Abstract
[Purpose]
The objective of this study was to evaluate the hepatoprotective effects of Hoveniae Semen Cum Fructus extract in ethanol induced hepatic damages.
[Methods]
Hepatic damages were induced by oral administration of ethanol and then Hoveniae Semen Cum Fructus extract was administered.
[Results]
Following Hoveniae Semen Cum Fructus extract administration, body and liver weights were increased, while aspartate aminotransferase, alanine aminotransferase, albumin, γ-glutamyl transferase, and triglyceride levels in the serum, triglyceride contents, tumor necrosis factor -α level, cytochrome (CY) P450 2E1 activity in the liver and mRNA expression of hepatic lipogenic genes, and Nitrotyrosine and 4-HNE-immunolabelled hepatocytes were decreased. However, mRNA expression of genes involved in fatty acid oxidation was increased. Also, as a protective mechanism for hepatic antioxidant defense systems, decreased liver MDA contents, increased glutathione contents, increased dismutase and catalase activities were observed when compared to the ethanol control.
[Conclusion]
Hoveniae Semen Cum Fructus extract favorably protected against liver damages, mediated by its potent anti-inflammatory and anti-steatosis properties through the augmentation of the hepatic antioxidant defense system by NF-E2-related factor-2 activation, and down-regulation of the mRNA expression of hepatic lipogenic genes or up-regulation of the mRNA expression of genes involved in fatty acid oxidation.
Keywords: Hoveniae, hepatoprotective, mouse, Nrf2, antioxidant
INTRODUCTION
The liver is an important organ actively involved in metabolic functions and is a frequent target of a number of toxicants1. It is well known that a substantial increase in steatosis and fibrosis usually leads to potentially lethal cirrhosis of the liver in humans2. Alcohol-related disorders are one of the challenging current health problems with far reaching medical, social, and economic consequences. Long-term alcohol use potentially results in serious illnesses, including alcoholic fatty liver, hypertriglyceridaemia, cirrhosis, cardiovascular disease, and inflammation of the pancreas3. Although much progress has been made in understanding the pathogenesis of alcoholic liver disease, there is no effective therapy for the disease. Novel therapeutic targets that successfully correct the fundamental cellular disturbances resulting from excessive alcohol consumption are effective4,5. Alcoholic liver disease is a result of complex pathophysiological events involving various types of cells, such as neutrophils, endothelial cells, Kupffer cells, and hepatocytes, and a variety of injurious factors such as endotoxin, oxidative stress, cytokines, and proteases6. Accumulated evidence has demonstrated that oxidative stress, abnormal cytokine production, especially tumor necrosis factor (TNF), and steatosis play important etiological roles in the pathogenesis of alcoholic liver disease. Therefore, agents that have antioxidant, anti-inflammatory and anti-steatosis properties, particularly anti-TNF production and decreasing lipid accumulation, represent promising therapeutic interventions for alcoholic liver disease4,5,7.
Ethanol (EtOH) administration causes the accumulation of reactive oxygen species (ROS), including superoxide, hydroxyl radical, and hydrogen peroxide8. ROS cause lipid peroxidation of cellular membranes, protein and DNA oxidation, which results in hepatocyte injury9,10. The potential harmful effects of these oxidant species are controlled by the cellular antioxidant defense system11. Reduced glutathione (GSH) is the predominant defense against ROS/free radicals in different tissues of the body12. In addition, antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutamate cystein ligase (GCL) and Hemeoxygenase-1 (HO-1) are essential in both scavenging ROS/free radicals and maintaining cellular stability13. Under normal conditions, the reductive and oxidative capacities of the cell (redox state) favor oxidation. However, when the generation of ROS in cells impairs antioxidant defenses or exceeds the ability of the antioxidant defense system to eliminate them, oxidative stress results14. It is conceivable that agents with a hepatic antioxidant property would have therapeutic potential for alcoholic liver disease4. Oxidative stress and lipid peroxidation are predominantly generated through the induction of cytochrome (CY) P450 2E1 15. A key role for this enzyme in EtOH-induced liver injury has been demonstrated by its inhibition through chlormethiazole and by the finding that CYP450 2E1 knock-out mice do not show evidence of EtOH-induced liver disease16.
It is well established that increased reactive oxygen species and electrophiles induce a series of antioxidant genes via the activation of antioxidant response elements (AREs). ARE-driven gene expression is mainly regulated by NF-E2-related factor-2 (Nrf2), a member of the cap’n’collar family of bZIP transcription factors, which are essential transcription factors that regulate the expression of major antioxidant enzymes including glutathione S-transferase A1/2, hemeoxygenase 1, UDP-glucuronosyl transferase 1A, NAD(P)H:quinone reductase, and -glutamylcysteine synthetase17. Nrf2-knockout mice are characterized by susceptibility to oxidative stress, including acetaminophen, UV irradiation and carcinogens 18. Therefore, Nrf2 plays a role as a multi-organ protector in oxidative stress-related diseases19.
Another major consequence of EtOH metabolism is lipid accumulation in the liver. EtOH metabolism changes the NAD/NADH ratio, which has important consequences on fuel utilization in the liver, favoring the synthesis of fatty acids and inhibiting their oxidation 20,21. Sterol regulatory element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor (PPAR) α, two nuclear transcription regulators controlling lipid metabolism, are involved in the development of alcoholic fatty liver5,15. EtOH administration activates hepatic SREBP-1c gene and its target genes: fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1), and acetyl-CoA carboxylase 1 (ACC1), which promotes de novo fatty-acid synthesis5,15. It also increases the expression of genes for PPARγ and diacylglycerol acyltransferase (DGAT) 2, which promotes triglyceride (TG) synthesis5,15,22-24. EtOH decreases the expression of mRNA encoding PPARα, acyl-CoA oxidase (ACO) and carnitine palmitoyltransferase1 (CPT1), which leads to the inhibition of fatty acid oxidation5,15,25.
EtOH-mediated experimental liver damaged rodents have been used for detecting the hepatoprotective effects of various herbal extracts or their chemical components based on the changes of body and liver weights. Histopathology of the liver with blood chemistry like serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), TG, γ-glutamyl transferase (γ-GTP), and albumin with hepatic TG contents, hepatic lipid peroxidative makers, mRNA expression of hepatic lipogenic genes or genes involved in fatty acid oxidation, and especially histopathological changes of hepatic parenchyma have been used as critical end points of EtOH-mediated hepatic damages in rodent models4,5,15,26-30.
Hoveniae Semen Cum Fructus (HSCF) is the dried peduncle of Hovenia dulcis Thunb. (Rhamnaceae). Various antioxidant based pharmacological effects of HSCF extracts have been reported including anti-adipogenic31, anti-fatigue32, neuroprotective33 and hepatoprotective34,35 effects. However, it seems more systemic evaluation of the hepatoprotective effects of HSCF extract with molecular targets is needed. In the present study, the beneficial potential of HSCF extract on subacute EtOH-induced hepatic damages in C57BL/6 mice was systemically investigated as well as the associated potent anti-oxidant, anti-inflammatory and anti-steatosis mechanisms.
METHODS
Preparations and administration of test materials
HSCF extracts (contains about 8.20ug/mg quercetin) were supplied by Aribio (Seoul, Korea) as a beige powder. HSCF was ground and extracted with hot water 2 times at 95°C for 4 hours then filtered and condensed using a rotary vacuum evaporator (EYELA N-1200B, USA). Finally, it was dried and standardized with dextrin using a spray drier (about 7.4ug/g quercetin). The HSCF extract was obtained as 26%. A reddish-yellow powder of silymarin was purchased from Sigma-Aldrich (St. Louise, MO, USA) as the reference drug. All test materials were stored at -20°C in a refrigerator to protect from light and humidity until used. In this study, 500 mg/kg was selected as the highest dose of the HSCF extract based on the clinical dosage in humans and 250 and 125 mg/kg were additionally selected as the middle and lowest doses with a common ratio of 2, respectively. HSCF extract (500, 250, and 125 mg/kg) and Silymarin (250 mg/kg) were suspended in distilled water and orally administered once a day after 1 hour of EtOH treatment for 14 days. In intact and EtOH control mice, equal volumes of distilled water were orally administered.
Animals and experimental design
A total of sixty-three healthy male SPF/VAF Inbred C57BL/6J mice (6-wk old upon receipt; OrientBio, Seungnam, Korea) were used after acclimatization for 10 days. Animals were allocated five per polycarbonate cage in a temperature (20-25°C) and humidity (50-55%) controlled room. The dark light cycle was 12hrs long. Commercial rodent feed (Samyang, Seoul, Korea) and tap water were supplied ad libitum. All animals were treated according to the international regulations for the usage and welfare of laboratory animals, and the protocol was approved by the Institutional Animal Care and Use Committee in Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) Approval No DHU2014-082. Eight mice in each group, with a total of six groups, were selected based on the body weights by ascending order after acclimatization: Intact control - Isocalorical maltose solution and distilled water administered mice, EtOH control: EtOH and distilled water administered mice, Silymarin group: EtOH and silymarin 250 mg/kg as reference drug treated mice, HSCF 500: mice administered EtOH and HSCF extracts at 500 mg/kg, HSCF 250: mice administered EtOH and HSCF extracts at 250 mg/kg, HSCF 125: mice administered EtOH and HSCF extracts at 125 mg/kg.
Induction of EtOH-mediated subacute hepatic damage
Subacute EtOH-induced hepatotoxicity was induced by oral administration of EtOH (0.8 g/ml concentration; Merck, Darmstadt, Germany) at 5g per kg once a day for 14 days, at 1 hr before oral administration of each test substance, according to previous established methods4,5,15 with some modifications. In intact control mice, isocalorical maltose solution was orally administered instead of EtOH.
Measurement of body weight and liver
Changes in body weight were measured at 1 day before initial test substance administration, the day of first test substance administration, and at 1, 7 10 and 14 days after initial HSCF or silymarin extracts administration using an automatic electronic balance (Precisa Instrument, Dietikon, Switzland). To reduce the individual differences, the body weight gains during 15 days of experiment were calculated as body weight on the last day of test substance administration – body weight on the first day of test substance administration. At sacrifice, the weights of the livers were measured (absolute wet-weights). To reduce the differences from individual body weights, relative weights were also calculated divided by body weight at sacrifice.
Measurement of serum biochemistry
At sacrifice, about 1ml of venous blood was collected from the caudal vena cava under anesthesia with isoflurane (Hana Pharm. Co., Hwasung, Korea). All blood samples were centrifuged at 13,000 rpm, 4°C for 10 min using clotting activated serum tubes. Serum AST, ALT, albumin and ALP levels were detected using a blood biochemistrical autoanalyzer (Hemagen Analyst, Hemagen Diagnostic, Columbia, MA, USA). In addition, serum TG and γ-GTP levels were measured using another type of automated blood biochemistrical analyzer (SP-4410, Spotochem, Kyoto, Japan).
