Abstract
[Purpose]
To determine whether resveratrol improves the adverse effects age on vascular function in mesenteric arteries (MAs), and diminishes the hyperactivity in adrenal gland with age.
[Methods]
Male F344 x Brown Norway rats were assigned to 6-month control (YC), 6-month resveratrol (YR), 24-month control (OC) and 24-month resveratrol (OR). Resveratrol (15 mg/kg) was provided to resveratrol groups in drinking water for 14 days.
[Results]
Concentration response curves to phenylephrine (PE, 10-9-10-5M), acetylcholine (Ach, 10-9-10-5M) and resveratrol (10-8-10-4M) were evaluated in pressurized isolated MAs. The Ach concentration-response curve was right shifted with maximal response diminished in OC compared with YC rats. These effects were reversed by resveratrol treatment. The resveratrol-mediated relaxant responses were unchanged with age or resveratrol suggesting an endothelium-independent mechanism. Resveratrol tended to increase endothelial nitric oxide synthase; caused no effect on copper-zinc superoxide dismutase; and normalized the age-related elevatation in DβH and NPY levels in adrenal medulla, two indicators of sympathetic activity
[Conclusion]
These data indicate that resveratrol reverses age-related dysfunction in endothelium-dependent vasodilation in MAs and partially reverses hyperactivity of adrenomedullary function with age. This treatment may have a therapeuticpotential in the treatment of cardiovascular diseases or hypertension in the elderly.
Keywords: Aging, resveratrol, adrenal, catecholamines, mesenteric artery
INTRODUCTION
Aging is associated with phenotypic and functional changes in vascular structure and functionand there is an age-related increase in the prevalence of hypertension, all of which elevate the risk for cardiovascular disease1-3. There is profound evidence for an increase in intima-media-thickness and vascular stiffness not only during healthy aging but also induced by cardiovascular risk factors4. Endothelial dysfunction which characterizes vascular aging is strictly associated with decreased nitric oxide (NO) bioavailibility, resulting in impaired vasodilatation, increased plaque formation and thrombosis 5-7. Several mechanisms are involved in reduced NO availability in aging. First, endothelial nitric oxide synthase (eNOS) activity and therefore eNOS derived NO production decline with increasing age 8; second, excess reactive oxygen species (ROS) produced by arteries during aging combine with NO to form peroxynitrite, a powerful oxidant, which is increased in the arterial media of aging vessels 9-11.
With aging, there is dysregulation of blood pressure, and the age-related increases in hypertension are associated with an increase in sympathetic nervous activity12-14, and sustained elevation of catecholamine biosynthesizing enzymes in the adrenal medulla and peripheral sympathetic ganglia. Protein levels and enzyme activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis, are elevated in the adrenal medulla of senescent rats compared with younger animals15-18. In addition, protein levels of another key catecholamine synthesizing enzyme, dopamine beta-hydroxylase (DβH) as well as NPY, a peptide, co-localized and co-released with catecholamines in central and peripheral nerves as well as the adrenal medulla, are also upregulated in the adrenal medulla with age19-22. Adrenal medullary levels of TH, DβH, and NPY are key indicators of sympathetic nervous system activity.
