Abstract
We present a case of clear-cell meningioma occurring in a 72-year-old female presenting with an infiltrative external auditory canal mass (with both intracranial and extracranial extension and extensive leptomeningeal involvement) and facial nerve paralysis.
Abbreviations: MRI, magnetic resonance imaging; CCM, clear-cell meningioma; CT, computed tomography
Introduction
Clear-cell meningioma (CCM) is a rare, recently recognized variant of meningioma that shows clinically aggressive behavior often associated with a high recurrence rate and metastatic potential. CCM occurs in a wide range of ages but, on average, in younger patients than are affected by typical meningiomas. The most common primary locations of involvement include the spinal canal and posterior fossa. Radiographic appearances may or may not be typical for meningioma, making imaging distinction difficult. Histologically, the tumor may mimic other clear-cell neoplasms, and attention to specific immunochemical and ultrastructural features is critical for accurate diagnosis.
Case report
A 72-year-old woman presented with an external auditory canal mass that was initially biopsied at an outside institution and diagnosed as an epidermoid cyst. No specific treatment was undertaken at that time. She represented several months later to our institution with ipsilateral facial paresis, persistent periauricular pain, and otorrhoea. Her medical history included ipsilateral middle-ear cholesteatoma, end-stage pulmonary sarcoidosis requiring home oxygen, and a family history of lymphoma. On clinical examination, her right external auditory canal was indurated and contained macroscopic debris.
The patient underwent imaging studies including contrast-enhanced CT of the temporal bones and 3-Tesla MRI of the brain and skull base.
CT of the temporal bone demonstrated a soft-tissue mass involving the external auditory canal extending to the middle ear cavity. Permeative bone destruction was noted of the right squamous temporal bone, mastoid, tegmentum, and ossicular chain. The pre-auricular soft-tissue component of the mass contained multiple small, calcified fragments (Fig. 1).
Figure 1.
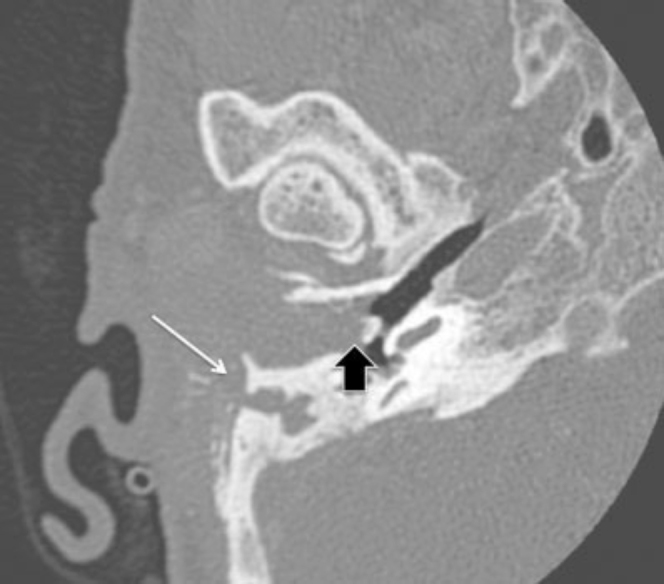
72-year-old woman with clear-cell meningioma. Axial CT, bone algorithm. Right external auditory canal (EAC) and middle ear. Soft-tissue mass filling and expanding the EAC and middle-ear cavity with involvement of the ossicles (black arrow). The mastoid air cells are infiltrated, and there is erosion of the lateral wall of the mastoid with dystrophic calcification involving the postauricular component of the mass (white arrow).
