P > 0.200 but Shapiro–Wilk statistic 0.981, P = 0.037). Mean zHgt of the matched autism and typically developing samples did not significantly differ (0.140 vs. 223, ns). Admixture analysis showed that a single distribution with one mean fit the data better than two or three distributions with differing means (chi-square = 1.67, df = 3, P = 0.64). The results were the same when all 224 subjects with ASD and height data were included in the analysis.
Fig. 2.
The distribution of standardized heights in autism probands.
The rate of tall stature (i.e., zHgt>1.88) was significantly increased in the total ASD sample (6.3%) but not in the autism sample (4.7%) compared to the expected rate of 3% (ASD: z = 2.89, P = 0.012). The rates of tall stature in the matched typical control and autism samples did not significantly differ (5.6% vs. 11.7%, z = −1.15, ns).
Short stature was increased in the total autism (8.7%) and ASD (6.3%) samples compared to the reference rate of 3% (z = 5.0, P < 0.0004), but there was no significant difference when matched autism (3.7%) and typical controls (1.4%) were compared (z = 0.79, ns). Short stature was found in 26.7% of females and 7% of males with autism. Individuals with autism and short stature appeared to differ from the autism group with normal stature in rate of history of seizures (45.4% vs.12.9%), percent from families of lower SES (SES III, IV, or V: 61.5% vs. 36.9%), and as expected given the correlation between zHC and zHgt, in mean zHC. The groups with short and normal stature did not differ in age, performance IQ, racial distribution, or fine and gross motor impairment. These are pilot findings only because only 13 subjects had short stature and height data were not available on all autism subjects.
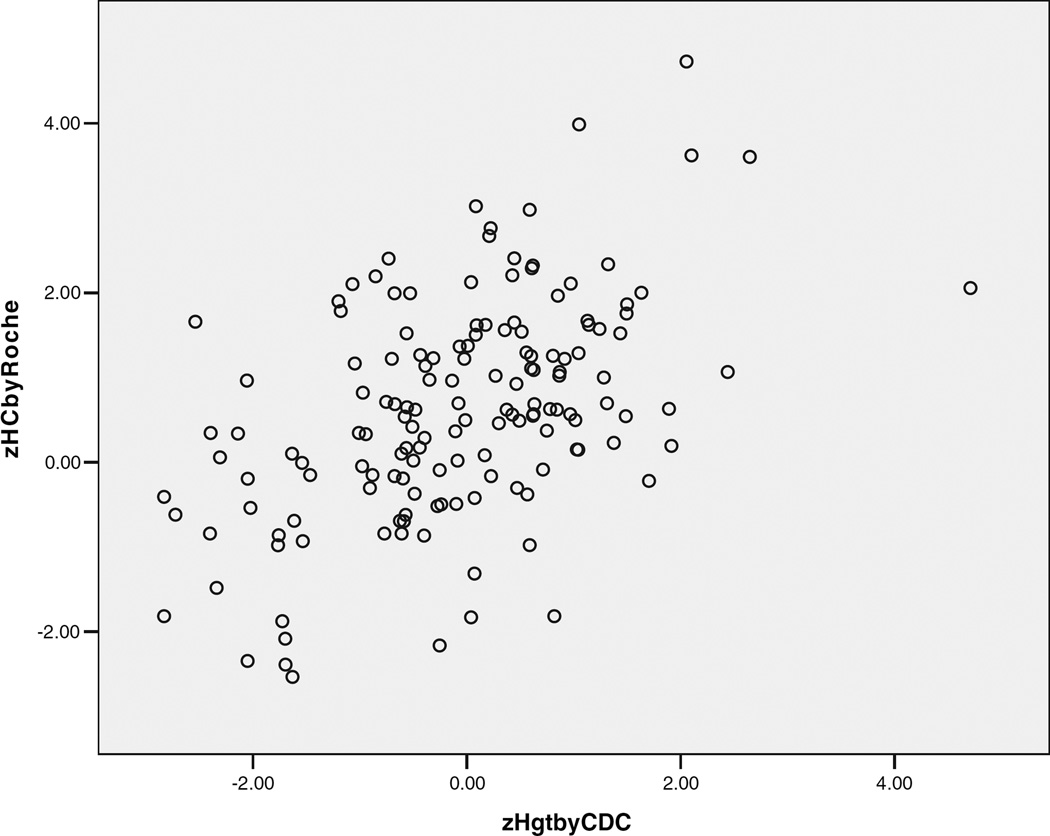
The Relationship Between Head Circumference and Height in Autism
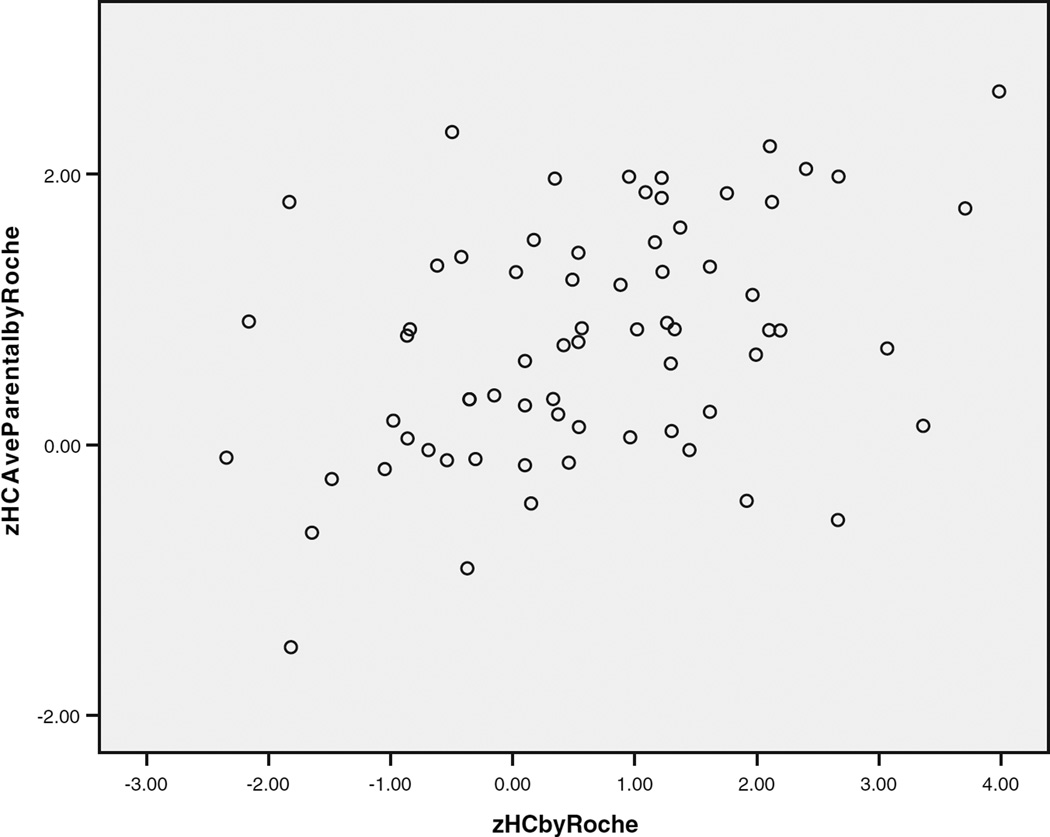
Standardized height and head circumference were significantly correlated in the typical controls (r = 0.459, P < 0.001) and autism samples (all autism: r = 0.495, P < 0.001; matched autism sample: r = 0.618, P < 0.001), as shown in Figure 3.