Resveratrol (3,5,4-trihydroxy-trans-stilbene) is a polyphenol phytoalexin, found in grape skins and red wine23,24. Accumulating reports have shown that resveratrol can prevent or slow the progression of a variety of diseases, including cancer, ischemic injuries, Alzheimer’s, as well as cardiovascular disease25,26. The mechanism of cardiovascular benefits probably include vasorelaxant, antioxidant, and antiplatelet effects of resveratrol27. In vitro studies have demonstrated that resveratrol has vasodilatory effects when applied to different isolated artery segments at pharmacological concentration. In organ chambers in vitro, resveratrol inhibits the contractile response to noradrenaline and causes relaxation of the phenylephrine-precontracted rat aorta28. The vasorelaxant activity of resveratrol has also been observed in isolated mesenteric and uterine arteries of guinea pigs29, in mesenteric resistance arteries of lean and obese rats30, in porcine coronary arteries31 and human internal mammary arteries32. Furthermore, resveratrol improves endothelial function in vivo. Endothelial dysfunction is an early event in the development of atherosclerosis and is present even before structural changes occur in the vasculature. All major risk factors for atherosclerosis such as hyperlipidemia, diabetes, hypertension, increasing age, and smoking are associated with endothelial dysfunction7,33. It was shown that oral treatment with resveratrol resulted in improvement in endothelial function in hypertensive rats34, diabetic rats and mice35, hypercholesterolemic rabbits36 as well as healthy rats37,38. These studies indicate the potential of resveratrol for health promotion and disease prevention. Therefore, in this study we aimed to evaluate whether resveratrol reverses the adverse effects of age on vascular function in mesenteric arteries (MAs) and adrenal medularry indicators of sympathetic activity. To this end, in young and old rats, we determined the vasorelaxant effects of resveratrol in isolated mesenteric arteries, eNOS and CuZnSOD in aorta, and the effects of resveratrol on TH, DβH, and NPY levels in adrenal medulla.
METHODS
Animal Preparation
Six-and 24-month-old male Fischer 344xBrown Norway rats were obtained from Harlan (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for one week. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals, and all procedures were approved by the local Institutional Animal Care and Use Committee (ACORP # 9811-010/Protocol # 003, dated 16 December 2008). Rats were housed individually with a 12:12 h light:dark cycle (06:00 to 18:00 hr) with water available ad libitum. The rats were randomly divided into 4 groups of 8 animals in each group; young control (YC), young resveratrol (YR), old control (OC) and old resveratrol (OR) rats. Resveratrol (90 mg/L, approximately 15 mg/kg) was given to YR and OR groups in their drinking water (bottles were covered and protected from light) for 14 days ad libitum. The concentration of resveratrol was chosen on the basis of previous in vivo observations in rats, rabbits, and mice in which the biological effects of resveratrol were observed at concentrations ranging from 3-22 mg/kg administered orally25,35,36. Resveratrol was obtained from Sigma (St. Louis, MO, USA) and prepared fresh every day during the experiment. Resveratrol was dissolved in absolute ethanol and diluted with water to a concentraion of 90mg/L. The fresh drinking water (with or without resveratrol) was provided once a day and total water intake was recorded daily. Body weights of the animals were recorded at the beginning and ending of the resveratrol treatment. Experiments were carried out on day 15 following resveratrol or vehicle consumption.
Tissue Harvesting and Preparation
After 2 weeks, rats were over-anesthetized with pentobarbital (120 mg/kg i.p.) and blood was collected through cardiac puncture. The mesenteric artery bed was removed immediately and placed in cold, oxygenated modified Krebs-Ringer bicarbonate solution (in mM: 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.18 KH2PO4, 1.17 MgSO4, 0.026 EDTA, 1.6 CaCl2, and 5.5 glucose). Next, the circulatory system was perfused with 60 ml of cold saline. The aorta and adrenal medula were rapidly dissected, immediately frozen in liquid nitrogen and stored at -80°C until subsequent analysis.
Isolation and Cannulation of MAs
These methods have been described previously in detail39. Briefly, with the aid of a dissecting microscope, a section of the MA (2 mm in length) was transferred to a vessel chamber and mounted and secured between two glass micropipettes with a 10-0 ophthalmic suture. The vessel chamber was transferred to an inverted light microscope stage coupled to a video dimension analyzer and a strip-chart recorder. The video dimension analyzer was connected to both a video monitor (for visualization of the vessel) and a strip-chart recorder for constant recording of the intraluminal diameter of the vessel. Oxygenated 20% O2-5% CO2-75% N2Krebs’ solution maintained at 37°C, was continuously circulated through the vessel bath. In addition, the lumen of the vessel was filled with Krebs solution through the micropipettes and maintained at a constant pressure of 80 mmHg. To facilitate the analysis of dilatory responses, appropriate amounts (10-9- 10-5M) of phenylephrine (PE) were added to constrict the arteries to about 40% of their initial diameter. This provided a wider range for analysis of diameter changes during relaxation and provided a similar level of initial vascular constriction. Experimental protocols were not initiated
Measurement of vascular responses
Concentration-response curves to PE (10-9-10-5M) or to acethylcholine (Ach 10-9-10-5M) and resveratrol (10-8-10-4M) in vessels preconstricted with PE were evaluated in pressurized isolated mesenteric arteries. The relaxant responses to sodium nitroprusside (SNP) (10-5M) were obtained after Ach responses. Relaxant responses were expressed as percentage of the precontraction response to PE.
