Abstract
The dynamics of Canadian lynx (Lynx canadensis) abundance are geographically structured according to the influence of large-scale climatic regimes. Here we demonstrate that this structuring matches zones of differential snow conditions, in particular surface hardness, as determined by the frequency of winter warm spells. Through a modified functional response curve, we show that various features of the snow may influence lynx interaction with its main prey species, the snowshoe hare (Lepus americanus). This study highlights the importance of snow, and exemplifies how large-scale climatic fluctuations can mechanistically influence population biological patterns.
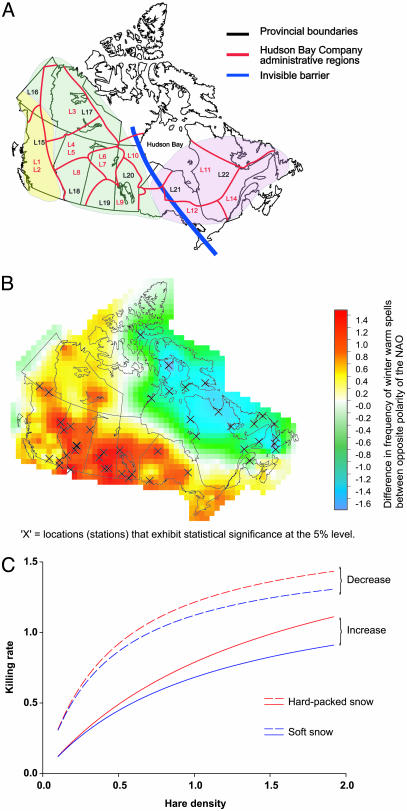
The Canadian subcontinent may be divided into three climatic regions, Pacific, Continental, and Atlantic (Fig. 1A), and the Canadian lynx (Lynx canadensis) seems to be influenced ecologically and evolutionary by the differential climatic conditions. Time-series data on the abundance of the cyclic-fluctuating lynx (1) show a similar large-scale spatial structuring, as do the genetic variability data, suggesting restricted gene flow in western and eastern Canada (2). Because there are no obvious geographic obstacles in eastern Canada, a hypothetical “invisible barrier” south of Hudson Bay was introduced to explain the lowered connectivity observed in the lynx population (2). Through numerical analysis of the climate forcing of ecological and evolutionary patterns (CEEP) model (3), it was later revealed that opposite effects of the North Atlantic oscillation (NAO) (4) on each side of this boundary could directly influence synchrony in the lynx dynamics, which again affect the rate of genetic diversification (3). Thus, a possible explanation for the genetic structuring was provided, but a direct link between climate and lynx ecology was still lacking. Here, we present data suggesting that differential snow conditions coupled to the hunting capacity of the lynx might generate the invisible barrier.
Fig. 1.
The relation between snow condition and lynx population dynamics and lynx hunting success. (A) The Pacific (yellow), Continental (green), and Atlantic (pink) regions; the old Hudson Bay time series are given as L1–L14 (red) and the modern Statistics Canada time series are given as L15–L22 (green) (1). The thick blue line separating the eastern Atlantic from the central Continental region represents the previously hypothesized [first on the basis of statistical modeling of time-series data on lynx (1) and later on the basis of genetic analyses (2)] invisible barrier. (B) Composite difference in the frequency of winter warm spells between the positive and negative polarity of the NAO during winter. A warm spell is defined as the persistence of the daily maximum temperature above the 80th percentile for at least 3 consecutive days. The NAO index is taken as the time series of the leading empirical orthogonal function of the Northern Hemisphere sea level pressure for the December-to-March period between 20–80°N, 90°Wto 40°E (www.cgd.ucar.edu/∼jhurrell/nao.html). Positive polarity of the NAO leads to lower frequency of warm spells in the Atlantic region and more frequent warm spells in the Continental region. Positive polarity occurs when there is anomalously low sea level pressure throughout the North Atlantic and higher than normal sea level pressure south of 55°N. Statistical significance is determined by a Monte Carlo technique in which the sample is shuffled 1,000 times. (C) The functional response curve (describing lynx hunting success under different conditions of hare abundance) including the phase and snow sinking depth (adjusted R2 = 95%); kill rate, or hunting success, is given as number of killed hares per predator per day, and hare density is given as number of hares per hectare.
Geographic Differences in Snow Properties Across Canada
Winter warm and cold spells are important climatic features in determining the hardness of the snow surface (5). Fig. 1B shows the relation between winter warm spells and NAO across Canada: when there are fewer warm spells in the Atlantic region, there are more warm spells in the Continental region, and vice versa. The frequency of cold spells is complementary: when there are fewer cold spells in one region, there are more cold spells in the other region. The surface properties of the snow determine the sinking depth of lynx. For instance, when there are few warm spells, the snow remains fluffy and the lynx sinks deep, whereas its main prey species, the snowshoe hare (Lepus americanus), does not so easily sink in the snow and will easily escape under such conditions.