Measurement of hepatic TG contents and TNF-α levels
To assess TG content, liver tissue (right lobes) was homogenized in an equal volume of normal saline and extracted with a mixture of chloroform and methanol (2:1) as described previously36. Zeolite (Sigma-Aldrich, St. Louise, MO, USA) was added to remove phospholipids. The resulting extract was dried under nitrogen and dissolved in Plasmanate (1ml; Sigma-Aldrich, St. Louise, MO, USA). TG were measured enzymatically using commercial kits (Kyowa Medex, Tokyo, Japan) as in previous studies37. Liver samples were disintegrated in 5 volumes of ice-cold radioimmunoprecipitation assay (RIPA) buffer. After incubation on ice for 30 min, samples were centrifuged twice at 20,000 × g for 15 min at 4°C. The supernatants were used for the assay. The contents of total protein were measured with the Lowry method38 using bovine serum albumin (Invitrogen, Carlsbad, CA, USA). The TNF-α levels were detected by enzyme-linked immunosorbent assay (ELISA) using a murine kit (BioSource International Inc., Camarillo, CA, USA) with a microplate reader (Tecan, Männedorf, Switzerland).
Splenic cytokine content measurements
Splenic concentrations of TNF-α, IL-1β, and IL-10 were measured with a mouse TNF-α ELISA kit (BD Biosciences/ Pharmingen, San Jose, CA, USA), mouse IL-1β ELISA kit (Genzyme, Westborough, MA, USA) and mouse IL-10 ELISA kit (Genzyme, Westborough, MA, USA), respectively39,40. Approximately 10-15 mg of tissue samples were homogenized in a tissue grinder containing 1 ml of lysis buffer (PBS containing 2 mM PMSF and 1mg/ml of aprotinin, leupeptin, and pepstatin A)41. Analysis was performed with 100ml of standard (diluted in lysis buffer) or 10, 50, or 100 ml of tissue homogenate. Each sample was run in duplicate, and a portion of the sample was analyzed for protein.
Determination of CYP450 2E1 Activity
Hydroxylation of p-nitrophenol to 4-nitrocatechol, a reaction catalyzed specifically by CYP2E1, was determined colorimetrically4. Liver tissue was homogenized in 0.15 KCl and was spun at 10,000×g for 30 min. Microsomes were isolated by further centrifugation at 105,000×g for 60 mins. For the assay, 300 ml of microsomal protein was incubated for 5 mins at 37°C, and absorbance at 535 nm was measured with 4-nitrocatechol (Sigma-Aldrich, St. Louise, MO, USA) as a standard using a UV/Vis spectrometer (OPTIZEN POP, Mecasys, Daejeon, Korea).
Measurement of liver lipid peroxidation
Liver tissues were weighed and homogenized in ice-cold 0.01M Tris-HCl (pH 7.4), and then centrifuged at 12,000×g for 15 mins as described by Kavutcu et al42. The concentrations of liver lipid peroxidation were determined by estimating MDA using the thiobarbituric acid test at the absorbance of 525 nm and represented by nM of MDA/mg protein43. Total protein was measured by the Lowry method38.
Measurement of hepatic antioxidant defense systems
Prepared homogenates were mixed with 0.1 ml of 25% trichloroacetic acid (Merck, West Point, CA, USA), and then centrifuged at 4,200 rpm for 40 min at 4 ºC. Glutathione (GSH) contents were measured at the absorbance of 412 nm using 2-nitrobenzoic acid (Sigma-Aldrich, St. Louise, MO, USA)44. Decomposition of H2O2 in the presence of catalase was performed at 240 nm45. Catalase activity was defined as the amount of enzyme required to decompose 1 nM of H2O2 per minute, at 25°C and pH 7.8. Measurements of SOD activities were made according to Sun et al.46.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the method described in previous studies5,15. The RNA concentrations and quality were determined with a CFX96TM Real-Time System (Bio-Rad, Hercules, CA, USA). To remove contaminating DNA, samples were treated with recombinant DNase I (DNA-free; Ambion, Austin, TX, USA). RNA was reverse transcribed using the reagent High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Briefly, the cDNA strand was first synthesized from the total RNA and then the mixture of the primers and the cDNA products was amplified by PCR. The conditions of PCR amplification were 58°C for 30 mins, 94°C for 2 mins, 35 cycles of 94°C for 15 sec, 60°C for 30 sec, 68°C for 1 min, and then 72°C for 5 mins. Finally, the PCR products were separated on 0.8% agarose gel. Analysis was carried out using a gel imaging system (Bio-Rad, Hercules, CA, USA). Expression levels of SREBP-1c, SCD1, ACC1, FAS, PPARγ, DGAT2, PPARα, ACO, CPT1 and Nrf2 were calculated as a percentage relative to the intact group using β-actin RNA as the internal control. The sequences of the PCR oligonucleotide primers are listed in Table 1.
Table 1. Oligonucleotides primers used for RT-qPCR in this study.
| Target | 5’ – 3’ | Sequence | Gene ID |
|---|---|---|---|
| SREBP-1c | Forward | GATGTGCGAACTGGACACAG | 6720 |
| Reverse | CATAGGGGGCGTCAAACAG | ||
| SCD1 | Forward | CCCCTGCGGATCTTCCTTAT | 20249 |
| Reverse | AGGGTCGGCGTGTGTTTCT | ||
| ACC1 | Forward | CCATTGGTATTGGGGCTTAC | 107476 |
| Reverse | CCCGACCAAGGACTTTGTTG | ||
| FAS | Forward | GCTGCGGAAACTTCAGGAAAT | 14102 |
| Reverse | AGAGACGTGTCACTCCTGGACTT | ||
| PPARγ | Forward | AGTGGAGACCGCCCAGG | 19016 |
| Reverse | GCAGCAGGTTGTCTTGGATGT | ||
| DGAT2 | Forward | AGTGGCAATGCTATCATCATCGT | 67800 |
| Reverse | AAGGAATAAGTGGGAACCAGATCA | ||
| PPARα | Forward | ATGCCAGTACTGCCGTTTTC | 19013 |
| Reverse | GGCCTTGACCTTGTTCATGT | ||
| ACO | Forward | GCCCAACTGTGACTTCCATT | 74121 |
| Reverse | GGCATGTAACCCGTAGCACT | ||
| CPT1 | Forward | GCACTGCAGCTCGCACATTACAA | 12894 |
| Reverse | CTCAGACAGTACCTCCTTCAGGAAA | ||
| Nrf2 | Forward | CGAGATATACGCAGGAGAGGTAAGA | 18024 |
| Reverse | GCTCGACAATGTTCTCCAGCTT | ||
| β-actin | Forward | CTGTCGAGTCGCGTCCA | 11461 |
| Reverse | CTCGCGGGTGGACGCGACTCGACAG |
SREBP-1c, Sterol regulatory element-binding protein-1c; SCD1, Stearoyl-CoA desaturase 1; ACC1, Acetyl-CoA carboxylase 1; FAS, Fatty acid synthase; PPAR, Peroxisome proliferator-activated receptor; DGAT2, Diacylglycerol acyltransferase 2; ACO, Acyl-CoA oxidase; CPT1, Carnitine palmitoyltransferase 1; Nrf2, NF-E2-related factor-2
Histopathological analysis
Left lateral lobes of the liver were fixed in 10% neutral buffered formalin (NBF), and embedded in paraffin, sectioned (3~4μm) and stained with Hematoxylin and eosin (H&E). Afterward, the histopathological profiles of each sample were observed under light microscope (Model 80i, Nikkon, Tokyo, Japan). For more detailed study, the number of hepatocytes, which occupied over 20% of lipid droplets in the cytoplasm, was calculated using an automated image analyzer (iSolution FL ver 9.1, IMT i-solution Inc., Vancouver, Canada). The value was reported as cells/1000 hepatocytes. The percentage of changed fatty regions (%/ mm2 of hepatic parenchyma) and the mean diameters of hepatocytes (μm/hepatocytes), with at least 10 hepatocytes per view field in the liver, were also calculated using an automated image analyzer in both the lateral and median lobes, according to the previously established method 47. The histopathologist was blinded to the group distribution when this analysis was conducted.
Immunohistochemistry
After deparaffinization of the prepared hepatic histological paraffin sections, citrate buffer antigen (epitope) retrieval pretreatment was conducted as previously described48,49. Briefly, a water bath with staining dish containing 10 mM citrate buffer (pH 6.0) was preheated until the temperature reached 95-100°C. The slides were immersed in the staining dish and a lid was placed loosely on the staining dish. Incubation was performed for 20 min and the water bath was turned off. The staining dish was placed at room temperature and the slides were allowed to cool for 20 minutes. After epitope retrieval, sections were immunostained using avidin-biotin complex (ABC) methods (Table 2) for NT and 4-Hydroxynonenal (4-HNE) according to the previous study48,50. Briefly, endogenous peroxidase activity was blocked by incubation in methanol and 0.3% H2O2 for 30 minutes, and non-specific binding of immunoglobulin was blocked with normal horse serum blocking solution (Vector Lab., Burlingame, CA, USA. Dilution 1:100) for 1 hr in a humidity chamber. Primary antiserum (Table 2) was applied overnight at 4ºC in the humidity chamber, followed by incubation with biotinylated universal secondary antibody (Vector Lab., Dilution 1:50) and ABC reagents (Vectastain Elite ABC Kit, Vector Lab., Burlingame, CA, USA; Dilution 1:50) for 1 hr at room temperature in the humidity chamber. Finally, reaction with a peroxidase substrate kit (Vector Lab., Burlingame, CA, USA) was conducted for 3 min at room temperature. All sections were rinsed in 0.01M PBS 3 times between steps. The cells that showed stronger immunoreactivities in the cytoplasm with over 20% of the density against each antiserum as compared with intact control hepatocytes were regarded as positive immunoreactive. The numbers of NT- and 4-HNE-positive cells were measured for a total of 1000 hepatocytes using a digital image analyzer, according to previous reports51-53. The histopathologist was blinded to the group distribution when this analysis was performed.