Fig. 3.
The relationship of standardized head circumference and height in autism probands.
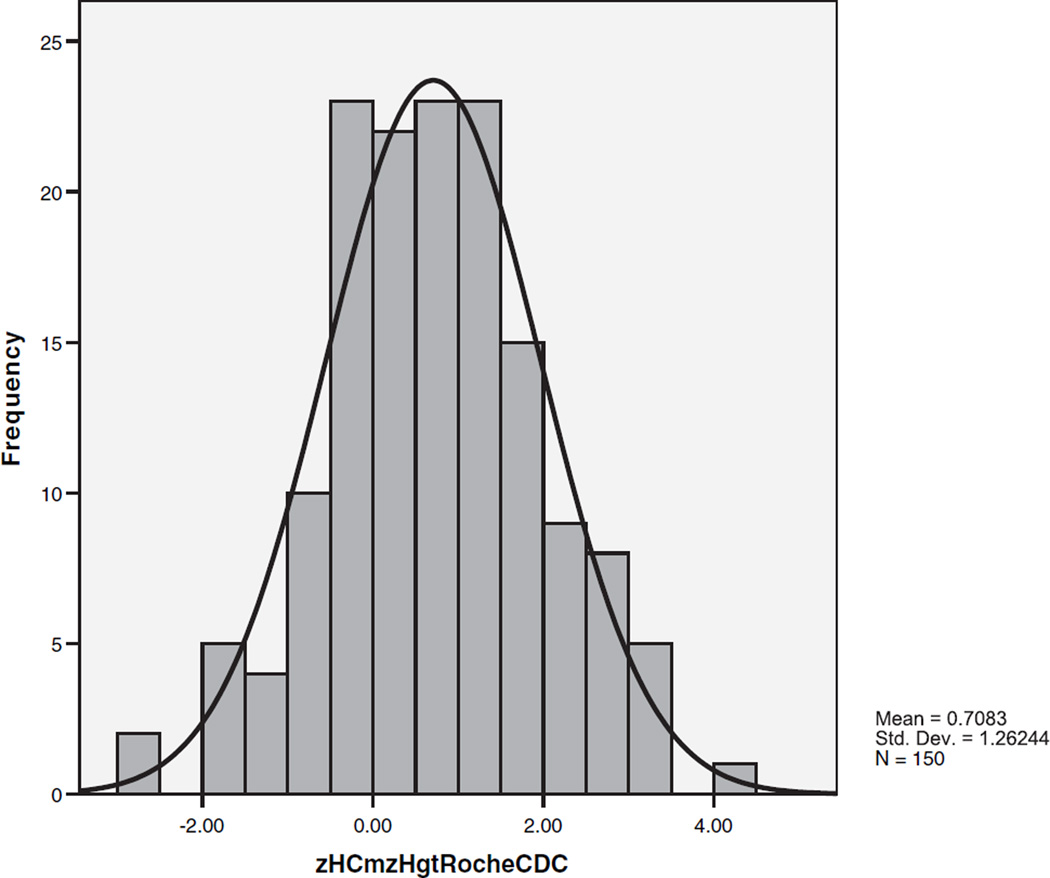
The difference between zHC and zHgt (zHC–zHgt) was calculated for each subject. Table III shows the mean discrepancies for the groups and subgroups. There was no significant discrepancy in the typical controls but in the autism sample the mean difference between zHC and zHgt was 0.708 standard deviations, significantly greater than the expected difference of 0 (autism vs. reference: t = 6.87, P < 0.004; matched autism 0.809 vs. typical controls 0.047: t = 3.71, P = 0.0004). Figure 4 shows the distribution of differences between zHC and zHgt in the autism probands. Similar to the zHC distribution, the mean is shifted upward and the variance increased. Differences between zHC and zHgt in the autism probands ranged from −2.66 to +4.20. To make sure that these findings were not an artifact resulting from the reference data we used, we recalculated the results using different reference data [Farkas, 1994]. The results were the same. We also examined the slopes of the regression line describing the relationship between zHC and zHgt in the matched autism and typically developing controls. The slopes of the best-fit regression lines were 0.709 for the autism group and 0.328 for the typical control group, again showing that the relationship is different for the two groups.
Fig. 4.
The distribution of the difference between standardized head circumference and height (zHC–zHgt) in autism probands.