The Functional Response Curve Describing the Trophic Relation Between the Lynx and the Hare Under Different Snow Conditions
To assess the effect of snow conditions on lynx hunting ability, we applied a functional response curve (6) modified to incorporate the effect of the stage of the density cycle (phase) (7) and the possible effect of snow conditions. The data on kill rates are derived from a large study on lynx and other boreal predators in Kluane (Yukon, Canada; ref. 8). Five snow variables were measured from 1987 to 1996: snow depth on/off lynx trail, sinking depth of lynx in snow, and snow hardness on/off lynx trail, all in centimeters. Snow variables were measured every 600 m while tracking lynx (see ref. 9 for details). Preliminary exploratory statistical analysis suggested that only the sinking depth is related to the kill rate (data not shown).
In addition to the hare abundance (given as x), two variables were used in the functional response model: snow sinking depth (given as Snow) and the phase of the lynx population cycle (given as Phase). The snow measurements reported in ref. 8 were “averages” of measurements over irregular dates from mid-September to late March; specifically, we fitted a simple linear regression of the sinking depth on the date of the measurement since September 1, and the fitted values of the sinking depth at day 150 since September 1 (i.e., at the end of January) were used in the model (as the variable Snow). The variable Phase represented the increase (=0) or decrease (=1) phase of the 10-year lynx cycle (7). As in our earlier study (7), data from eight complete winters were used.
We have fitted seven functional response curves to the data by incorporating different combinations of coefficients of Snow and Phase in the numerator and/or the denominator to be zero. The kill rates were modeled as ϕ(x) + ε, where the noise term, ε, is assumed to be independent and identically normally distributed with zero mean and constant variance, σ2. All models were fitted by the method of minimum least squares via the nlm function in r (www.r-project.org). The resulting best model is given as
 |
where standard errors are shown in parentheses below the corresponding point estimates. All coefficients, except that of Snow, are significant at the 5% level. However, when the snow variable is removed from the above model, the residual sum of squared errors increases by 10% [AIC(Phase) = –40.1, RSS(Phase) = 0.0195; AIC(Phase, Snow) = –38.9, RSS(Phase, Snow) = 0.0177), where AIC is the Akaike information criterion and RSS is the residual sum of squares; Snow thus accounts for (0.0195–0.0177)/0.0177 = 10.2% of the residual variation over the model with only Phase included]. Given that data for only 8 years are available (6), we interpret this 10% improvement to be biologically real. Clearly, ϕ decreases with Snow. Furthermore, with Snow and x fixed, the differences ϕ(x; Phase = 1, Snow)–ϕ(x; Phase = 0, Snow) are positive for all eight data points, (mean, 0.3; standard deviation, 0.093), indicating that the adverse effects of Snow on the lynx hunting success rate are stronger during the increase phase than the decrease phase.
Fig. 1C shows the functional response curves for lynx preying on snowshoe hare under different snow conditions and during the two main density phases. It suggests that this trophic interaction is affected by the snow sinking depth. Specifically, the lynx killing rate is lowered when the sinking depth is deeper, and more so during the increase phase than during the decrease phase of the population cycle.
Discussion and Conclusion
The concordance between the ecological (1) and genetic (2, 3) border between the Atlantic and the Continental region (Fig. 1 A) and the geographic pattern of warm spells across Canada (Fig. 1B) is striking. Stenseth et al. (3) demonstrated how NAO acts as a synchronizer on lynx dynamics within each geographic region. Here, we approach an ecological understanding of how lynx is actually affected by the climate.
The abundance of lynx, as a specialized top predator, is inseparable from the abundance of snowshoe hare. The phase-dependent structure of the lynx dynamics, in the second autoregressive component of the model used in Stenseth et al. (1), may be seen as a result of the interaction between the hare and the lynx (7). The fact that this trophic interaction is influenced by properties of the snow, which result from temperature changes (i.e., the frequency of warm/cold spells) correlated to the NAO, suggests a specific process generating the large-scale pattern observed in lynx dynamics across Canada.
The frequency of warm spells is correlated to the hardness of the snow surface, and we have shown that the snow sinking depth affects the killing capacity of lynx (Fig. 1C). However, further data on hunting success by lynx in hard and soft snow at different phases of the hare cycle should be obtained. Further analyses, both meteorological and ecological, are certainly needed to determine to what extent this mechanism explains the geographic pattern observed.