Western Blot analysis
The aortas were homogenized to examine copper-zinc superoxide dismutase (CuZnSOD) and eNOS, whereas adrenal medullae samples were used for the assesment of tyrosine hydroxlase (TH) and dopamine beta-hydroxylase (DβH) using Western blot analysis. Tissue samples were homogenized in 50 mM Tris (pH 7.0) with leupeptin and protein content was assessed by the DC protein assay (Bio-Rad, Hercules, CA). An equal amount of protein for each sample was separated by polyacrylamide gel electrophoresis via 12.5% gradient polyacrylamide gels containing 0.1% sodium dodecyl sulfate for 1 h at 100 mA. After electrophoresis, the proteins were transferred to nitrocellulose membranes at 90 V for 1.5h. To control for protein loading and transfer differences, membranes were stained with Ponceau S and analyzed. The membranes were washed and subsequently blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h at room temperature and subsequently incubated overnight at 4°C with a primary antibody. This step was followed by incubation at room temperature with a secondary antibody directed against the primary for 1 h. All bound antibodies were detected by chemifluorescence (ECL Plus Western Blotting Detection System; GE Healthcare), scanned (Storm 860 Phosphorimager Scanner; GE Healthcare) and analyzed using Image Quant software.
Primary antibodies
Copper zinc superoxide dismutase (Anti-CuZnSOD, Calbiochem/ EMD Chemicals Inc. Gibbstown, NJ USA. Catalog number 574597); (Anti-eNOS, AbCam Cambridge, MA USA. Catalog number ab5589); TH (Pel Freez Biologicals, Rogers, AR); and DβH (Novus Biologicals, Littleton, CO).
Reverse transcriptase-PCR for NPY in adrenal medulla
NPY mRNA expression was identified in the adrenal medulla by using relative quantitative reverse transcriptase-PCR through the use of Quantum RNA 18s Internal Standards kit (Ambion, Austin, TX). PCR was performed by multiplexing NPY primers (sense 5’-ATGGGGCTGTGTGGACTGACC; antisense, 5’-GTCAGGAGAGCAAGTTTCATTT) and 18S primers. The optimum ratio of 18S primer to competimer was 1:9. PCR was performed at 94°C denaturation for 60 s, 59°C annealing temperature for 60 s and 72°C elongation temperature for 60 s for 23 cycles.
Statistical Analysis
All data are expressed as mean ± SE. Amplitude of vascular relaxations were calculated as a percentage of preconstriction values. Concentration-response curves were evaluated at each concentration for differences between treated and untreated arteries from young and old animal groups. Differences between experimental groups were evaluated using of two-way analysis of variance, followed by Bonferroni’s post hoc test. The criterion for significance was p<0.05.
RESULTS
Animal body weight and resveratrol intake
Mean body weight was significantly higher in the old rats, and the two-week treatment with resveratrol did not alter mean body weight in either age group (Table 1). Resveratrol was administered in the drinking water. Water consumption was greater in the aged compared with the young rats, and thus, the daily dose of resveratrol consumed by the old rats was 33% greater than that consumed by the young. However daily dose of resveratrol adjusted for body weight was nearly identical in the young and old (Table 1).