Given probable intracranial extension, brain MR imaging was ordered. It demonstrated a large, infiltrative, multicompartmental lesion with both intracranial and extracranial extension. Intracranially, the mass was clearly extra-axial, with a CSF cleft between the lesion and the underlying cortex. The mass was predominately solid but also contained cystic components. Adjacent dural thickening and enhancement were seen (as expected with meningioma); however, there was also extensive leptomeningeal enhancement, unusual for typical meningioma. The lesion encroached on the sigmoid sinus, resulting in partial stenosis of the sinus without occlusion. Although no macroscopic intra-axial extension was appreciated, vasogenic edema was noted within the adjacent right temporal lobe, likely due to leptomeningeal extension of the tumor. The lesion was low signal on T2 and isointense to grey matter on T1. The solid component enhanced avidly and demonstrated mild diffusion restriction. Abnormal enhancement of the tympanic and mastoid segments of the facial nerve was observed, suggestive of perineural spread of disease (Figure 2, Figure 3, Figure 4, Figure 5).
Figure 2.
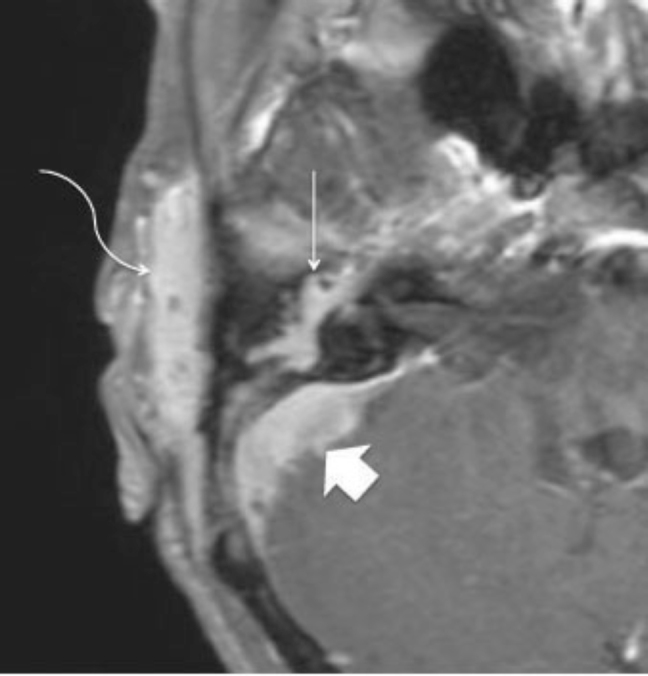
72-year-old woman with clear-cell meningioma. Axial T1 post-Gadolinium MRI. Lateral arrow indicates the enhancing soft-tissue mass involving the peri-auricular subcutaneous tissues. Note peri-auricular soft-tissue infiltration (curved arrow), middle ear involvement incorporating the facial nerve (straight arrow), and intracranial extension involving the sigmoid sinus (arrowhead).
Figure 3.

72-year-old woman with clear-cell meningioma. Coronal T1 post-Gadolinium MRI demonstrating the intracranial and extracranial extension of the meningioma. Arrow indicates leptomeingeal enhancement involving the right middle cranial fossa.
Figure 4.
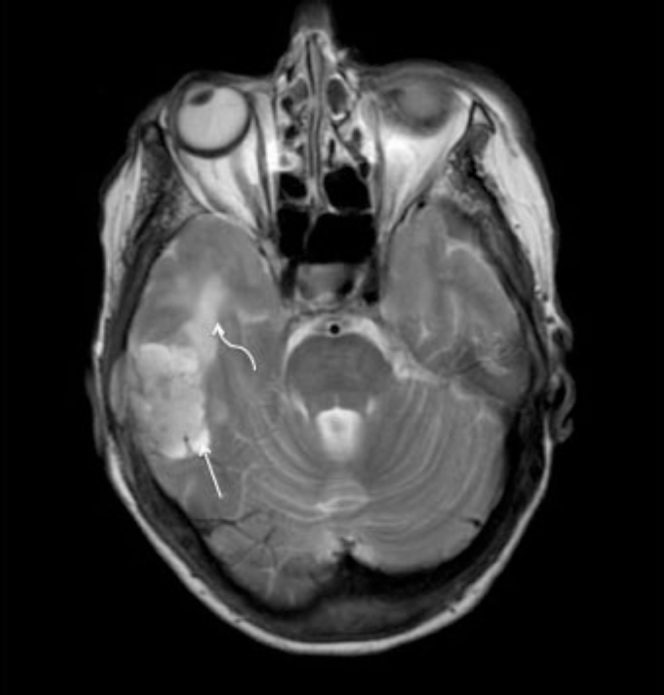
72-year-old woman with clear-cell meningioma. Axial T2 MRI. CSF cleft surrounding the mass confirms the extra-axial location of the disease (straight arrow). Note vasogenic edema within the right temporal lobe (curved arrow).