Age, Sex, Socioeconomic Status, IQ, and Standardized Head Circumference in Autism
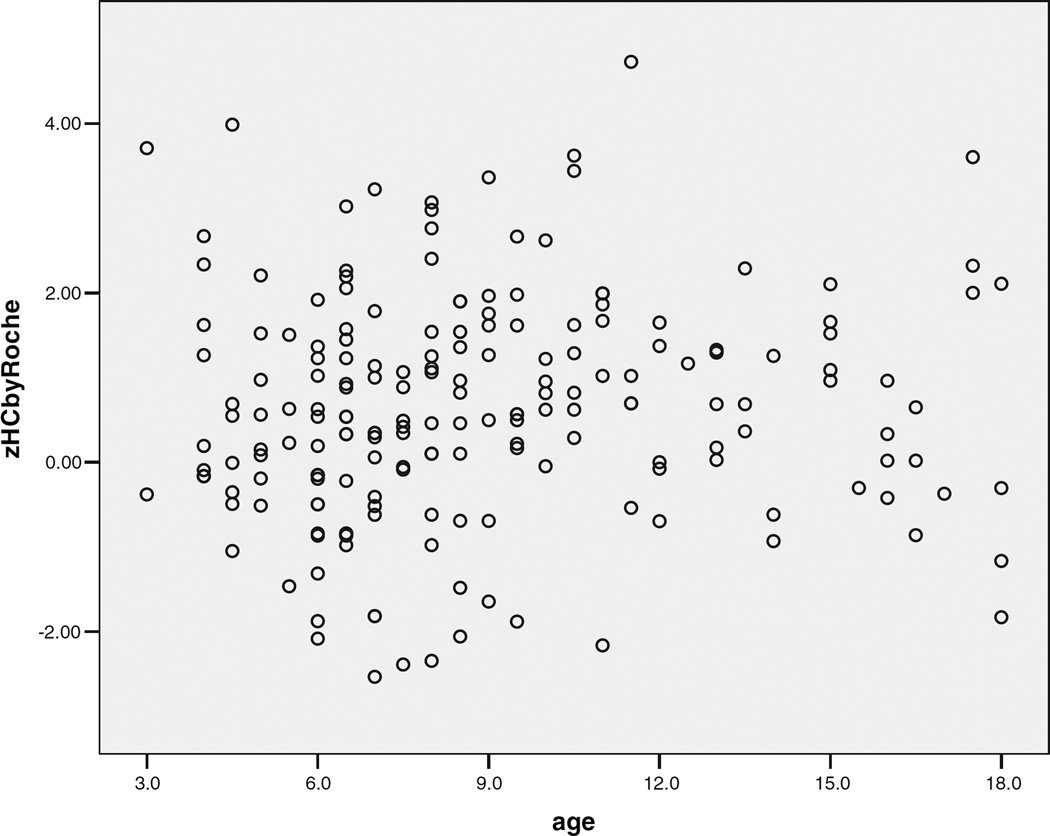
SES and IQ, but not age or sex, had independent effects on zHC in typically developing controls when zHgt was controlled (SES: t = 2.42, P = 0.02; vIQ: t = 2.36, P = 0.02, df 49). This was not the case for the autism subjects. Table III shows mean zHC values and rates of microcephaly and macrocephaly in the autism subjects by age, sex, and IQ group. There was a small, significant, positive correlation of zHC with age between 3 and 12 years of age (r = 0.173, P = 0.028) but not when older subjects were included (Fig. 5). Mean zHCs and rates of macrocephaly did not significantly differ in the four age groups of autism subjects (F = 0.228, ns; X2 = 0.475, ns). Mean zHC was 50% less in females than in males with autism, but the difference was not significant and the effect size (ES) was small (ES = 0.27). Similarly, mean zHgt, the mean discrepancy between zHC and zHgt, and the rates of macrocephaly and microcephaly did not significantly differ between male and female probands with autism or ASD.
Fig. 5.
Relationship of standardized head circumference and age in autism probands during childhood and adolescence.
There was no significant correlation between pIQ or vIQ and zHC in the individuals with autism. When pIQ was used to categorize probands as high-functioning (pIQ ≥ 70) and low-functioning (pIQ < 70), mean zHC was increased in both subgroups compared to the reference mean (t = 7.29, P < 0.0004; t = 2.54, P = 0.035). The difference in mean zHC between high- and low-functioning individuals with autism was, however, not significant (t = 1.15, ns, ES = 0.19). The discrepancy between vIQ and pIQ (vIQ-pIQ) had a small correlation with zHC when the discrepancy was considered as one of three groups (vIQ-pIQ = < −14, −14 to 14, and >14; n = 208, r = −0.209, P = 0.007) but not when it was analyzed dimensionally. Mean vIQ-pIQ significantly differed between cognitively high- and low-functioning autism subjects (HFA −8.49, LFA −0.078, t = −3.02, P = 0.01).
Head Size in Parents of Autism Probands
Parental head circumference data were available for 76 of the autism probands and 121 of the ASD probands. Autism probands with and without parent data did not differ on age, sex, race, pIQ, parental SES, total ADI algorithm score, zHC, zHgt, or rates of macrocephaly and microcephaly. Probands with parent data were more often multiplex (56% vs. 9%) and had significantly lower verbal IQs (66.9 vs. 79.5). Table III shows that multiplex status and IQ have insignificant effects on mean zHC in autism.
Table IV shows the mean standardized head circumferences and rates of macrocephaly and microcephaly for the 76 mothers and 71 fathers. The mean zHCs of mothers and fathers of autism probands was greater than the reference mean of 0 (z = 6.34 and z = 6.95, both P < 0.0004) and strikingly similar to the mean zHC of their offspring with autism. The rate of macrocephaly was increased in both mothers and fathers (P < 0.0004) and was similar to the 20.8% rate in their proband offspring. Three percent of the autism offspring had both parents with macrocephaly. Thirty-three percent (33%) of the autism offspring had either a mother or a father with macrocephaly. The rate of macrocephaly in either parent was similar for autism probands with macrocephaly (35.7%) and those without macrocephaly (32.7%). The results were the same when data from parents of all ASD probands (121 mothers, 106 fathers) were examined.