Table 1. Body weight, water consumption and resveratrol consumption.
| Body Weight (initial,g) |
Body Weight (final, g) |
Water Intake (ml/day) |
Resveratrol Intake (mg/day) |
Resveratrol (mg/day/kg) |
|
|---|---|---|---|---|---|
| Young Control | 356 ± 3.2 | 362 ± 3.1 | 0 | 0 | |
| Young Res | 359 ± 9.4 | 362 ± 3.9 | 60.3 ± 2.6 | 5.42 ± 0.23 | 15.0 ± 0 |
| Old Control | 545 ± 1.6a | 546 ± 1.3 | 0 | 0 | |
| Old Res | 554 ± 1.0a | 552 ± 2.0 | 89.8 ± 2.8* | 8.08 ± 0.25* | 14.6 ± 0 |
Data represent the mean ± SE of 8 rats per group. Resveratrol intake calculated from water consumption times resveratrol concentration in water (90mg/L).
*P<0.0001 for difference with age by t-test.
Vascular reactivity
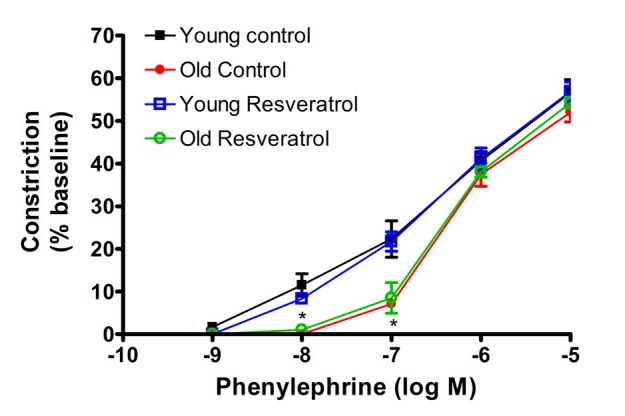
PE contracted the cannulated MAs in a concentration-dependent manner with decreased sensitivity demonstrated in the aged rats. Constriction at 10-8M and 10-7M PE were significantly diminished in aged rats with the receptor activation constant Kact of 2x10-7M and 5x10-7M, respectively for young and old (Figure 1). There was no change in sensitivity with resveratrol treatment, and maximal constriction with PE was similar in all four groups. Young MAs constricted to 40.86 ± 2.56% and 39.13 ± 3.29% of the initial diameter in the control and resveratrol groups, respectively, while old control MAs constricted to 38.50 ± 2.67% and old resveratrol MAs to 38.33 ± 1.33%.
Figure 1. Cumulative dose-response curves to phenylephrine-dependent constriction in mesenteric arteries.
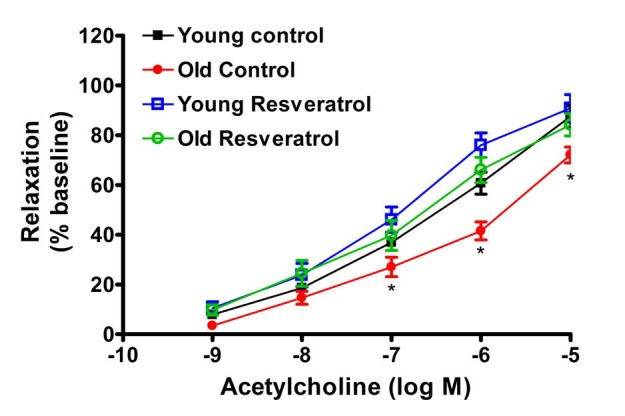
The concentration-response relaxation was determined for Ach and resveratrol. Ach-mediated relaxant responses were significantly diminished in OC rats compared to YC group (p<0.05) with both a decrease in maximal relaxation and a right shift in the concentration response curve (Figure 2). Two-week treatment with resveratrol did not significantly alter relaxation in the young group, however, reservatrol treatment reversed the impaired Ach-mediated relaxant responses in the aged group, such that there were no longer any significent differences with age (Figure 2). Endothelium-independent relaxantation responses using SNP (10-5 M) in YR, OC and OR groups fully relaxed MA and were not significantly different between groups (data not shown).