Table 2. Primary antisera and detection kits used in Immunohistochemistry.
| Antisera or detection kits | Code | Source | Dilution |
|---|---|---|---|
| Primary antisera* | |||
| Anti-4-Hydroxynonenal polyclonal antibody | ab46545 | Abcam, Cambridge, UK | 1:100 |
| Anti-Nitrotyrosine polyclonal antibody | 06-284 | Millipore, Temecula, CA, USA | 1:200 |
| Detection kits | |||
| Vectastain Elite ABC Kit | PK-6200 | Vector Lab., Burlingame, CA, USA | 1:50 |
| Peroxidae substrate kit | SK-4100 | Vector Lab., Burlingame, CA, USA | 1:100 |
*All antiserum were diluted using 0.01M phosphate buffered saline (pH 7.2)
Data Analysis
All numerical data were expressed as mean ± standard deviation (SD) of eight mice. Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test54. If the Levene test indicated no significant deviations from variance homogeneity, the obtained data were analyzed by one-way ANOVA test followed by least-significant differences multi-comparison (LSD) test to determine which pairs of group comparisons were significantly different. In the event of significant deviations from variance homogeneity in the Levene test, a non-parametric comparison test, Kruskal-Wallis H test, was conducted. When a significant difference was observed in the Kruskal-Wallis H test, the Mann-Whitney U (MW) test was conducted to determine the specific pairs of group comparison, which are significantly different. Statistical analyses were conducted using SPSS for Windows (Release 14.0K, IBM SPSS Inc., Armonk, NY, USA)55. In addition, the perpercent-point changes between intact vehicle and EtOH control were calculated to observe the severities of hepatic damages induced by 2 weeks of continuous oral treatment of EtOH in this study. The percent-point changes as compared with EtOH control and test substances treated mice were also calculated for understanding of the hepatoprotective effects of the test materials as in Equations 1 and 2, respectively, also according to our previous established method56.
Equation 1. Percent-point Changes as Compared with Intact Vehicle Control (%)
= ((Data of EtOH control – Data of intact control)/Data of intact control) × 100
Equation 2. Percent-point Changes as Compared with EtOH Control (%)
= ((Data for test substance administered group – Data of EtOH control)/Data for EtOH control) × 100.
RESULTS
Changes of the body weights
Significant (p<0.01 or p<0.05) decreases of body weight were detected from 7 days after EtOH administration in the EtOH control. The body weight gains during 15 days of experimentation were also significantly (p<0.01) decreased in the EtOH control as compared with the intact control. However, significant (p<0.01 or p<0.05) increases of body weights were observed from the 10th day of test substance administration in the mice treated with HSCF extracts at 500mg/kg and silymarin at 250 mg/kg, and from the 13th day in those treated with HSCF extracts at 250 and 125 mg/ kg as compared with the EtOH control. In addition, the body weight gains during 15 days of experiment were significantly (p<0.01) increased in HSCF and silymarin administered mice as compared with the EtOH control (Table 3).
Table 3. Body weight in mice with subacute EtOH-induced intoxication.
| Groups | Body weights (g) | Weight gains | Liver weight | |||
|---|---|---|---|---|---|---|
| Day - 1 | Day 0 [A]* | Day 14 [B]* | [B-A] | absolute | relative | |
| Controls | ||||||
| Intact | 21.96±1.58 | 19.86±1.73 | 23.21±2.04 | 3.35±0.87 | 0.881±0.095 | 3.801±0.351 |
| EtOH | 21.99±1.47 | 19.83±1.50 | 17.98±0.82a | - 1.85±0.94a | 0.478±0.038a | 2.661±0.207a |
| Silymarin | 21.90±1.81 | 19.85±1.74 | 20.65±1.32ab | 0.80±0.51ab | 0.669±0.038ab | 3.246±0.196ab |
| HSCF treated | ||||||
| 500 mg/kg | 21.96±1.69 | 19.88±1.86 | 20.66±1.98ab | 0.79±0.51ab | 0.665±0.047ab | 3.243±0.364ab |
| 250 mg/kg | 22.05±1.54 | 20.01±1.68 | 20.31±1.54ab | 0.30±0.38ab | 0.623±0.036ab | 3.076±0.218ab |
| 125 mg/kg | 21.99±1.47 | 20.03±1.40 | 19.93±1.48ac | - 0.10±0.86ac | 0.594±0.027ab | 2.995±0.240ac |
Values are expressed as Means ± SD of eight mice
*All animals were overnight fasted. Day 0 means the day of first test substance administration. Day 14 means 24 hrs after the last (14th) test substance administration
a p<0.01 as compared with intact control by LSD test. b p<0.01 and c p<0.05 as compared with EtOH control by LSD test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts
Changes in the liver weights
Significant (p<0.01) decreases of liver absolute wet- and relative weights were detected in EtOH control mice as compared with the intact control. However, these EtOH-induced decreases of liver absolute and relative weights were dose-dependently and significantly (p<0.01) inhibited by treatment with HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced liver weight decreases as compared with silymarin at 250 mg/kg in this experiment (Table 3).
Changes in the serum biochemistry
Significant (p<0.01) increases of serum AST, ALT, albumin, ALP, TG and γ-GTP levels were observed in the EtOH control as compared with the intact control. However, the serum chemistries were significantly (p<0.01) decreased by treatment with HSCF extracts at all dosages, and the effect was dose-dependent (Table 4).
Table 4. Changes in the serum biochemistry in Subacute EtOH-treated mice.
| Groups | Serum biochemistrical levels | |||||
|---|---|---|---|---|---|---|
| AST (IU/l) | ALT (IU/l) | Albumin (g/dl) | ALP (IU/l) | TG (mg/dl) | γ-GTP (IU/l) | |
| Controls | ||||||
| Intact | 90.00±21.78 | 46.38±13.96 | 3.88±0.82 | 66.75±14.42 | 148.88±28.03 | 2.00±0.76 |
| EtOH | 343.75±46.91a | 158.13±37.16c | 11.74±1.98a | 246.38±27.84a | 397.63±32.44a | 7.50±1.60a |
| Silymarin | 215.13±59.76ab | 93.75±14.87cd | 6.65±1.08ab | 170.13±28.49ab | 223.50±46.58ab | 4.25±1.39ab |
| HSCF treated | ||||||
| 500 mg/kg | 221.63±36.79ab | 93.25±16.06cd | 6.69±1.49ab | 169.75±19.04ab | 229.00±49.34ab | 4.38±1.19ab |
| 250 mg/kg | 252.50±34.97ab | 103.75±11.61cd | 7.98±1.36ab | 188.13±18.61ab | 286.38±22.53ab | 5.13±1.46ab |
| 125 mg/kg | 277.63±35.43ab | 117.88±12.37ce | 8.56±1.23ab | 204.13±13.51ab | 329.00±41.19ab | 5.75±0.89ab |
Values are expressed as Means ± SD of eight mice.
a p<0.01 as compared with intact control by LSD test. c p<0.01 as compared with intact control by MW test. b p<0.01 as compared with EtOH control by LSD test; d p<0.01 and e p<0.05 as compared with EtOH control by MW test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; TG, Triglyceride; γ-GTP, γ-Glutamyl Transferase.
Changes in the hepatic TG, TNF- α contents and CYP 450 2E1 activity
Significant (p<0.01) increases of liver TG contents were observed in the EtOH control as compared with the intact control mice. However, the liver TG contents were significantly (p<0.01) and dose-dependently decreased in HSCF extracts treated mice at all dosages. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic TG content elevation as compared with silymarin at 250 mg/kg (Table 5).
Table 5. Hepatic TG and TNF-α contents with hepatic CYP450 2E1 activity in subacute EtOH-treated mice.
| Groups | Hepatic contents | Hepatic CYP450 2E1 activity (4-nitrocatechol μM/min/mg protein) |
|
|---|---|---|---|
| TG (mg/g tissue) | TNF-α (pg/mg protein) | ||
| Controls | |||
| Intact | 18.94±3.51 | 27.70±15.99 | 1.91±0.49 |
| EtOH | 136.74±21.06a | 90.46±17.04a | 8.03±1.55a |
| Silymarin | 79.93±16.24ab | 52.81±13.12ab | 4.40±1.49ab |
| HSCF treated | |||
| 500 mg/kg | 81.19±17.88ab | 53.63±12.32ab | 4.45±1.10ab |
| 250 mg/kg | 98.73±15.49ab | 61.89±17.33ab | 5.34±0.88ab |
| 125 mg/kg | 110.26±17.64ab | 67.60±13.40ab | 6.06±0.97ab |
Values are expressed means ±SD of ten mice.
a p<0.01 as compared with intact control mice by LSD test. b p<0.01 as compared with EtOH control mice by LSD test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; CY, Cytochrome; TG, Triglyceride;TNF, Tumor necrosis factor.
Significant (p<0.01) increases of liver TNF-α contents were observed in EtOH control as compared with intact control mice. However, the liver TNF-α contents were dose-dependently and significantly (p<0.01) decreased by treatment with HSCF extracts at all dosages. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic TNF-α elevation as compared with silymarin at 250 mg/kg in this study (Table 5).
Significant (p<0.01) increases of liver CYP450 2E1 activity and hydroxylation of p-nitrophenol to 4-nitrocatechol were observed in the EtOH control as compared with the intact control mice. However, the liver CYP450 2E1 activity was significantly (p<0.01) decreased by treatment with all dosages of HSCF extracts as compared with the EtOH control, dose-dependently. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic CYP450 2E1 activity increases as compared with silymarin at 250 mg/kg in this experiment (Table 5).
Changes in the hepatic lipid peroxidation and antioxidant defense systems
Significant (p<0.01) increases in hepatic lipid peroxidation and increases of MDA contents in liver parenchyma were observed in the EtOH control mice as compared with the intact control mice. However, these increases in liver lipid peroxidation were significantly (p<0.01) and dose-dependently inhibited by treatment with HSCF extracts at 500, 250 and 125 mg/kg as compared with EtOH control mice. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic lipid peroxidation as compared with silymarin at 250 mg/kg in our experiment (Table 6).
Table 6. Hepatic lipid peroxidation and antioxidant defense systems in subacute EtOH-treated mice.
| Groups | Malondialdehyde (nM/mg protein) |
Glutathione (nM/mg protein) |
Superoxide dismutase (U/mg protein) |
Catalase (U/mg protein) |
|---|---|---|---|---|
| Controls | ||||
| Intact | 1.47±0.66 | 41.08±12.23 | 416.67±104.78 | 251.99±49.21 |
| EtOH | 5.98±1.55a | 6.27±2.14d | 95.23±18.89a | 85.39±16.77d |
| Silymarin | 3.04±0.84ab | 11.24±1.67de | 197.15±61.73ab | 177.36±32.38de |
| HSCF treated | ||||
| 500 mg/kg | 3.01±0.94ab | 11.29±1.84de | 199.87±85.74ab | 178.87±18.32de |
| 250 mg/kg | 3.61±0.76ab | 10.13±1.36de | 186.96±79.78ac | 170.08±22.94de |
| 125 mg/kg | 4.13±0.66ab | 8.92±1.23df | 173.68±18.98ac | 158.35±22.90de |
Values are expressed as Means ± SD of eight mice.
a p<0.01 as compared with intact control by LSD test. d p<0.01 as compared with intact control by MW test. b p<0.01 and c p<0.05 as compared with EtOH control by LSD test. e p<0.01 and f p<0.05 as compared with EtOH control by MW test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts.