TABLE IV.
Mean Standardized Head Circumference and Rates of Macrocephaly and Microcephaly in Parents of Autism Probands
| Characteristic | Parents | ||
|---|---|---|---|
| Parents of autism probands |
Mothers | Fathers | Average parent |
| Parents of all autism probands |
76 | 71 | 69 |
| Mean zHC (sd) | 0.729 (1.32) | 0.759 (1.15) | 0.770 (0.87) |
| Range | −3.02, 2.41 | −1.04, 5.15 | −1.44, 3.16 |
| Microcephaly | 2.6% | 0 | 0 |
| Macrocephaly | 19.7% | 16.9% | Both 2.9% Either 33.3% |
| Parents of macrocephalic autism probands |
|||
| N | 15 | 15 | 14 |
| Mean zHC (sd) | 1.06 (1.21) | 1.10 (1.00) | 1.12 (.98) |
| Range | −0.98, 3.42 | −0.70, 3.11 | −0.56, 2.61 |
| Microcephaly | 0 | 0 | 0 |
| Macrocephaly | 20% | 20% | Both 7.1% Either 35.7% |
Maternal, paternal, and mid-parental zHCs were significantly correlated with zHCs of the autism offspring (shown in Table VI). Figure 6 shows a scatterplot of the relationship between autism pro-band and average parental zHC. Most of the autism offspring had zHCs within two standard deviations of the average zHC of their parents. More autism probands (11.6%) than expected (2.5%) had zHCs two or more standard deviations greater than their parents (z = 9.1, P < 0.004). No autism proband had zHC more than two standard deviations below the parent average. The 11.6% rate in autism probands was not significantly different from the 7% of typically developing individuals whose zHCs are two or more standard deviations away from the average zHC of their parents [Weaver and Christian, 1980].
TABLE VI.
Correlates of Macrocephaly and Standardized Head Circumference (zHC)
| Variable | Association with macrocephaly | Correlation with zHC |
|---|---|---|
| Standardized height (zHgt) | t = 3.32 P = 0.0036 | r = 0.495 P < 0.0001 |
| Difference between zHC–zHgt | t = 5.68 P = 0.0004 | r = 0.548 P < 0.0001 |
| Difference between proband and average parent zHC |
t = 6.41 P = 0.003 | r = 0.793 P < 0.0001 |
| ADI-R social algorithm score | t = 2.66 P = 0.022 | r = 0.180 P = 0.009 |
| Onset single words (delayed or not) |
X2 = 6.83 P = 0.024 | ns |
| Average parental zHC | ns | r = 0.399 P = 0.001 |
| Mother zHC | ns | r = 0.271 P = 0.018 |
| Father zHC | ns | r = 0.272 P = 0.022 |
| ADI-R communication algorithm score (verbal children) |
ns | r = 0.199 P = 0.012 |
| ADI-R degree of overactivity | ns | r = −0.197 P = 0.038 |
| Total ADI algorithm score | ns | r = 0.164 P = 0.025 |
Fig. 6.
Scatterplot of autism proband zHC and average parental zHC.
Table V shows parent-child resemblance in zHC variance in the autism families measured by linear regression. Their resemblance is similar to the heritability reported in typical families in whom 50% of the variance is due to familial factors [Weaver and Christian, 1980]. The results were similar when data from all probands with ASD and their parents were examined.
TABLE V.
Familial Resemblance in zHC Between Autism Probands and Their Parents
| Expected value |
Observed value |
h2 | |
|---|---|---|---|
| Parent-offspring regressions | |||
| Autism Probands | |||
| Midparent proband | h2 | 0.399 | 0.399 |
| Father proband | h2/2 | 0.272 | 0.544 |
| Mother proband | h2/2 | 0.271 | 0.542 |
Correlates of Macrocephaly and Standardized Head Circumference (zHC) in Autism
Correlates of zHC may be either continuous, for example, associated with zHC across the entire distribution including macrocephaly or discontinuous, for example, associated with the macrocephaly part of the zHC distribution but not the entire distribution. Table VI shows variables that were associated with macrocephaly, used as a categorical variable, correlated with zHC dimensionally across the head circumference distribution, or both. The only variable uniquely associated with macrocephaly in the autism sample was the categorical variable delay in the onset of spoken words (n = 189). Seventy percent of macrocephalic subjects and 44% of non-macrocephalic autism subjects had a reported delay in the onset of single words used communicatively. The continuous variable, age at onset of words, however, was not correlated with zHC. Subjects with macrocephaly and subjects without macrocephaly did not differ on the following variables: age at the time of evaluation, mean age at clinical onset of autism, history of early developmental regression, male:female ratio, percent with an affected sibling with ASD (multiplex), parental SES, maternal education, verbal and performance IQ, vIQ-pIQ discrepancy, ADI-R stereotyped repetitive behavior algorithm score, percent who were nonverbal, or who had a history of fine or gross motor problems or seizures. The number of microcephalic children (n = 7) in the autism sample was too small for meaningful analysis.