The NAO-linked periodicity of the warm spells might play a key factor in the phase-structuring of the functional response curve and ought to be further evaluated empirically. Moreover, the geographic extent of the proposed invisible barrier should be identified. This might be done by on-the-ground surface studies, or through analyses of surface snow–melt events from passive microwave retrievals from satellites (9).
It has been observed (although the data are scarce) that lynx familiar with certain snow and prey conditions tend to stay within an area of similar conditions when dispersing (10), most likely because there is a cost to learning how to use a new habitat, reducing the probability of successful migration. More comprehensive studies are needed to eventually verify this, and whether the dispersal length is reduced in softer snow conditions should be measured. Theoretical analyses (3) demonstrate that density dependence of migration is an important factor for genetic diversification of lynx between the Atlantic and Continental region. However, it is reasonable to assume that differential snow conditions could lead to additional population structuring, also in other large predators found in habitats with long, snowy winter seasons, a proposition that certainly can be tested.
Although snow is generally appreciated as an important factor in the ecology of the Canadian lynx, this study clearly indicates that snow indeed may be the key factor in the spatial, ecological, and genetic structuring of this species. Based on the sum of information available thus far, we hypothesize that the invisible barrier between the Atlantic and the Continental region is generated by differential snow quality. Field studies of lynx are logistically challenging, time consuming, and expensive because of the high mobility of the individuals, and further complicated by the cyclicity of the species, because several demographic traits seem to be phase-dependent (11). Nevertheless, the lynx–hare interaction represents a unique and most fascinating ecological system that has intrigued biologist for decades. The observed similarity between large-scale climatic, ecological (1), and genetic (2, 3) data raises new questions and will hopefully inspire new studies.
Acknowledgments
Input from two anonymous reviewers improved this article. We thank Dr. Richard Armstrong for help with some key references. This study was supported by the University of Oslo.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: NAO, North Atlantic oscillation.
References
- 1.Stenseth, N. C., Chan, K. S., Tong, H., Boonstra, R., Boutin, S., Krebs, C. J., Post, E., O'Donoghue, M., Yoccoz, N. G., Forchhammer, M. C., et al. (1999) Science 285, 1071–1073. [DOI] [PubMed] [Google Scholar]
- 2.Rueness, E. K., Stenseth, N. C., O'Donoghue, M., Boutin, S., Ellegren, H. & Jakobsen, K. S. (2003) Nature 425, 69–72. [DOI] [PubMed] [Google Scholar]
- 3.Stenseth, N. C., Ehrich, D., Rueness, E. K., Lingjærde, O. C., Chan, K.-S., Boutin, S., O'Donoghue, M., Robinson, D. A., Viljugrein, H. & Jakobsen, K. S. (2004) Proc. Natl. Acad. Sci. USA 101, 6056–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenseth, N. C., Ottersen, G., Hurrell, J. W., Mysterud, A., Lima, M., Chan, K.-S., Yoccoz, N. G. & Ådlandsvik, B. (2003) Proc. R. Soc. London Ser. B 270, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colbeck, S., Akitaya, E., Armstrong, R. L., Gubler, H., Lafeuille, J., Lied, K., McClung, D. & Morris, E. (1990) International Classification for Seasonal Snow on the Ground (Int. Commission on Snow and Ice, Int. Assoc. Hydrol. Sci., and the World Data Center for Glaciology, Boulder, CO).
- 6.Holling, C. S. (1959) Can. Entomol. 91, 293–320. [Google Scholar]
- 7.Stenseth, N. C., Falck, W., Chan, K.-S., Bjørnstad, O. N., O'Donoghue, M., Tong, H., Boonstra, R., Boutin, S., Krebs, C. J. & Yoccoz, N. G. (1998) Proc. Natl. Acad. Sci. USA 95, 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donoghue, M., Boutin, S., Krebs, C. J., Zuleta, G., Murray, D. L. & Hofer, E. J. (1998.) Ecology 79, 1193–1208. [Google Scholar]
- 9.Walker, A. & Goodison, B. E. (1993) Ann. Glaciol. 17, 307–311. [Google Scholar]
- 10.O'Donoghue, M., Boutin, S. Murray, D. L., Krebs, C. J., Hofer, E. J., Breitenmoser, U., Breitenmoser-Wüersten, C., Zuleta, G., Doyle, C. & Nams, V. O. (2001) in Ecosystem Dynamics of the Boreal Forest, eds. Krebs, C. J., Boutin, S. & Boonstra, R. (Oxford Univ. Press, Oxford), pp. 275–323.
- 11.Murray, D. L. & Boutin, S. (1991) Oecologia 88, 463–469. [DOI] [PubMed] [Google Scholar]