Significant (p<0.01) decreases of hepatic GSH contents, SOD and CAT activities were detected in the EtOH control mice as compared with the intact control. However, hepatic antioxidant defense systems were significantly (p<0.01 or p<0.05) and dose-dependently enhanced by treatment with all dosages of HSCF extracts as compared with the EtOH control, resulting in significantly (p<0.01 or p<0.05) increased hepatic GSH contents, SOD and CAT activities as compared with EtOH control. Similar enhancement effects on the hepatic endogenous antioxidant defense systems were observed in mice treated with HSCF extracts at 500 mg/kg as compared with silymarin at 250 mg/kg (Table 6).
Changes in the mRNA expression of hepatic lipogenic genes
To elucidate the molecular mechanism involved in the aggravation of EtOH-induced steatosis in HSCF extracts treated mice, the expression of genes regulating hepatic lipid synthesis was determined by quantitative RT-PCR, including SREBP-1c, SCD1, ACC1, FAS, PPARγ and DGAT2 in the present study.
Hepatic SREBP-1c mRNA expression: In the EtOH control mice, significant (p<0.01) increases of hepatic SREBP-1c mRNA expression (SREBP-1c/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) dose dependent decreases of the hepatic SREBP-1c mRNA expression were demonstrated in mice treated with HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced increases of hepatic SREBP-1c mRNA expression as compared with silymarin at 250 mg/kg in the present study (Table 7).
Table 7. Hepatic TG and TNF-α contents with hepatic CYP450 2E1 activity in subacute EtOH-treated mice.
| Controls | HSCF treated | |||||
|---|---|---|---|---|---|---|
| Intact | EtOH | Silymarin | 500 mg/kg | 250 mg/kg | 125 mg/kg | |
| Hepatic lipogenic genes | ||||||
| SREBP-1c | 1.00±0.19 | 3.79±0.96d | 1.90±0.36de | 1.91±0.42de | 2.25±0.44de | 2.41±0.30de |
| SCD1 | 1.01±0.11 | 3.84±1.01d | 2.05±0.47de | 2.16±0.47de | 2.48±0.42de | 2.81±0.25de |
| ACC1 | 0.99±0.10 | 2.32±0.43d | 1.50±0.29de | 1.51±0.18de | 1.61±0.26de | 1.82±0.18de |
| FAS | 1.00±0.09 | 3.84±0.79d | 2.13±0.48de | 2.16±0.50de | 2.46±0.47de | 2.83±0.47de |
| PPARγ | 1.00±0.06 | 5.34±1.51d | 2.36±0.50de | 2.47±0.54de | 3.00±0.84de | 3.51±0.69df |
| DGAT2 | 1.09±0.20 | 3.68±0.89d | 2.09±0.42de | 2.00±0.19de | 2.43±0.60de | 2.75±0.32de |
| Genes involved in fatty acid oxidation | ||||||
| PPARα | 0.96±0.19 | 0.41±0.16a | 0.77±0.16bc | 0.76±0.10ac | 0.69±0.16ac | 0.63±0.09ac |
| ACO | 1.11±0.16 | 0.35±0.14a | 0.70±0.14ac | 0.69±0.11ac | 0.62±0.09ac | 0.54±0.09ac |
| CPT1 | 0.99±0.20 | 0.27±0.10a | 0.63±0.15ac | 0.65±0.11ac | 0.52±0.12ac | 0.48±0.11ac |
| Master transcription factor of antioxidant genes | ||||||
| Nrf2 | 1.03±0.11 | 0.29±0.13a | 0.61±0.17ac | 0.63±0.12ac | 0.58±0.10ac | 0.47±0.07ac |
Values are expressed as Means ± SD of eight mice.
a p<0.01 and b p<0.05 as compared with intact control by LSD test. d p<0.01 as compared with intact control by MW test. c p<0.01 as compared with EtOH control by LSD test. e p<0.01 and f p<0.05 as compared with EtOH control by MW test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; RT-PCR = reverse transcription polymerase chain reaction; SREBP-1c = Sterol regulatory element-binding protein-1c; SCD1 = Stearoyl-CoA desaturase 1; ACC1 = Acetyl-CoA carboxylase 1; FAS= Fatty acid synthase; PPAR = Peroxisome proliferator-activated receptor; DGAT2 = Diacylglycerol acyltransferase 2; ACO = Acyl-CoA oxidase; CPT1 = Carnitine palmitoyltransferase 1; Nrf2 = NF-E2-related factor-2.
Hepatic SCD1 mRNA expression: In the EtOH control mice, significant (p<0.01) increases of hepatic SCD1 mRNA expression (SCD1/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) and dose-dependent decreases of the hepatic SREBP-1c mRNA expression were observed in mice treated with all three doses of HSCF extracts as compared with the EtOH control mice. Similar inhibitory effects on the hepatic SREBP-1c mRNA expression were demonstrated in mice treated with HSCF extracts at 500 mg/kg as compared with silymarin at 250 mg/kg in this study (Table 7).
Hepatic ACC1 mRNA expression: Significant (p<0.01) increases of liver ACC1 mRNA expression (ACC1/β-actin mRNA) were observed in the EtOH control as compared with the intact control mice. However, the hepatic ACC1 mRNA expression was significantly (p<0.01) and dose-dependently decreased by treatment with HSCF extracts at 500, 250 and 125 mg/kg, respectively. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic ACC1 mRNA expression increases as compared with silymarin at 250 mg/kg in this experiment (Table 7).
Hepatic FAS mRNA expression: In the EtOH control mice, significant (p<0.01) increases of hepatic FAS mRNA expression (FAS/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) and dose-dependent decreases of the hepatic FAS mRNA expression were observed with all three doses of HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice. HSCF extracts at 500 mg/ kg showed similar inhibitory effects on the EtOH-induced increases of hepatic FAS mRNA expression as compared with silymarin at 250 mg/kg (Table 7).
Hepatic PPARγ mRNA expression: Significant (p<0.01) increases of liver PPARγ mRNA expression (PPARγ/ β-actin mRNA) were observed in the EtOH control as compared with the intact control mice. However, the hepatic PPARγ mRNA expression was significantly (p<0.01 or p<0.05) decreased by treatment with all three doses of HSCF extracts. Similar inhibitory effects on the hepatic PPARγ mRNA expression were observed in mice treated with HSCF extracts at 500 mg/kg as compared with silymarin at 250 mg/kg, in our study (Table 7).
Hepatic DGAT2 mRNA expression: In the EtOH control mice, significant (p<0.01) increases of hepatic DGAT2 mRNA expression (DGAT2/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) dose dependent decreases of the hepatic DGAT2 mRNA expression were observed in mice treated with HSCF extracts at 500, 250 and 125 mg/kg as compared with EtOH control mice. HSCF extracts at 500 mg/ kg showed similar inhibitory effects on the EtOH-induced hepatic DGAT2 mRNA expression increases as compared with silymarin at 250 mg/kg in our experiment (Table 7).
Changes in the hepatic mRNA expression of genes involved in fatty acid oxidation
To elucidate the molecular mechanism involved in the aggravation of EtOH-induced steatosis in HSCF extracts treated mice, the expression of genes involved in fatty acid oxidation was also determined by quantitative RT-PCR, including PPARα, ACO and CPT1 in the present study.
Hepatic PPARα mRNA expression: Significant (p<0.01) decreases of hepatic PPARα mRNA expression (PPARα/ β-actin mRNA) were observed in the EtOH control as compared with the intact control mice. However, the hepatic PPARα mRNA expression was significantly (p<0.01) and dose-dependently increased by treatment with all three doses of HSCF extracts at 500, 250 and 125 mg/kg. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced decreases of hepatic PPARα mRNA expression as compared with silymarin at 250 mg/kg (Table 7).
Hepatic ACO mRNA expression: In the EtOH control mice, significant (p<0.01) decreases of hepatic ACO mRNA expression (ACO/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) dose dependent increases of the hepatic ACO mRNA expression were observed in mice treated with all three doses of HSCF extracts as compared with the EtOH control mice. Similar up-regulatory effects on the hepatic ACO mRNA expression were observed in mice treated with HSCF extracts at 500 mg/kg as compared to those treated with silymarin at 250 mg/kg (Table 7).
Hepatic CPT1 mRNA expression: In the EtOH control mice, significant (p<0.01) decreases of hepatic CPT1 mRNA expression (CPT1/β-actin mRNA) were observed as compared with the intact control mice. However, significant (p<0.01) and dose-dependent increases of the hepatic CPT1mRNA expression were observed in mice treated with HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic CPT1 mRNA expression decreases as compared with silymarin at 250 mg/kg in our experiment (Table 7).
Changes in the hepatic mRNA expression of Nrf2
To elucidate the molecular mechanism involved in the aggravation of EtOH-induced oxidative stress in HSCF extracts treated mice, the expression of the master transcription factor of antioxidant gene, Nrf2, was also determined by quantitative RT-PCR in the present study. Significant (p<0.01) decreases of hepatic Nrf2 mRNA expression (Nrf2/ β-actin mRNA) were demonstrated in the EtOH control as compared with the intact control mice. However, the hepatic Nrf2 mRNA expression was significantly (p<0.01) and dose-dependently increased by treatment with all three doses of HSCF extracts at 500, 250 and 125 mg/kg. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced decreases of hepatic Nrf2 mRNA expression as compared with silymarin at 250 mg/kg (Table 7).
Effects on the liver histopathology
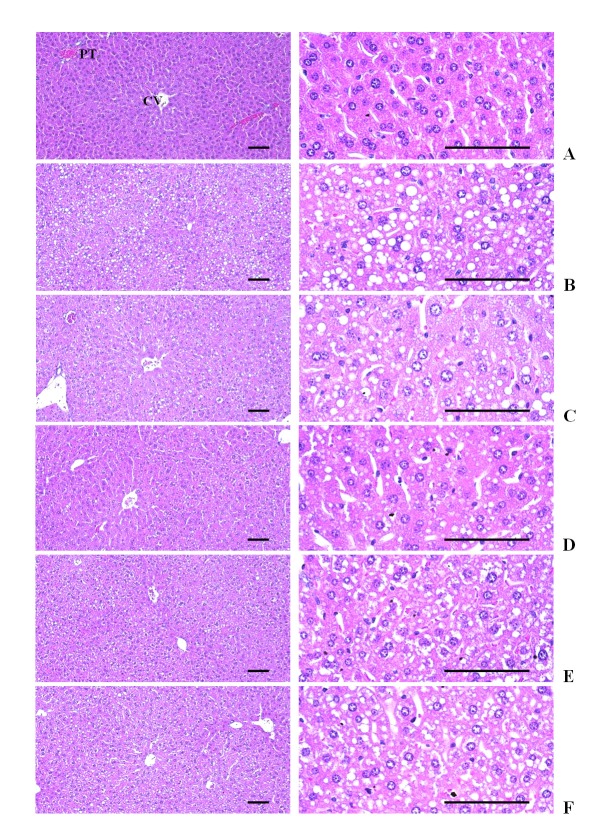
Severe deposition of lipid droplets in the cytoplasm of hepatocytes and hepatosteatosis were observed in all EtOH-dosing groups in the present study. This EtOH-induced hepatosteatosis was re-confirmed with histomorphometry based on the number of changed fatty hepatocytes, mean diameters of hepatocytes and percentage of changed fatty regions, which were significantly (p<0.01) increased in the EtOH control mice as compared with the intact control mice. However, the EtOH treatment-related histopathological hepatosteatosis was significantly (p<0.01 or p<0.05) inhibited by treatment with all three doses of HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice, and the effect was dose-dependent. Similar inhibitory effects on the EtOH-induced histopathological hepatosteatosis were observed in mice treated with HSCF extracts at 500 mg/kg as compared to those treated with silymarin at 250 mg/kg in the present study (Table 8, Fig 1).