Head Circumference and Height in PDD-NOS
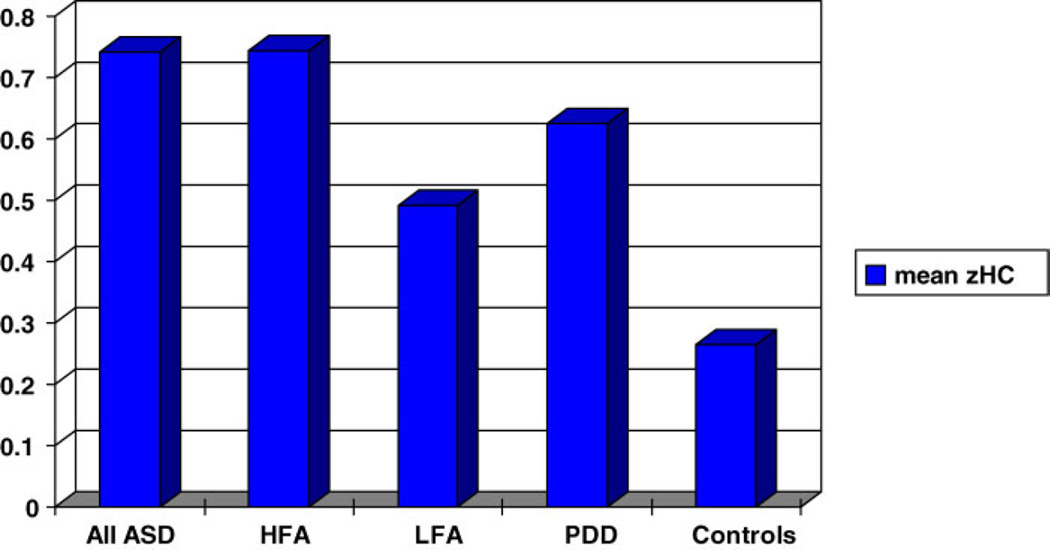
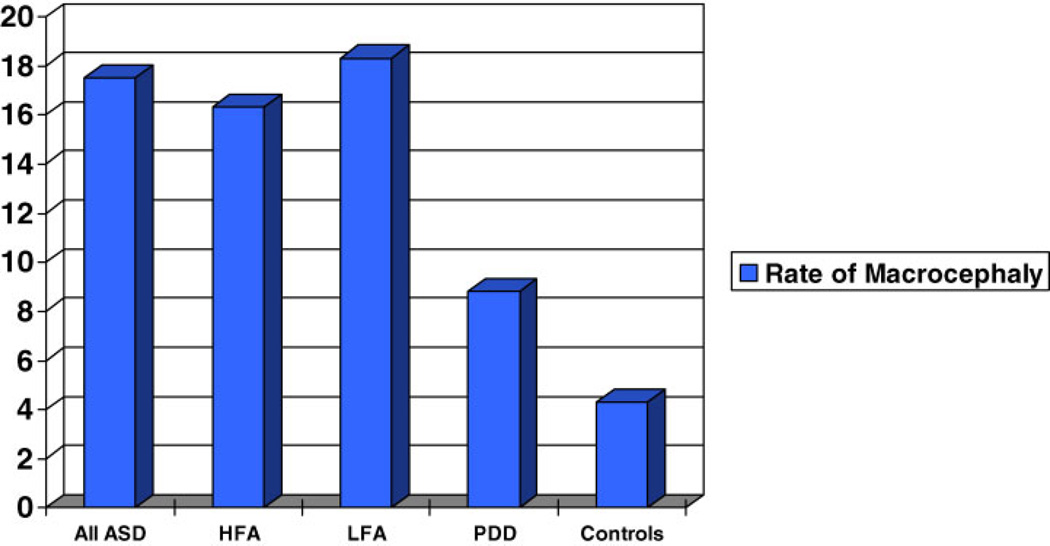
There were 34 probands with pervasive developmental disorder—not otherwise specified (PDD-NOS). Figures 7 and 8 show mean zHC and rate of macrocephaly for the PDD-NOS sample compared to the autism and typically developing samples. Mean zHC was similar in the PDD-NOS and combined autism samples (mean (sd): PDD-NOS = 0.625 (0.97), autism = 0.651 (1.30), t = 0.110, ns). The rate of macrocephaly in the PDD-NOS probands was half the rate in the autism probands but the difference did not reach significance although the effect size was large (PDD-NOS = 8.8%, autism = 17.3%, X2 = 1.56, P = 0.212, ES = 1.53; odds ratio = 2.16, CI: 0.627, 7.46). None of the PDD-NOS probands were microcephalic and their standardized height (zHgt) did not significantly differ from the other groups. The number of probands with sufficient data for a CPEA diagnosis of Asperger’s disorder (n = 7) was too small for analysis.
Fig. 7.
Mean zHC of all ASD probands, cognitively high-functioning and low-functioning autism probands, PDD-NOS probands, and typically developing controls. ASD, all autism-spectrum disorder probands; HFA, cognitively higher-functioning autism probands (pIQ > 70); LFA: cognitively lower-functioning autism probands (pIQ < 70); PDD, probands with pervasive developmental disorder, not otherwise specified (PDD-NOS); Controls: typically developing individuals.
Fig. 8.
Rate of macrocephaly (percent) in all ASD probands, cognitively high-functioning and low-functioning autism probands, PDD-NOS probands, and typically developing controls.