Figure 5.
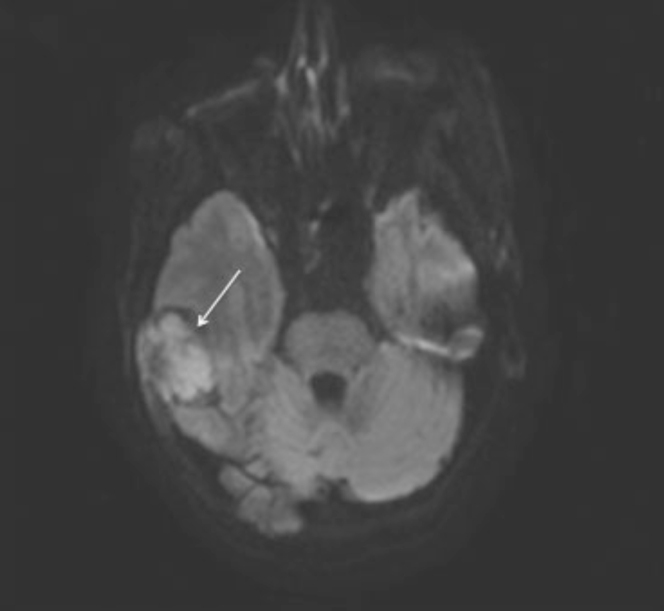
72-year-old woman with clear-cell meningioma. Axial DWI MRI demonstrates diffusion restriction within the right temporal mass consistent with high cellularity (straight arrow). Restricted diffusion was confirmed on ADC.
We subsequently performed a CT-guided biopsy of the lesion in our department for histologic diagnosis. Multiple 18g core biopsies were obtained through the preauricular soft-tissue component of the mass. The lesion was rubbery in consistency and macroscopically pale in appearance.
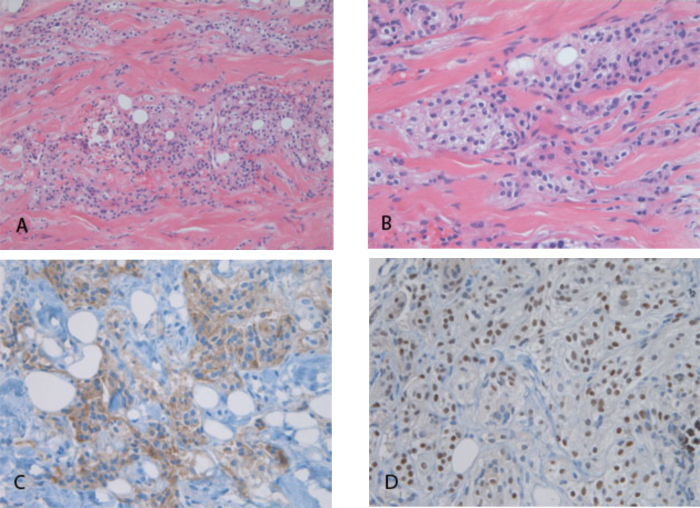
Histologic sections of the biopsy cores showed neoplastic cells with clear cytoplasm and relatively bland, uniform nuclei (Fig. 6, A and B). The neoplastic cells infiltrated as irregular groups and single cells through fibrous stroma. Clear cytoplasm is a distinctive feature; the differential diagnosis included a clear-cell carcinoma—such as metastatic renal-cell carcinoma or myoepithelial carcinoma—as well as CCM. Metastatic renal-cell carcinoma was excluded by negative immunohistochemistry for high- and low-molecular-weight cytokeratins and the transcription factor Pax2; the neoplastic cells also lacked the characteristic capillary network of renal-cell carcinoma. A diagnosis of myoepithelial carcinoma was supported by strong nuclear immunoreactivity for p63 (Fig. 6D); however, cells did not stain with antibodies directed against low-molecular-weight cytokeratin and smooth-muscle actin, making this diagnosis much less likely. While the neoplasm generally lacked the typical “whorled” architecture of classic meningiomas, the diagnosis of a CCM was supported by strong membranous immunoreactivity for epithelial membrane antigen (EMA; Fig. 6C), and by positive cytoplasmic staining with periodic acid-Schiff (PAS) stain. Furthermore, there was weak nuclear immunoreactivity for progesterone receptor, also typical of meningiomas. While p63 positivity is not usually a feature of meningiomas, the rate of p63 nuclear expression in meningiomas increases with the grade of the lesion, and is reported to be present in 92% of grade-II meningiomas, a class that includes the CCM variant (1).