Table 8. Hepatic TG and TNF-α contents with hepatic CYP450 2E1 activity in subacute EtOH-treated mice.
| Fatty change regions (%) |
Changed fatty hepatocyte numbers (cells/1000 hepatocyte) |
Mean hepatocyte diameters (μm) |
Numbers NT- immunolabeled cells (cells/1000 hepatocyte) |
Numbers 4-HNE immunopositive cells (cells/1000 hepatocyte) |
|
|---|---|---|---|---|---|
| Controls | |||||
| Intact | 8.44±2.78 | 86.75±31.11 | 18.05±2.42 | 44.00±18.08 | 77.50±27.67 |
| EtOH | 77.93±10.79a | 743.75±138.164d | 32.52±3.25a | 562.63±92.50d | 685.13±112.77a |
| Silymarin | 36.37±10.93ac | 387 00±102.73de | 23.91±2.83ac | 228.50±68.96de | 195.88±61.02ac |
| HSCF treated | |||||
| 500 mg/kg | 35.74±8.05ac | 365.25±119.80de | 23.28±3.26ac | 215.50±82.25de | 171.88±47.04bc |
| 250 mg/kg | 52.88±10.89ac | 513.63±101.61de | 26.10±3.64ac | 319.88±63.24de | 263.50±76.53ac |
| 125 mg/kg | 59.01±13.03ac | 557.63±99.95df | 27.88±2.92ac | 430.00±66.80de | 472.25±84.84ac |
Values are expressed as Means ± SD of eight mice.
a p<0.01 and b p<0.05 as compared with intact control by LSD test. d p<0.01 as compared with intact control by MW test. c p<0.01 as compared with EtOH control by LSD test. e p<0.01 and f p<0.05 as compared with EtOH control by MW test. EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; 4-HNE, 4-Hydroxynonenal; NT, Nitrotyrosine
Figure 1. Representative histological images of the liver, taken from subacute EtOH-treated mice.
Severe deposition of lipid droplets in cytoplasm of hepatocytes, hepatosteatosis were observed in all EtOH-treated mice in the present study, and these EtOH-induced hepatosteatosis are re-confirmed with histomorphometry based on the number of changed fatty hepatocytes, mean diameters of hepatocytes and percentages of changed fatty regions, which were significantly increased in EtOH control mice as compared with intact control mice. However, this EtOH treatment-related histopathological hepatosteatosis was significantly inhibited by treatment with all three doses of HSCF extracts at 500, 250 and 125 mg/kg as compared with EtOH control mice, dose-dependently. Similar inhibitory effects on the EtOH-induced histopathological hepatosteatosis were demonstrated in mice treated with HSCF extracts at 500 mg/kg as compared with silymarin at 250 mg/kg in the present study.
(A) Intact control, (B) EtOH control, (C) Silymarin control, (D) HSCF 500, (E) HSCF 250, (F) HSCF 125
EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; CV, Central vein; PT, Portal triad
Hematoxylin-eosin stain. Scale bars, 200μm.
Effects on the hepatic NT and 4-HNE-immunoreactivities
The immunoreactivities of NT as a marker of iNOS related oxidative stress57 and 4-HNE as a marker of lipid peroxidation58 in hepatic parenchyma were assessed to determine the liver oxidative stress.
Changes in the NT-immunolabeled hepatocytes: Marked and significant (p<0.01) increases of an iNOS related oxidative stress marker, NT-immunoreactive hepatocytes, were observed in the EtOH control mice as compared with the intact control mice. HSCF extracts at 500, 250 and 125 mg/kg dose-dependently and significantly (p<0.01) normalized the EtOH-related increases of NT-immunoreactive hepatocytes. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic NT-immunolabeled cell increases as compared with silymarin at 250 mg/kg in this study (Table 8, Fig 2).
Figure 2. Representative images of NT and 4-HNE-immunoreactivities in the liver sections, taken from subacute EtOH-treated mice.
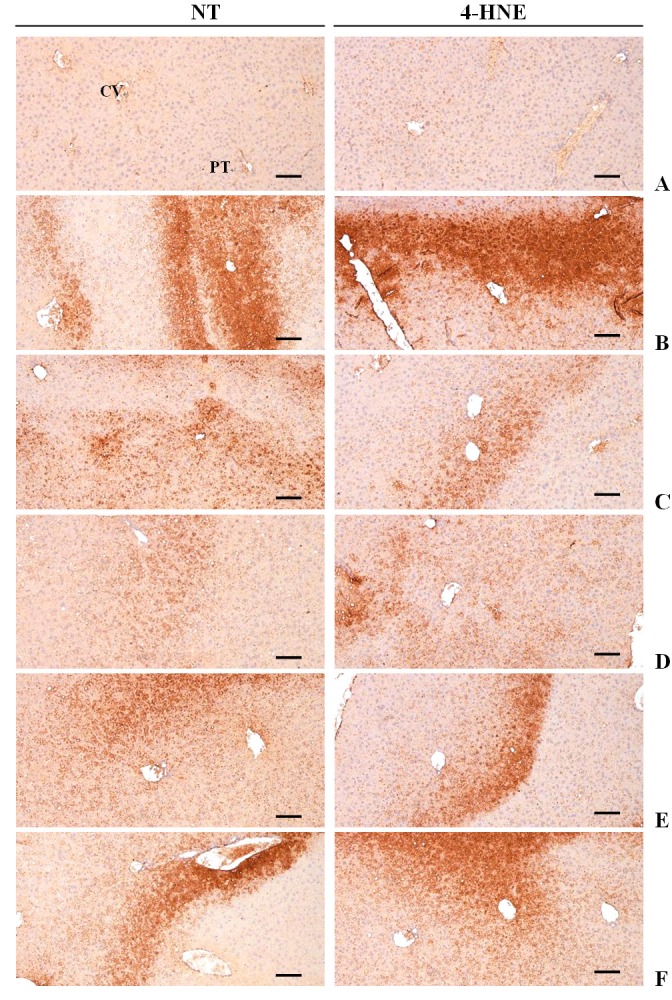
Marked and significant increases of an iNOS related oxidative stress marker, nitrotyrosine-immunoreactive hepatocytes and also significant increases of a lipid peroxidation marker, 4-HNE-immunoreactive hepatocytes were observed in EtOH control mice as compared with intact control mice. HSCF extracts at 500, 250 and 125 mg/kg dose-dependently and significantly normalized these EtOH-related increases of NT- and 4-HNE-immunoreactive hepatocytes. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic NT- and 4-HNE-immunolabeled cell increases as compared with silymarin at 250 mg/kg in this study.
(A) Intact control, (B) EtOH control, (C) Silymarin control, (D) HSCF 500, (E) HSCF 250, (F) HSCF 125
EtOH, Ethanol; HSCF, Hoveniae Semen Cum Fructus extracts; NT, Nitrotyrosine; 4-HNE, 4-hydroxynonenal; iNOS, inducible nitric oxide synthase, CV, Central vein; PT, Portal triad. ABC immunohistochemistrical methods. Scale bars, 200μm.
Changes in the 4-HNE-positive hepatocytes: Marked and significant (p<0.01) increases of a lipid peroxidation marker, 4-HNE-immunoreactive hepatocytes, were observed in the EtOH control mice as compared with the intact control mice. However, significant (p<0.01) decreases of the 4-HNE-immunopostive hepatocytes were demonstrated in mice treated with all three doses of HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control mice, and the effect was dose-dependent. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the increases of the hepatic 4-HNE-immunolabeled cells induced by 2 weeks of continuous oral administration of EtOH as compared with silymarin at 250 mg/kg (Table 8, Fig 2).
DISCUSSION
Alcoholic liver disease remains one of the most common causes of chronic liver disease in the world59. Alcoholic fatty liver is the earliest and most common response of the liver to heavy alcohol use, and it is a precursor of more severe forms of liver injury5,15,16,60. Accumulated evidence has demonstrated that oxidative stress, abnormal cytokine production, and steatosis play important etiological roles in the pathogenesis of alcoholic liver disease. Therefore, agents that have antioxidant, anti-inflammatory and anti-steatosis properties, particularly anti-TNF production and decreasing lipid accumulation, represent a promising therapeutic intervention for alcoholic liver disease4,5,7. Various antioxidant-based pharmacological effects of HSCF extracts have been reported including anti-adipogenic31 and hepatoprotective34,35 effects. However, it seems that more systemic evaluation of the hepatoprotective effects of HSCF extract with molecular targets is needed. In the present study, the beneficial potential of HSCF extract on the subacute EtOH-induced hepatic damages in mice was systemically evaluated as well as the corresponding potent anti-oxidant, anti-inflammatory and anti-steatosis mechanisms.
The body weight decrease after EtOH treatment was considered a result of the direct toxicity of EtOH and/or indirect toxicity related to liver damage. The body weight can also decrease due to malnutrition, secondary to food intake decreases61,62. Therefore, the increased body weight and gains detected in the silymarin group and HSCF extracts treated group are considered indirect evidence of hepatoprotective effects as compared with the EtOH control, since body weight is considered a putative indicator of health. In addition, dose-dependent inhibitory effects on the EtOH-induced liver weight decreases following treatment with HSCF extracts were also considered evidence that HSCF extracts have hepatoprotective effects against acute EtOH intoxication. In chronic alcoholics, the liver weight is generally decreased due to necrotic and inflammatory processes that occur in the hepatic parenchyma and the substitution of hepatic parenchyma with lipids 63-66. This is also the case in acute EtOH-induced liver damaged mice67. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced liver weight decreases as compared with silymarin at 250 mg/kg in this experiment.
Generally, AST, ALT, albumin, γ-GTP and ALP are used as serum markers to represent various types of liver damage 68. These markers were markedly elevated following EtOH-induced hepatic damages in previous reports69,70 and also in this experiment. In addition, serum TG levels are generally increased with EtOH-induced hepatic damage due to decreased TG utilization in hepatocytes71,72. Therefore, it is considered evidence that HSCF extracts have favorable hepatoprotective effects against EtOH-induced liver injuries because marked inhibition of the EtOH-induced serum AST, ALT, albumin, ALP, γ-GTP and TG levels, and hepatic TG contents were dose-dependently improved in these groups as compared with the EtOH control mice in the present study. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced serum AST, ALT, albumin, ALP, TG and γ-GTP elevation as compared with silymarin at 250 mg/kg.