Figure 2. Cumulative dose-response curves to acetylcholine-dependent relaxation in mesenteric arteries.
In contrast to Ach-mediated relaxation, resveratrol-induced vasodilation was indistinguishable between YC and OC both with respect to sensitivity and effiacy (Figure 3). Again, relaxant responses to SNP (10-5 M) were not significantly different between YC and OC groups.
Figure 3. Cumulative dose-response curves to resveratrol-dependent relaxation.

eNOS and CuZnSOD in the Aorta
Due to insufficient amount of mesenteric arterial tissue, aortas were used for analysis for eNOS and CuZnSOD protein levels. Expression of eNOS in aorta was not significantly different with either age or resveratrol treatment, although there is a trend for an increase with age and with resveratrol (Table 2). Similarly, neither age nor resveratrol had any detectable effect on the expression of CuZnSOD protein (Table 2).
Table 2. eNOS and CuZnSOD in the Aorta.
| Young | Old | |||
|---|---|---|---|---|
| Control | Resveratrol | Control | Resveratrol | |
| eNOS | 100 ± 17 | 130 ± 17 | 136 ± 19 | 162 ± 15 |
| CuZnSOD | 100 ± 9.1 | 105 ± 4.6 | 92.1± 4.7 | 101 ± 5.9 |
Data represent the mean ± SE of 8 rats per group. The value of Control for each age is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in respective Control.
Tyrosine hydroxylase, dopamine beta hydroxylase and NPY in adrenal medul
Tyrosine hydroxylase (TH) and dopamne beta hydroxylase (DβH) protein levels were examined in the adrenal medulla after two weeks of resveratrol treatment. Both TH and DβH protein levels were augmented with age, and for DβH, these elevated levels were restored to young control levels with resveratrol treatment (Figure 4, top and middle, respectively). In addition, mRNA levels of NPY, a peptide co-synthesised and released with cathecolamines from the adrenal medulla, were elevated with age and resveratrol treatment in the old rats decreased NPY expression. Interestingly, resveratrol adminiatration increased NPY expression in the young, such that there was no longer a age related difference between the young and old resveratrol treated rats (Figure 4, bottom).
Figure 4. Protein levels of TH (top) and DβH (middle) and mRNA levels of NPY (Bottom) in young and old rats with and without resveratrol treatment.
DISCUSSION
This study demonstrates that two-week resveratrol treatment reverses impaired endothelium-dependent Ach-mediated relaxant responses in aged rats. In contrast, resveratrol-induced concentration-dependent vasorelaxation was not different between young and aged rats, suggesting an endothelium independent mechanism. Although SOD expression in aorta was not changed either by aging or twoweek resveratrol treatment, eNOS protein levels in aorta tended towards an increase with resveratrol treatment. Finally, our results demonstrated for the first time that resveratrol administration partially normalized adrenal medullary function with age as related to DβH and NPY content.