Head Circumference and Height in Affected Versus Unaffected Siblings
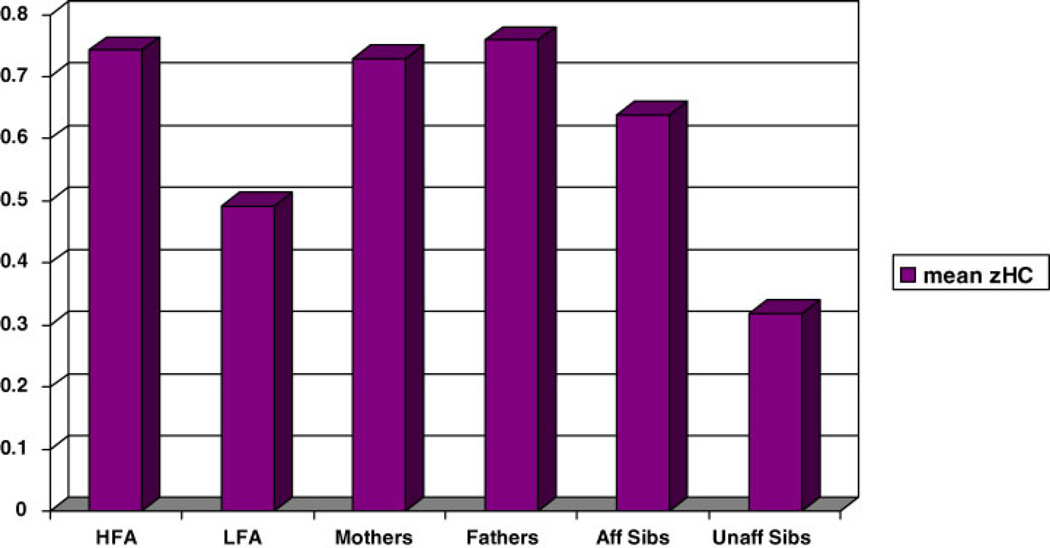
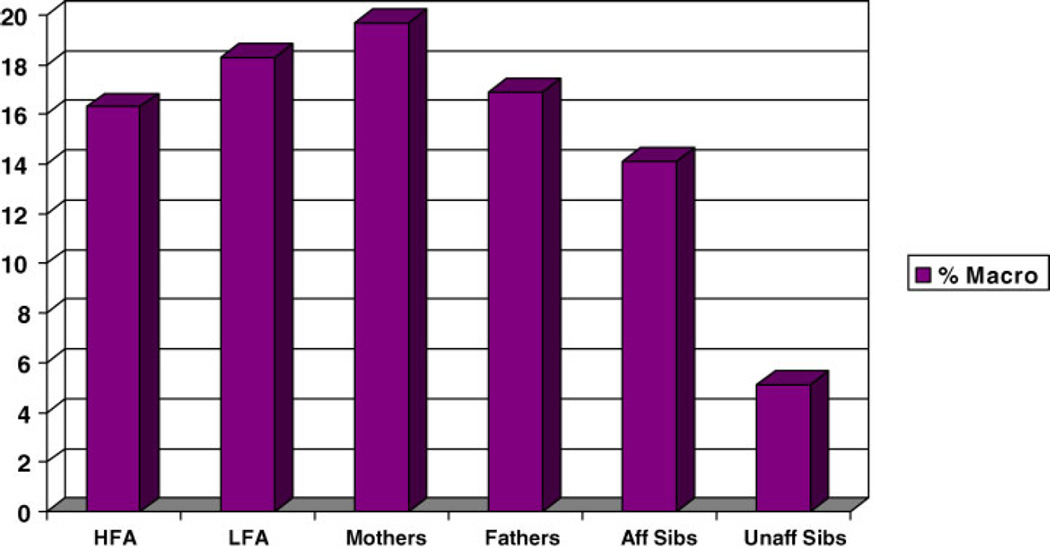
“Affected” was defined as having any ASD (autism, Asperger, PDD NOS, or broad ASD) and “unaffected” as not meeting criteria for any ASD. Figures 9 and 10 show the mean zHCs and rates of macrocephaly in 71 affected siblings and 78 unaffected siblings of the autism proband. Affected siblings had a higher rate of macrocephaly than unaffected sibling (14.1% vs. 5.1%,z = 3.21, P = 0.0047). Mean zHC was increased in affected siblings (0.638) but the difference from unaffected siblings (0.318) did not reach significance (z = 1.76, ns).
Fig. 9.
Mean zHC in Autism Families: High- and low-functioning Autism probands, mothers, fathers, affected, and unaffected siblings.
Fig. 10.
Rate of macrocephaly (percent) in Autism families: high- and low-functioning autism probands, mothers, fathers, affected, and unaffected siblings.
DISCUSSION
The findings of this study add several important pieces of information to our understanding of head circumference, height, and macrocephaly in persons with autism. The distribution of standardized head circumference (zHC) in individuals with autism is unimodal, normal in shape, with the mean shifted to the right and the variance increased. Mean standardized height (zHgt) is normal in autism. Across the entire distribution, head circumference tends to be larger than expected relative to height. Mean head circumference and rate of macrocephaly are increased in the parents of autism probands with and without macrocephaly.
The Head Circumference Distribution
The distribution of zHC in the autism probands in our study was normal in shape. This result contrasts with the findings of three other studies [Lainhart et al., 1997; Fombonne et al., 1999; Miles et al., 2000]. All three of these studies found evidence that the distribution of head circumference in autism deviated from normal. The sample sizes in these studies were 34%–56% smaller than in the current study, diagnostic classification methods were not as stringent, not all of the subjects had idiopathic autism, and the proportion of subjects with mental retardation was twice as high as in the current study. All of these factors may explain the different findings. When we performed a simulation on our dataset, randomly excluding subjects with high-functioning autism to achieve a rate of mental retardation of 60%, similar to the rates in other three studies and the autism population, the distribution of zHC was still not statistically different from a normal distribution. Family of origin SES of the subjects in the current study was higher than in two of the other three studies and in the autism population. But our data show that while SES is related to standardized head size in typically developing individuals, no such relationship is found in autism. In addition, our data show that the increased mean of the zHC distribution is not due to a secular effect in our sample or simply due to the increased rate of macrocephaly. Using the most stringent inclusion and exclusion criteria and diagnostic classification to date, the findings show that the tendency toward increased head circumference in idiopathic autism affects most of the distribution. It appears that some factors are operating in idiopathic autism to shift the distribution of standardized head circumference upward and increase the normal degree of biological variation in this measure.
Height and its Relationship to Head Circumference
Increased mean height is not a factor shifting head size upward in persons with autism. Examination of the distribution of standardized heights in autism shows that the mean and the rate of tall stature are not increased. This finding is in agreement with another study [Miles et al., 2000]. Although standardized head circumference and height are correlated in the autism sample, a key finding of this study is that head circumference tends to be larger than expected for height. The normal link between head size and height found in typically developing individuals is perturbed; proportionality between head size and height is decreased in autism. This suggests that factors are differentially affecting head and brain size in autism, rather than affecting body size as a whole.