Abnormal metabolism of cytokines, especially TNF-α, is another major feature of alcoholic liver disease4,7. It was initially observed that cultured monocytes from alcoholic hepatitis patients spontaneously produced TNF-α and produced significantly more TNF-α in response to a lipopolysaccaride stimulus than control monocytes73. Subsequently, earlier researchers demonstrated that anti-TNF antibody prevented liver injury in alcohol-fed rats and mice lacking the TNF-type I receptor also did not develop alcoholic liver injury74,75. Consistent with chronic alcohol effects, increased hepatic TNF-α production by acute EtOH exposure has recently been reported4,7,76. In vitro studies demonstrated that silymarin inhibited Kupffer cell functions and TNF-α production in lipopolysaccaride-stimulated RAW264.7 cells77,78. Our results also showed that 2 weeks of continuous subacute EtOH administration enhanced hepatic TNF-α production. In vivo HSCF extracts administration dose-dependently attenuated this increased TNF-α production, similar to silymarin at 250 mg/kg, suggesting that the hepatoprotective effects of HSCF extracts on EtOH-induced subacute hepatic damages may be mediated by anti-inflammatory effects through suppression of the hepatic TNF-α production.
Although there are many potential sources of ROS in response to EtOH exposure, CYP450 2E1 is one of the major sites involved in ROS production in the liver in response to alcohol4. It has been reported that long-term alcohol exposure increased CYP450 2E1 activities76,79. Furthermore, investigations using CYP450 2E1 inhibitors, including diallyl sulfide or chlormethiazole, have shown that inhibition of CYP450 2E1 activity inhibits alcohol-induced liver injury, indicating the importance of CYP450 2E1 in alcohol- induced ROS accumulation and liver injury80,81. Similarly, genetic overexpression of CYP450 2E1 in the liver causes enhanced alcohol-induced liver injury in mice82. To investigate the possible mechanisms by which HSCF extracts attenuated subacute EtOH-induced liver injury, we first evaluated the effect of HSCF extracts on CYP450 2E1 enzymatic activity in response to acute EtOH exposure. Our study indicated that 2 weeks of continuous oral administration of EtOH increased hepatic CYP450 2E1 activity, but this increase of CYP450 2E1 activity was dose-dependently diminished by treatment with HSCF extracts. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic CYP450 2E1 activity increases as compared with silymarin at 250 mg/kg.
Considerable experimental and clinical evidence has contributed to support a key role of oxidative stress in the pathophysiological processes of liver injury related to excessive alcohol consumption83,84. The metabolism of EtOH gives rise to the generation of excess amounts of ROS and has a detrimental effect on the cellular antioxidant defense system85,86 that leads to hepatic cellular necrosis, inflammation and steatohepatitis64,87. Thus, numerous interventions have been put forward to counteract the vulnerability of the liver to oxidative challenges during alcohol consumption by reinforcing the endogenous antioxidant defense system86,88. Lipid peroxidation is an autocatalytic mechanism leading to oxidative destruction of cellular membranes89,90. Such destruction can lead to cell death and to the production of toxic and reactive aldehyde metabolites called free radicals, with MDA as the most important91,92. It is known that ROS leads to oxidative damage of biological macromolecules, including lipids, proteins, and DNA91,93, and oxidative stress influences body adipocytes, resulting in decreases in body fat mass and related body weight decreases94. MDA is a terminal product of lipid peroxidation. So the content of MDA can be used to estimate the extent of lipid peroxidation91. Marked increases of liver MDA contents have been observed in alcoholic rodents4,5,7,15,64, and liver MDA content was increased in this study by treatment with EtOH. GSH is a representative endogenous antioxidant that prevents tissue damage by keeping the ROS at low levels and at certain cellular concentrations, and is accepted as a protective antioxidant factor in tissues95. SOD is one of the antioxidant enzymes that contributes to enzymatic defense mechanisms, and catalase is an enzyme that catalyzes the conversion of H2O2 to H2O96. The decrease of antioxidant enzyme activities such as SOD and catalase, and GSH contents may be indicative of the failure to compensate for the oxidative stress induced by EtOH64,85-87. In this experiment, the hepatic antioxidant defense system was dose-dependently enhanced by treatment with HSCF extracts at 500, 250 and 125 mg/kg as compared with the EtOH control, along with up-regulation of Nrf2, a master transcription factor of antioxidant genes17,19, which was down-regulated by EtOH supply. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced hepatic lipid peroxidation, and enhancement effects on the hepatic endogenous antioxidant defense systems as compared with silymarin at 250 mg/kg. This suggests that the hepatoprotective effects of HSCF extracts against EtOH intoxication are mediated by augmentation of the hepatic antioxidant defense system, which may be mediated by Nrf2 activation and related inhibitory effects on lipid peroxidation.
There are multiple mechanisms underlying EtOH-induced development of fatty liver. Enhanced lipogenesis and impaired fatty-acid oxidation have long been proposed as important biochemical mechanisms underlying the development of alcoholic fatty liver5,15. Previous studies demonstrated that EtOH administration activates SREBP- 1c and its target genes like SCD1, ACC1 and FAS, which promote de novo fatty-acid synthesis5,15,23. SREBP-1cnull mice fed EtOH by intragastric infusion for 4 weeks showed significantly lower TG concentration than that in wild typed mice97. In this experiment, EtOH treatment also significantly up-regulated the hepatic SREBP-1c mRNA expression, and its target genes – FAS, SCD1 and ACC1. However, all dosages of HSCF extracts dose-dependently down regulated the hepatic mRNA expression of SREBP- 1c, SCD1, ACC1 and FAS. This suggests that the hepatoprotective effects of HSCF extracts against EtOH-induced hepatic steatosis are mediated by down regulation of SREBP-1c and its target genes, FAS, SCD1 and ACC1. HSCF extracts at 500 mg/kg showed similar inhibitory effects on the EtOH-induced increases of hepatic lipogenic genes - SREBP-1c, FAS, SCD1 and ACC1 mRNA expression as compared with silymarin at 250 mg/kg.
PPARγ and DGAT2 are involved in TG synthesis5,15,22-24. DGAT is involved in TG synthesis in the liver, and the levels of DGAT1 and DGAT2 mRNAs were increased in response to EtOH5,15. PPARγ is a member of the nuclear receptor superfamily of ligand-activated transcription factors that regulate the expression of genes associated with lipid metabolism. Adenovirus-mediated delivery of PPARγ to hepatocytes leads to fatty liver, and PPARγ RNA interference is reported to decrease hepatic TG levels22,24. PPARγ and DGAT are significantly up-regulated after acute EtOH administration and are involved in EtOH-induced fatty liver in mouse5,15,23. In this study, hepatic mRNA levels of both PPARγ and DGAT2 were up-regulated by EtOH stimulation. HSCF extracts at 500, 250 and 125 mg/kg significantly and dose-dependently impaired the elevation of these genes, similar to silymarin at 250 mg/kg, well corresponding to the results of hepatic and serum TG levels. These results suggested that oral treatment of HSCF extracts dose-dependently inhibits hepatic lipogenesis in response to EtOH by suppressing genes related to TG synthesis.
In addition to increased lipogenesis, decreased fatty acid metabolism also contributes to EtOH-induced fatty liver5,15,23,60. PPARα and its target genes, including ACO and CPT1, are involved in fatty-acid β-oxidation5,15,25. Administration of Wy14643, a PPARα agonist, prevented fatty liver in mice fed EtOH for 4 weeks98,99. In this experiment, subacute treatment of EtOH 5 g/kg decreased the expression of these genes and impaired fatty-acid β-oxidation in the liver. However, HSCF extracts at 500, 250 and 125 mg/ kg up-regulated the hepatic mRNA expression of PPARα and its target genes, including ACO and CPT1, similar to silymarin at 250 mg/kg. Oral treatment of HSCF extracts at dose levels of 500, 250 and 125 mg/kg not only down regulated the expression of genes related to fatty-acid and TG synthesis, but also increased fatty acid metabolism through up-regulation of genes involved in fatty-acid β-oxidation in the liver.
Acute or chronic alcohol consumption can cause severe histopathological liver injury100. Alcohol is known to impair fat oxidation and to stimulate lipogenesis in the liver101-103. Thus, alcohol consumption can lead to the development of hepatic steatosis104. In this experiment, severe deposition of lipid droplets in the cytoplasm of hepatocytes and hepatosteatosis were also observed in all EtOH treated mice. This EtOH-induced hepatosteatosis was re-confirmed with histomorphometry based on the number of changed fatty hepatocytes, mean diameters of hepatocytes and percentages of changed fatty regions, which were significantly increased in EtOH control mice as compared with intact control mice in the left lateral lobes. However, this EtOH treatment-related histopathological hepatosteatosis was significantly and dose-dependently inhibited by treatment of HSCF extracts at 500, 250 and 125 mg/kg, similar to silymarin 250 mg/kg, as compared with EtOH control mice in this experiment. These findings are considered direct evidence that HSCF extracts have favorable hepatoprotective effects against EtOH-induced hepatic steatosis.
NT is a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide. It is detected in a large number of pathological conditions including EtOH-induced liver damages, and is considered a marker of nitric oxide-dependent, reactive nitrogen species-induced nitrative stress51,57,105. Most studies on alcoholic hepatic steatosis have focused on the ability of EtOH to shift the redox state in the liver and to inhibit fatty acid oxidation101,106. Indeed, previous studies have shown the repression of some enzymes involved in fatty acid oxidation and induction of lipogenic enzymes in EtOH-fed animals102,103. Sustained exposure to ROS leads to prolonged oxidative stress and increases of NT107,108. In this experiment, marked and significant increases of NT-immunoreactive cells were observed in the hepatic tissues of EtOH control mice as compared with intact control mice, but they were significantly reduced by treatment of HSCF extracts at 500, 250 and 125 mg/kg, dose-dependently, similar to silymarin at 250 mg/kg. It is suggested that HSCF extracts favorably inhibit iNOS related oxidative stress and protect against hepatocyte necrotic changes from EtOH at dose levels of 500, 250 and 125 mg/kg.
4-HNE is an α, β-unsaturated hydroxyalkenal which is produced by lipid peroxidation in cells. It is considered a possible causal agent of numerous diseases, such as chronic inflammation, neurodegenerative diseases, adult respiratory distress syndrome, atherogenesis, diabetes and different types of cancer58,109,110. Sustained exposure to EtOH mediated ROS leads to prolonged oxidative stress, which promotes lipid peroxidation and generation of reactive aldehydes, such as 4-HNE107,111. In the present study, marked and significant increases of 4-HNE-positive cells were also observed in the left lateral hepatic lobes of EtOH control mice as compared with intact control mice, but they were significantly and dose-dependently normalized by treatment of all dosages of HSCF extracts, similar to silymarin at 250 mg/kg. This corresponded to the results of NT-immunolabeled cells and is considered as direct evidence that HSCF extracts effectively inhibited lipid peroxidation and the formation of 4-HNE to protect against hepatocyte necrotic changes from EtOH.