Figure 6.
72-year-old woman with clear-cell meningioma. Hematoxylin- and eosinstained sections of biopsy core show an infiltrative clear-cell neoplasm at 200X (A) and 400X (B) magnification. Immunohistochemical staining for epithelial membrane antigen (C) and p63 (D) show cytoplasmic membrane and nuclear reactivity, respectively.
Discussion
Given our patient’s clinical background, differential diagnoses based on imaging included meningioma, lymphoma, metastases, and sarcoidosis. The positive diffusion restriction in our case was felt to be representative of high cellularity and could be seen in the setting of either meningioma or lymphoma. Any of the above lesions could result in bony lysis. The dystrophic calcification would be unusual in the setting of lymphoma but could be seen in the setting of metastatic renal-cell carcinoma, meningioma, or sarcoidosis. Leptomemingeal disease can be seen with sarcoidosis, metastases, and atypical meningioma. Recurrent cholesteatoma and epidermoid cyst were excluded from our imaging differential, as these two entities would not enhance. Final histologic diagnosis confirmed clear-cell variant meningioma.
CCM is a rare histological meningioma variant classified as Grade II of IV by the World Health Organization. The tumor is distinct from more typical meningiomas in that it is more locally aggressive, with a high recurrence rate and metastatic potential (2). The tumor has distinct histologic features requiring differentiation from other clear-cell neoplasms including metastatic renal-cell carcinoma, pleomorphic xanthroastrocytoma, oligodendroglioma, hemangioblastoma, germinoma, lipid-rich glioblastoma, and clear-cell ependymoma (2, 3, 4).
CCM has no sex predilection. The average age of presentation reported in the literature is younger than that for typical meningioma, with a mean age in the mid to late twenties (2, 5, 6, 7). CCM also differs in the frequency of sites of occurrence compared with typical meningioma. Typical meningioma most commonly involves the lateral convexities, parasagittal/parafalcine dura, and the sphenoid and middle cranial fossa (8). In comparison, in the majority of cases, CCM presents as an intradural spinal lesion. Other frequent sites of involvement include the supratentorium, cerebellopontine angle, foramen magnum, and skull base (2, 5, 9). There have been reports of spinal lesions lacking a dural attachment, as well as cases presenting primarily as intraparenchymal masses (2, 10).
Typical meningioma has the propensity to induce an osteoblastic reaction, resulting in adjacent bony sclerosis: however, in our case the pattern was one of permeative destruction, presumably reflecting bone invasion (8). These tumors may induce vasogenic edema even in the absence of histologic parenchymal invasion, likely from pia-arachnoid invasion of tumor, but this feature is not seen exclusively in CCM. Evidence of leptomeningeal enhancement on pre-operative neuroimaging may raise the possibility of CCM, but this has also been reported as associated with other meningioma subtypes (2). Perineural disease, as seen in our case, has previously been described with atypical meningioma (11). Macroscopic cysts are also uncommon for typical meningioma, seen in up to 23% of cases (8), but this finding is also not specific for the clear-cell variant.