Although the tendencies toward larger head size, macrocephaly, and head size larger than expected for height are strong in autism, the variability in all of these measures is striking; they are not universal features in persons with idiopathic autism. In absolute terms, the head (and likely the brain) is not always large in autism. Abnormal enlargement is present in a minority (17%) of cases. Our data show that head size in autism may be normal or even smaller than normal, proportionate to height or small relative to height. These results suggest that research aimed at determining why head and brain size vary so widely, in what is still clinically recognized as one disorder, may be as important as the recent focus on why the head and brain are sometimes abnormally large in autism. Research is also needed to determine if head size across the entire zHC distribution and brain size at some time during development, are larger than they would have been if the child had not had autism. The stepwise effect of zHC in the affected and unaffected siblings in our study suggests the possibility that increased brain size may be one liability factor influencing whether a child has a subclinical phenotype or full autism.
Factors Associated With Variation in Standardized Head Circumference in Autism
We identified factors that contribute to the wide variability in standardized head size in autism. Some of these are the same factors that contribute to head size variability in typically developing individuals: height and parental head size. The only other study to examine height relative to head circumference in older children and adults with autism also found an association [Miles et al., 2000]. Our findings about parent head size are in agreement with the results of three studies that found an increased rate of macrocephaly in parents of autism probands including the only study that examined the relationship between proband and parent head size [Stevenson et al., 1997; Fidler et al., 2000; Miles et al., 2000]. We found an increased rate of macrocephaly in parents of macrocephalic and non-macrocephalic probands. In our sample, maternal, paternal, and mid-parental standardized head sizes significantly correlated with proband head size. The independent effect of parent head size on proband head size appeared to be strong; when it was entered into a regression model no other factors, including proband zHgt, had independent effects. The parent-proband resemblance of zHC in our autism sample is similar to the estimated heritability reported in typical families. Because parental head size precedes and affects proband head size, the findings highlight an important question in autism research: why is parental head size increased in idiopathic autism?
Several factors related to head size in typically developing individuals were not correlated with, or independently contributed to, standardized head size (zHC) in our autism sample. These included non-verbal and verbal IQ, family of origin SES, and maternal education. Several others studies did not find a relationship between zHC or macrocephaly and IQ in autism [Lainhart et al., 1997; Stevenson et al., 1997; Fombonne et al., 1999; Miles et al., 2000; Gillberg and De Souza, 2002]. Two studies found increased rates of mental retardation in autism subjects with microcephaly, but IQ was not a significant independent predictor [Fombonne et al., 1999; Miles et al., 2000]. Because we used reference data and z-scores to standardized subject values for age and sex, we did not expect these factors to affect zHC in our autism sample. Mean zHC in our small sample of females with autism was 50% less than in the males, but the differences in mean zHC and rates of macrocephaly were not significant. The later findings are in agreement with several other studies [Fombonne et al., 1999; Miles et al., 2000; Dementieva et al., 2005].
Retrospective studies comparing birth to later head circumference in autism and neuroimaging studies of brain volume have suggested an age effect on zHC and rate of macrocephaly with higher values early in childhood compared to later [Lainhart et al., 1997, 2005; Courchesne et al., 2001, 2003; Sparks et al., 2002]. Instead of a negative correlation between zHC and age in children, we found a small positive correlation between 3 and 12 years. Rates of macrocephaly were not significantly greater in younger versus older children with autism in our sample. Our data suggest that the rate of macrocephaly in autism reaches 15%–20% by 3–5 years of age and remains stable thereafter. Our finding is in agreement with the results of a recent analysis of data pooled from other published studies [Lainhart, 2006].
Continuous Versus Discontinuous Correlates of Standardized Head Circumference in Autism
Our hypothesis that macrocephaly identifies a unique subtype of autism was not supported by analysis of the head circumference distribution or examination of correlates. Most of the factors associated with macrocephaly were correlated with zHC across the distribution rather than being unique correlates. Variables whose means were increased in subjects with macrocephaly compared to subjects without macrocephaly and positively correlated with zHC in a continuous manner across the distribution were standardized height, the discrepancy between standardized head circumference and height, the discrepancy between proband and average parental zHC, and the ADI social algorithm score. The only variable uniquely associated with macrocephaly was parental report that the proband had had delayed onset in meaningful use of single words, a key index of onset of language. This finding must be considered preliminary pending replication in independent samples. Macrocephaly in autism is very likely multifactorial, and it is still possible that other unique correlates of subgroups of autism probands with macrocephaly will be found. Biological correlates, such as variants of the HOXA1, PTEN, and other genes, are likely candidates [Conciatori et al., 2004; Butler et al., 2005; McCaffery and Deutsch, 2005].
PDD-NOS and Affected and Unaffected Siblings
Preliminary examination of head size in subjects with PDD-NOS probands shows no difference from autism subjects in mean standardized head circumference. Rate of macrocephaly was lower but it did not reach statistical significance. Examination of affected and unaffected sibling data suggests a step-wise effect in autism families. Mean standardized head circumference tends to be the largest in autism probands and their parents, followed by affected siblings and then unaffected siblings.
Implications
The most important observation of our study is the very wide distribution of head circumference in persons with autism. The increased variance of the distribution underscores the clinical heterogeneity of autism, and it supports recent thinking about the potential importance of studying dimensional features of autism, rather than just categorical features [Tager-Flusberg and Joseph, 2003]. Neurobiological theories of autism must be consistent with this wide variation. Similarities in the shapes of the zHC distributions in autism and typically developing individuals raises the possibility that alterations in head and brain size and growth in autism may be indirect, rather than direct, effects of primary neuropathological mechanisms operating in autism.