Results corresponding to previous reports4,5,7,15 regarding marked decreases of body and liver weights, increases of serum AST, ALT, Albumin, γ-GTP and TG levels, hepatic TG contents, TNF-α level, CYP450 2E1 activity and mRNA expression of hepatic lipogenic genes (SREBP- 1c, SCD1, ACC1, FAS, PPARγ and DGAT2), decreases mRNA expression of genes involved in fatty acid oxidation (PPARα, ACO and CPT1) or master transcription factor of antioxidant gene (Nrf2) were observed with histopathological changes related to hepatosteatosis (noticeable increases of the percentages of changed fatty regions, the number of changed fatty hepatocytes and mean hepatocyte diameters) and increases of NT and 4-HNE-immunolabelled hepatocytes, following continuous oral administration of EtOH for 2 weeks in the present study. Also, the destruction of hepatic antioxidant defense systems (the increase of hepatic lipid peroxidation, increase of liver MDA contents, and decreases of GSH contents, SOD and CAT activities) were demonstrated in EtOH control mice as compared with intact control. However, these EtOH treatment related liver inflammatory damages, steatosis, increases of mRNA expression of hepatic lipogenic genes, decreases of mRNA expression of genes involved in fatty acid oxidation, and destruction of antioxidant defense systems, may be mediated by down-regulation of Nrf2, which was markedly and dose-dependently inhibited by pretreatment of HSCF extracts at 500, 250 and 125 mg/kg. The overall effects of HSCF extracts at 500 mg/kg were similar to those of silymarin at 250 mg/kg in this experiment.
CONCLUSION
This study found that oral administration of 500, 250, and 120 mg/kg of HSCF favorably protected against liver damages from subacute mouse EtOH intoxication. Hoveniae Semen Cum Fructus extract demonstrated potent anti-inflammatory and anti-steatosis properties through augmentation of the hepatic antioxidant defense system, mediated by Nrf2 activation and down-regulation of the mRNA expression of hepatic lipogenic genes or up-regulation of the mRNA expression of genes involved in fatty acid oxidation. Hoveniae Semen Cum Fructus extract seems to be a new potent hepatoprotective agent or ingredient for liver diseases, with less toxicity.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No.2012R1A5A2A42671316)
REFERNCES
- 1.Meyer SA, Kulkarni AP. et al. Hodgson E, Smart RC. Introduction to biochemical toxicology. 3rd ed. New York: John Wiley & Sons; 2001. Hepatotoxicity; pp. 487–90. [Google Scholar]
- 2.Uličná O, Greksák M, Vančová O, Zlatoš L, Galbavỳ Ŝ, Božek P, Nakano M. Hepatoprotective effect of rooibos tea (Aspalathus linearis) on CCl4-induced liver damage in rats. Physiol Res. 2003;52:461–6. [PubMed] [Google Scholar]
- 3.Ponnappa BC, Rubin E. Modeling alcohol’s effects on organs in animal models. Alcohol Res Health. 2000;24:93–104. [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30:407–13. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P, Wang Z, Zhan Y, Wang T, Zhou M, Xia L, Yang X, Zhang J. Endogenous A1 adenosine receptor protects mice from acute ethanol-induced hepatotoxicity. Toxicology. 2013;309:100–6. doi: 10.1016/j.tox.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–4. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 7.Xing WW, Zou MJ, Liu S, Xu T, Wang JX, Xu DG. Interleukin-22 protects against acute alcohol-induced hepatotoxicity in mice. Biosci Biotechnol Biochem. 2011;75:1290–4. doi: 10.1271/bbb.110061. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994;29:513–22. [PubMed] [Google Scholar]
- 9.Kurose I, Higuchi H, Kato S, Miura S, Watanabe N, Kamegaya Y, Tomita K, Takaishi M, Horie Y, Fukuda M, Mizukami K, Ishii H. Oxidative stress on mitochondria and cell membrane of cultured rat hepatocytes and perfused liver exposed to ethanol. Gastroenterology. 1997;112:1331–43. doi: 10.1016/S0016-5085(97)70147-1. [DOI] [PubMed] [Google Scholar]
- 10.Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–5. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 11.Bondy SC, Orozco J. Effects of ethanol treatment upon sources of reactive oxygen species in brain and liver. Alcohol Alcohol. 1994;29:375–83. [PubMed] [Google Scholar]
- 12.DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-L. [DOI] [PubMed] [Google Scholar]
- 13.Somani SM. et al. Somani SM. Pharmacology in Exercise and Sports. Boca Raton: FL: CRC Press; 1996. Exercise, drugs and tissue specific antioxidant system; pp. 57–95. [Google Scholar]
- 14.Jenkins RR, Goldfarb A. Introduction: oxidant stress, aging, and exercise. Med Sci Sports Exerc. 1993;25:210–2. doi: 10.1249/00005768-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Yang P, Zhan Y, Xia L, Hua Z, Zhang J. Deletion of circadian gene Per1 alleviates acute ethanol-induced hepatotoxicity in mice. Toxicology. 2013;314:193–201. doi: 10.1016/j.tox.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Zhuge J, Wang X, Bai J. Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005;7:385–94. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 18.Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol. 2011;85:273–84. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–6. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 20.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 21.Zeng T, Xie KQ. Ethanol and liver: recent advances in the mechanisms of ethanol-induced hepatosteatosis. Arch Toxicol. 2009;83:1075–81. doi: 10.1007/s00204-009-0457-4. [DOI] [PubMed] [Google Scholar]
- 22.Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–3. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 23.Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior toethanol administration prevents acute ethanol-induced fatty liver in mice. J Hepatol. 2008;49:441–50. doi: 10.1016/j.jhep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expressionand adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 25.Reddy JK, Mannaerts GP. Peroxisomal lipid metabolism. Annu Rev Nutr. 1994;14:343–70. doi: 10.1146/annurev.nu.14.070194.002015. [DOI] [PubMed] [Google Scholar]
- 26.Devipriya N, Srinivasan M, Sudheer AR, Menon VP. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: a drug dose dependent study. Singapore Med J. 2007;48:311–8. [PubMed] [Google Scholar]
- 27.Jafri MA, Jalis Subhani M, Javed K, Singh S. Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J Ethnopharmacol. 1999;66:355–61. doi: 10.1016/S0378-8741(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 28.Kaviarasan S, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed polyphenols protect liver from alcohol toxicity: a role on hepatic detoxification system and apoptosis. Pharmazie. 2007;62:299–304. [PubMed] [Google Scholar]
- 29.Kumar RS, Ponmozhi M, Viswanathan P, Nalini N. Effect of Cassia auriculata leaf extract on lipids in rats with alcoholic liver injury. Asia Pac J Clin Nutr. 2002;11:157–63. doi: 10.1046/j.1440-6047.2002.00286.x. [DOI] [PubMed] [Google Scholar]
- 30.Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78:713–8. doi: 10.1016/j.lfs.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 31.Kim HL, Sim JE, Choi HM, Choi IY, Jeong MY, Park J, Jung Y, Youn DH, Cho JH, Kim JH, Hwang MW, Jin JS, Hong SH, Cho HW, Um JY. The AMPK pathway mediates an anti-adipogenic effect of fruits of Hovenia dulcis Thunb. Food Funct. 2014;5:2961–8. doi: 10.1039/C4FO00470A. [DOI] [PubMed] [Google Scholar]
- 32.Na CS, Yoon SY, Kim JB, Na DS, Dong MS, Lee MY, Hong CY. Anti-fatigue activity of Hovenia dulcis on a swimming mouse model through the inhibition of stress hormone expression and antioxidation. J Chin Med. 2013;41:945–55. doi: 10.1142/S0192415X13500638. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Min BS, Zheng C, Lee J, Oh SR, Ahn KS, Lee HK. Neuroprotective and free radical scavenging activities of phenolic compounds from Hovenia dulcis. Arch Pharm Res. 2005;28:804–9. doi: 10.1007/BF02977346. [DOI] [PubMed] [Google Scholar]
- 34.Hase K, Ohsugi M, Xiong Q, Basnet P, Kadota S, Namba T. Hepatoprotective effect of Hovenia dulcis THUNB. on experimental liver injuries induced by carbon tetrachloride or D-galactosamine/ lipopolysaccharide. Biol Pharm Bull. 1997;20:381–5. doi: 10.1248/bpb.20.381. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Zhu P, Jiang C, Ma L, Zhang Z, Zeng X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem Toxicol. 2012;50:2964–70. doi: 10.1016/j.fct.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Butler WM, Maling HM, Horning MG, Brodie BB. The direct determination of liver triglycerides. J Lipid Res. 1961;2:95-6. Bondy SC, Orozco J. Effects of ethanol treatment upon sources of reactive oxygen species in brain and liver. Alcohol Alcohol. 1994;29:375–83. [PubMed] [Google Scholar]
- 37.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 38.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Osborne DF, Lappas GD, Karl IE. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock. 1995;3:337–42. [PubMed] [Google Scholar]
- 40.Yoon HS, Kim JW, Cho HR, Moon SB, Shin HD, Yang KJ, Lee HS, Kwon YS, Ku SK. Immunomodulatory effects of Aureobasidium pullulans SM-2001 exopolymers on the cyclophosphamide-treated mice. J Microbiol Biotechnol. 2010;20:438–45. [PubMed] [Google Scholar]
- 41.Clark BD, Bedrosian I, Schindler R, Cominelli F, Cannon JG, Shaw AR, Dinarello CA. Detection of interleukin 1 alpha and 1 beta in rabbit tissues during endotoxemia using sensitive radioimmunoassays. J Appl Physiol (1985) 1991;71:2412–8. doi: 10.1152/jappl.1991.71.6.2412. [DOI] [PubMed] [Google Scholar]
- 42.Kavutcu M, Canbolat O, Oztürk S, Olcay E, Ulutepe S, Ekinci C, Gökhun IH, Durak I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: effects of vitamins E and C. Nephron. 1996;72:269–74. doi: 10.1159/000188853. [DOI] [PubMed] [Google Scholar]
- 43.Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase superoxidase dismutase and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol. 1985;80:33–42. doi: 10.1016/0041-008X(85)90098-5. [DOI] [PubMed] [Google Scholar]
- 44.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 45.Aebi H. Bergmeyer HU. Methods in Enzymatic Analysis. New York: Academic Press; 1974. Catalase; pp. 673–86. [Google Scholar]
- 46.Sun Y, Larry WO, Ying L. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 47.Jung YM, Lee SH, Lee DS, You MJ, Chung IK, Cheon WH, Kwon YS, Lee YJ, Ku SK. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011;31:387–96. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Ki SH, Yang JH, Ku SK, Kim SC, Kim YW, Cho IJ. Red ginseng extract protects against carbon tetrachloride-induced liver fibrosis. J Ginseng Res. 2013;37:45–53. doi: 10.5142/jgr.2013.37.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi SR, Chaiwun B, Young L, Cote RJ, Taylor CR. Antigen retrieval technique utilizing citrate buffer or urea solution for immunohistochemical demonstration of androgen receptor in formalin-fixed paraffin sections. J Histochem Cytochem. 1993;41:1599–604. doi: 10.1177/41.11.7691930. [DOI] [PubMed] [Google Scholar]