The importance of distinguishing CCM from other histologic subtypes of meningioma is that it has different biologic behavior, with higher propensity to recur or metastasize. This is paradoxical, as the neoplastic histology is relatively bland; anaplastic cytologic features are absent, and histologic mitotic indices are low (6, 7, 9). Recurrence rates average 61%, with local recurrence being the most common manifestation. CSF dissemination and leptomemingeal spread have also been described. Intracranial CCM tends to recur more often than spinal CCM, with recurrence rates of 80% and 46%, respectively (2, 9).
The mainstay of treatment is complete surgical excision. Radiotherapy/radiosurgery is usually reserved for tumor recurrence (2). Our patient’s treatment was challenging, given her poor respiratory reserve and extensive intracranial and extracranial involvement, requiring extensive debridement and reconstructive surgery. She underwent pre-operative arterial particle embolization, followed by subtotal resection.
In conclusion, the combination of a dural-based, extra-axial mass with leptomeningeal and perineural involvement, and cystic degeneration with or without an associated soft-tissue or osseous component should raise suspicion of CCM. Immunohistochemical stains may be essential for definitive diagnoss, to exclude other neoplasms with clear-cell features. Correct diagnosis is critical, as these patients require close surveillance, given the high risk of recurrence.
Footnotes
Published: February 23, 2012
References
- 1.Rushing EJ, Olsen C, Man YG. Correlatio of P63 Immunoreactivity with tumor grade in meningiomas. International Journal of Surgical Pathology. 2008;16(1):38–42. doi: 10.1177/1066896907306772. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Le W, Chang KH, Choe G, Chi J, Chung CK. MR imaging features of clear-cell meningioma with diffuse leptomeningeal seeding. American Journal of Neuroradiology. 2000;21:130–132. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzeyli K, Cakir E, Usul H, Karaarslan G, Reis AK. Clear cell meningioma: case report and literature review. Journal of Clinical Neuroscience. 2003;10(2):264–266. doi: 10.1016/s0967-5868(02)00287-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Pizzoni C, Sarandria C, Pierangeli E. Clear-cell meningioma of the anterior cranial fossa. Case report and review of literature. Journal of Neurosurgical Science. 2009;53(3):113–117. [PubMed] [PubMed] [Google Scholar]
- 5.Tena-Suck ML, Salinas-Lara C, Gomez C, Bojorquez DR. Frontotemporal clear cell meningioma. Report of 3 cases. Annals of Diagnostic Pathology. 2007;11:182–189. doi: 10.1016/j.anndiagpath.2006.03.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Sharma MC, Sarkar C, Suri V, Garg A. Clear cell meningioma, an uncommon variant of meningioma: a clinical study of nine cases. Journal of Neurooncology. 2007;81(3):315–321. doi: 10.1007/s11060-006-9237-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Rousselot C, Francois P, Jan M, Bergemer AM. Report of seven cases of clear-cell meningioma and a literature review. Annals of Pathology. 2010;30(2):73–82. doi: 10.1016/j.annpat.2010.01.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Buetow MP, Buetow PC, Smirnioopoulos JC. Typical, atypical and misleading features in meningioma. Radiographics. 1991;11:1087–1106. doi: 10.1148/radiographics.11.6.1749851. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. American Journal of Surgical Pathology. 1995;19(5):493–505. [PubMed] [PubMed] [Google Scholar]
- 10.Teo JG, Goh KY, Rosenblum MK, Muszynski CA, Epstein FJ. Intraparenchymal clear cell meningioma of the brainstem in a 2-year-old child. Case report and literature review. Pediatric Neurosurgery. 1998;28(1):27–30. doi: 10.1159/000028614. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Wei FY, Wu CT, Lin KL, Wong AM, Ng SH. Childhood atypical meningioma with perineural spread: MR findings. Pediatric Radiology. 2005;35(9):895–898. doi: 10.1007/s00247-005-1472-0. [PubMed] [DOI] [PubMed] [Google Scholar]