The wide distribution may help explain why the findings of neuroimaging and post-mortem studies of autism have not been more consistent [Bigler et al., 2003]. Small samples sizes randomly drawn from the very wide distribution may, by chance alone, vary in how representative they are of the population of individuals with idiopathic autism. Many regional brain volumes are related to total brain volume. Scaling of regional brain volumes to each other and to total brain volume may be non-uniformly different in large versus small brains [Casanova, 2004; Lainhart et al., 2005]. All of these reasons underscore the importance of large collaborative studies that have high statistical power to sort out what has gone awry in the brains of children and adults with autism.
Limitations
The majority of individuals with autism in this study were high-functioning. This focus increased the homogeneity of the sample and decreased the likelihood of undetected medical causes of autism. In some analyses of subgroups, sample sizes were not large enough to provide definitive tests for some of the findings. This uncertainty applies to gender effects, adults with autism, probands with PDD-NOS, and parents.
Acknowledgments
This work was supported by the NICHD: U19s HD35476 (University of Utah), HD35469 (University of Pittsburgh), HD34565 (University of Washington), HD35468 (University of California at Davis), HD035470 (UCLA), HD35466 (University of Rochester) and NICHD/NIDCD DC03610 (Boston University), HD 35482 (Yale University), and the NICHD/NIDCD Collaborative Program of Excellence in Autism (CPEA). We thank Jubel Morgan RN, JoAnn Petrie, Pamela Flodman, and the other staff of the CPEA sites who helped in collection of the data. We express our sincere gratitude to the children and adults with ASDs and their families and the typically developing individuals who participated in this CPEA Network.
REFERENCES
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Biol B. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83. [Google Scholar]
- Bigler ED, Tate DF, Neeley ES, Wolfson LJ, Miller MJ, Rice SA, Cleavinger H, Anderson C, Coon H, Ozonoff S, Johnson M, Dinh E, Lu J, McMahon W, Lainhart JE. Temporal lobe, autism, and macrocephaly. Am J Neuroradiol. 2003;24:2066–2076. [PMC free article] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Good S, Crowson M, Bailey A, Rutter M. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Petti TA, Green WH, Cohen H, Genieser NB, David R. Some physical parameters of young autistic children. J Am Acad Child Psychiatry. 1980;19:193–212. doi: 10.1016/s0002-7138(09)60697-x. [DOI] [PubMed] [Google Scholar]
- Casanova MF. White matter volume increase and mini-columns in autism. Ann Neurol. 2004;56:453. doi: 10.1002/ana.20196. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: Confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Conciatori M, Stodgell CJ, Hyman SL, O’Bara M, Militerni R, Bravaccio C, Trillo S, Montecchi F, Schneider C, Melmed R, Elia M, Crawford L, Spence SJ, Muscarella L, Guarnieri V, D’Agruma L, Quattrone A, Zelante L, Rabinowitz D, Pascucci T, Puglisi-Allegra S, Reichelt KL, Rodier PM, Persico AM. Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biol Psychiatry. 2004;55:413–419. doi: 10.1016/j.biopsych.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Davidovitch M, Patterson B, Gartside P. Head circumference measurements in children with autism. J Child Neurol. 1996;11:389–393. doi: 10.1177/088307389601100509. [DOI] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, DeLong GR, Abramson RK, Wright HH, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Deutsch CK, Joseph RM. Brief report: Cognitive correlates of enlarged head circumference in children with autism. J Autism Dev Disord. 2003;33:209–215. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales: Introductory and technical handbook. New York: The Psychological Corporation; 1990. [Google Scholar]
- Farkas LG. Anthropometry of the head and face. New York: Raven Press; 1994. p. 405. [Google Scholar]
- Fidler DJ, Bailey JN, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol. 2000;42:737–740. doi: 10.1017/s0012162200001365. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Roge B, Claverie J, Courty S, Fremolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Simmons H, Ford T, Meltzer H, Goodman R. Prevalence of pervasive developmental disorders in the British nationwide survey of child mental health. J Am Acad Child Adolesc Psychiatry. 2001;40:820–827. doi: 10.1097/00004583-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Gillberg C, De Souza L. Head circumference in autism, Asperger syndrome, and ADHD: A comparative study. Dev Med Child Neurol. 2002;44:296–300. doi: 10.1017/s0012162201002110. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropath Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC growth charts for the United States: Methods and development. Vital Health Stat Series. 2002;11:1–190. [PubMed] [Google Scholar]
- Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet Part C Semin Med Genet. 2006;142C:33–39. doi: 10.1002/ajmg.c.30080. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Lazar M, Bigler E, Alexander A. The brain during life in autism: Advances in neuroimaging research. In: Casanova M, editor. Recent Developments in Autism Research. Hauppauge, New York: NOVA Science Publishers; 2005. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Aut Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- McCaffery P, Deutsch CK. Macrocephaly and the control of brain growth in autistic disorders. Prog Neurobiol. 2005;77:38–56. doi: 10.1016/j.pneurobio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Miles JH, Hadden LL, Takahashi TN, Hillman RE. Head circumference is an independent clinical finding associated with autism. Am J Med Genet. 2000;95:339–350. [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidence Service Inc; 1995. [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Roche AF, Mukherjee D, Guo SM, Moore WM. Head circumference reference data: Birth to 18 years. Pediatrics. 1987;79:706–712. [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International performance scale-revised. Wood Dale, IL: Stoelting Company; 1997. [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ. Autism and macrocephaly. Lancet. 1997;349:1744–1745. doi: 10.1016/S0140-6736(05)62956-X. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DD, Christian JC. Familial variation of head size and adjustment for parental head. J Pediatrics. 1980;96:990–994. doi: 10.1016/s0022-3476(80)80623-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scales for Children-Third Edition (WISC-III) San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-third edition (WAIS-III) San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Woodhouse W, Bailey A, Rutter M, Bolton P, Baird G, Le Couteur A. Head circumference in autism and other pervasive developmental disorders. J Child Psychol Psychiatry. 1996;37:665–671. doi: 10.1111/j.1469-7610.1996.tb01458.x. [DOI] [PubMed] [Google Scholar]