- 50.Li SY, Yang D, Fu ZJ, Woo T, Wong D, Lo AC. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol Dis. 2012;45:624–32. doi: 10.1016/j.nbd.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Chen JH, Tipoe GL, Liong EC, So HS, Leung KM, Tom WM, Fung PC, Nanji AA. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am J Clin Nutr. 2004;80:742–51. doi: 10.1093/ajcn/80.3.742. [DOI] [PubMed] [Google Scholar]
- 52.Hartley DP, Kolaja KL, Reichard J, Petersen DR. 4-Hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: immunochemical detection and lobular localization. Toxicol Appl Pharmacol. 1999;161:23–33. doi: 10.1006/taap.1999.8788. [DOI] [PubMed] [Google Scholar]
- 53.Noyan S, Cavusoglu I, Minbay FZ. The effect of vitamin A on EtOH-induced hepatic injuries in rats: a histochemical, immunohistochemical and ultrastructural study. Acta Histochem. 2006;107:421–34. doi: 10.1016/j.acthis.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Levene A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin Otolaryngol Allied Sci. 1981;6:145–51. doi: 10.1111/j.1365-2273.1981.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 55.Ludbrook J. Update: microcomputer statistics packages. A personal view. Clin Exp Pharmacol Physiol. 1997;24:294–6. doi: 10.1111/j.1440-1681.1997.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 56.Kang SJ, Lee JE, Lee EK, Jung DH, Song CH, Park SJ, Choi SH, Han CH, Ku SK, Lee YJ. Fermentation with Aquilariae Lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients. 2014;6:3536–71. doi: 10.3390/nu6093536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact. 2011;192:107–12. doi: 10.1016/j.cbi.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/S0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 60.Hu M, Yin H, Mitra MS, Liang X, Ajmo JM, Nadra K, Chrast R, Finck BN, You M. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology. 2013;58:1953–63. doi: 10.1002/hep.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saravanan N, Nalini N. Inhibitory effect of Hemidesmus indicus and its active principle 2-hydroxy 4-methoxy benzoic acid on ethanol-induced liver injury. Fundam Clin Pharmacol. 2007;21:507–14. doi: 10.1111/j.1472-8206.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 62.Saravanan N, Rajasankar S, Nalini N. Antioxidant effect of 2-hydroxy-4-methoxy benzoic acid on ethanol-induced hepatotoxicity in rats. J Pharm Pharmacol. 2007;59:445–53. doi: 10.1211/jpp.59.3.0015. [DOI] [PubMed] [Google Scholar]
- 63.Gopumadhavan S, Rafiq M, Azeemuddin M, Mitra SK. Ameliorative effect of Partysmart in rat model of alcoholic liver disease. Indian J Exp Biol. 2008;46:132–7. [PubMed] [Google Scholar]
- 64.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–9. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 65.Pari L, Suresh A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem Toxicol. 2008;46:1627–34. doi: 10.1016/j.fct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhang R, Hu Y, Yuan J, Wu D. Effects of Puerariae radix extract on the increasing intestinal permeability in rat with alcohol-induced liver injury. J Ethnopharmacol. 2009;126:207–14. doi: 10.1016/j.jep.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, Kim SJ, Hwang I, Bae KC, Bae JH, Song DK. Green tea extract co-administered with a polymer effectively prevents alcoholic liver damage by prolonged inhibition of alcohol absorption in mice. Alcohol Alcohol. 2013;48:59–67. doi: 10.1093/alcalc/ags118. [DOI] [PubMed] [Google Scholar]
- 68.Sodikoff CH. Laboratory profiles of small animal diseases, A guide to laboratory diagnosis. St. Louise: Mosby. 1995:1–36. [Google Scholar]
- 69.Das SK, Varadhan S, Gupta G, Mukherjee S, Dhanya L, Rao DN, Vasudevan DM. Time-dependent effects of ethanol on blood oxidative stress parameters and cytokines. Indian J Biochem Biophys. 2009;46:116–21. [PubMed] [Google Scholar]
- 70.Li YM, Chen SH, Yu CH, Zhang Y, Xu GY. Effect of acute alcoholism on hepatic enzymes and oxidation/antioxidation in rats. Hepatobiliary Pancreat Dis Int. 2004;3:241–4. [PubMed] [Google Scholar]
- 71.Ho WY, Yeap SK, Ho CL, Abdul Rahim R, Alitheen NB. Hepatoprotective activity of Elephantopus scaber on alcohol-induced liver damage in mice. Evid Based Complement Alternat Med. 2012;2012:417953. doi: 10.1155/2012/417953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiang J, Zhu W, Li Z, Ling S. Effect of juice and fermented vinegar from Hovenia dulcis peduncles on chronically alcohol-induced liver damage in mice. Food Funct. 2012;3:628–34. doi: 10.1039/c2fo10266h. [DOI] [PubMed] [Google Scholar]
- 73.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–51. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 74.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997 Dec;26(6):1530–7. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 75.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/S0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–22. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- 77.Dehmlow C, Erhard J, Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–54. doi: 10.1002/hep.510230415. [DOI] [PubMed] [Google Scholar]
- 78.Cho JY, Kim PS, Park J, Yoo ES, Baik KU, Kim YK, Park MH. Inhibitor of tumor necrosis factor-alpha production in lipopolysaccharide- stimulated RAW264.7 cells from Amorpha fruticosa. J Ethnopharmacol. 2000;70:127–33. doi: 10.1016/S0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- 79.Lieber C. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 80.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr., French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–9. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 81.McCarty MF. Inhibition of CYP2E1 with natural agents may be a feasible strategy for minimizing the hepatotoxicity of ethanol. Med Hypotheses. 2001;56:8–11. doi: 10.1054/mehy.1999.1015. [DOI] [PubMed] [Google Scholar]
- 82.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–34. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 83.Cahill A, Cunningham CC, Adachi M, Ishii H, Bailey SM, Fromenty B, Davies A. Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrion. Alcohol Clin Exp Res. 2002;26:907–15. doi: 10.1111/j.1530-0277.2002.tb02621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castilla R, González R, Fouad D, Fraga E, Muntané J. Dual effect of ethanol on cell death in primary culture of human and rat hepatocytes. Alcohol Alcohol. 2004;39:290–6. doi: 10.1093/alcalc/agh065. [DOI] [PubMed] [Google Scholar]
- 85.Navasumrit P, Ward TH, Dodd NJ, O’Connor PJ. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis. 2000;21:93–9. doi: 10.1093/carcin/21.1.93. [DOI] [PubMed] [Google Scholar]
- 86.Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J Gastroenterol. 2003;9:125–8. doi: 10.3748/wjg.v9.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;25:89–97. doi: 10.1016/S0741-8329(01)00176-8. [DOI] [PubMed] [Google Scholar]
- 88.Koch O, Farré S, De Leo ME, Palozza P, Palazzotti B, Borrelo S, Palombini G, Cravero A, Galeotti T. Regulation of manganese superoxide dismutase (MnSOD) in chronic experimental alcoholism: effects of vitamin E-supplemented and –deficient diets. Alcohol Alcohol. 2000;35:159–63. doi: 10.1093/alcalc/35.2.159. [DOI] [PubMed] [Google Scholar]
- 89.Subudhi U, Das K, Paital B, Bhanja S, Chainy GB. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem Biol Interact. 2008;173:105–14. doi: 10.1016/j.cbi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Videla LA. Energy metabolism, thyroid calorigenesis, and oxidative stress: functional and cytotoxic consequences. Redox Rep. 2000;5:265–75. doi: 10.1179/135100000101535807. [DOI] [PubMed] [Google Scholar]
- 91.Messarah M, Boumendjel A, Chouabia A, Klibet F, Abdennour C, Boulakoud MS, Feki AE. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp Toxicol Pathol. 2010;62:301–10. doi: 10.1016/j.etp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 92.Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci. 2006;63:414–34. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das K, Chainy GB. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim Biophys Acta. 2001;1537:1–13. doi: 10.1016/S0925-4439(01)00048-5. [DOI] [PubMed] [Google Scholar]
- 94.Voldstedlund M, Tranum-Jensen J, Handberg A, Vinten J. Quantity of Na/K-ATPase and glucose transporters in the plasma membrane of rat adipocytes is reduced by in vivo triiodothyronine. Eur J Endocrinol. 1995;133:626–34. doi: 10.1530/eje.0.1330626. [DOI] [PubMed] [Google Scholar]
- 95.Odabasoglu F, Cakir A, Suleyman H, Aslan A, Bayir Y, Halici M, Kazaz C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103:59–65. doi: 10.1016/j.jep.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 96.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 97.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–24. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35–8. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:7997–8004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 100.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 101.Donohue TM., Jr. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13:4974–8. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004a;34:39–43. doi: 10.1016/j.alcohol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 103.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004b;287:G1–6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 104.Chen YH, Yang CM, Chang SP, Hu ML. C/EBP beta and C/EBP delta expression is elevated in the early phase of ethanol-induced hepatosteatosis in mice. Acta Pharmacol Sin. 2009;30:1138–43. doi: 10.1038/aps.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133:143–9. doi: 10.1016/j.jss.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 106.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790–7. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 107.Leung TM, Lu Y, Yan W, Morón-Concepción JA, Ward SC, Ge X, Conde de la Rosa L, Nieto N. Argininosuccinate synthase conditions the response to acute and chronic ethanol-induced liver injury in mice. Hepatology. 2012;55:1596–609. doi: 10.1002/hep.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–90. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dubinina EE, Dadali VA. Role of 4-hydroxy-trans-2-nonenal in cell functions. Biochemistry (Mosc) 2010;75:1069–87. doi: 10.1134/S0006297910090014. [DOI] [PubMed] [Google Scholar]
- 110.Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003;24:281–91. doi: 10.1016/S0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 111.Galligan JJ, Smathers RL, Shearn CT, Fritz KS, Backos DS, Jiang H, Franklin CC, Orlicky DJ, Maclean KN, Petersen DR. Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease. J Toxicol. 2012;207594 doi: 10.1155/2012/207594. [DOI] [PMC free article] [PubMed] [Google Scholar]