With respect to vascular activity, the PE-mediated concentration-dependent constriction was right-shifted with age, however, the maximum constriction was similar in young and aged rats. Subsequent Ach-mediated vasodilation demonstrated both a rightward shift in the relaxation-dependent concentration curve and a decrease in maximum relaxation at the Ach concentration of 10-5M. Most interestingly, the short two-week treatment with resveratrol reversed these age-related impairments in Ach-mediated relaxation. In constast, to the diminished vasodilatory responses with age to Ach, resveratrol-mediated concentration-dependent relaxation in both young and old MAs were similar. These data suggest that the resveratrol-mediated relaxation is an endothelium independent mechanism. Previous studies have indicated that resveratrol-induced vasorelaxation may be either endothelium-dependent or endothelium-independent26,29,30,40. The endothelium-dependent vasorelaxation is largely attributable to NO, whereas the endothelium-independent relaxation is likely to be mediated by ion channels including voltage-gated K+ channels, big Ca++-activated K+ channels, 4-AP and margatoxin-sensitive K+ channels or voltage-gated Ca++ channels 41-43. Resveratrol might also become incorporated into the smooth muscle membrane, where it could either couple with a membrane receptor31 or interact directly with membrane calcium channels44, thus inducing endothelium-independent vasorelaxation30. It is well known that polyphenols inhibit cyclic nucleotide phosphodiesterases, which hydrolyse the vasorelaxants cAMP and cGMP45. It is possible that such a mechanism might be involved in the endothelium-independent relaxation induced by resveratrol. Furthermore, the mechanism of resveratrol-induced vasorelaxations may depend on the concentration of resveratrol. Chen and coworkers28 concluded that resveratrol exerts both indirect and direct vasodilatory effects on the blood vessels by NO-mediated and non-NO-mediated mechanisms in rat aortic rings. These authors reported that the former effect is apparent at low resveratrol concentrations (10-30 μM/L) and is blocked by inhibitors of NOS activity, whereas endothelium-independent effects appear at high resveratrol concentrations (>60 μM/L) and are not blocked by endothelial denudation or NOS inhibitors28. On the other hand, Naderali and coworkers30 found that L-NAME attenuated the responses to resveratrol of arteries from lean animals, but notdietary-obese animals with impaired endothelial function, suggesting resveratrol relaxaton is mediated via NO in lean rodents.
In the present study, resveratol appears to be involved in both endothelium-dependent and endothelium-independent functions. The resveratrol-mediated direct vasorelaxation appears to be an endothelium independent, but resveratol treatment, in vivo, improves Ach-mediated endothelium dependent relaxation. Moreover, resveratrol treatment had no effect on the endothelium-independent relaxation induced by SNP. Aged rats5,6, similar to dietary-obese rats, have impaired endothelial function, as evidenced by the diminished vasorelaxation to Ach. Resveratrol treatment tended to stimulate the synthesis or availability of NO in MAs, and this may underlie the restoration of Ach-medated relaxation in the aged rats with resveratrol treatment.
With regards to eNOS expression in aging, conflicting data have been reported, showing reduced9, unchanged46 or increased11,47 eNOS protein levels during aging. Therefore, it is possible that changes in eNOS expression and activity are responsible either for preserved NO availability, or for improved vasorelaxation in aged animals with resveratrol treatment. Endothelial cell culture studies demonstrate an upregulation of eNOS expression and activity as a result of acute or short-term resveratrol exposure48-50. Animal studies indicated that long-term treatment of resveratrol augmented basal release of NO and nitrite/nitrate levels in aorta, and potentiated endothelial function in healthy animals37,38. Another study reported that administration of resveratrol to rabbits with high-cholesterol improved flow-mediated vasodilation and increased plasma NO level36. Similar to the present study, Rush and coworkers34 reported that resveratrol treatment improved the maximal Ach-induced, endothelium-dependent, NO-mediated relaxation of aorta in spontaneous hypertensive rats34. Moreover, this occured in the absence of significant changes in aorta eNOS protein levels34. In the present study, the restoration of the Ach-mediated vasorelaxation in aged rats is consistent with resveratrol-induced elevation of eNOS synthesis or NO availability, however, the observed increase in eNOS was minor and without significance. It is possible that there is increased NO availablity or that other mechanisms may be involved.
Possible mechanisms include NO-guanylyl cyclase interactions or subsequent cGMP-dependent mechanism in the MAs smooth muscle cells or changes in oxidative stree or inflamation. Oxidative stress is attributable to excessive production of reactive oxygen species (ROS) and is known to be the key factor in the pathogenesis of age-related vascular dysfunction5,10.The inactivation of NO by ROS is recognized to be a crucial factor in reducing NO bioavailability and the development of endothelial dysfunction51. Furthermore, resveratrol has been shown to reduce superoxide-mediated NO breakdown, and thus enhance NO bioavailability leading to improved endothelial function35,38,52. Although levels of CuZnSOD were not significantly different with age in the present study, these findings do not eliminate the possibility that resveratrol treatment might change the activities of other antioxidant processes.
In addition, inflammation is beleived to play an essential role in the etiology of vascular aging. The pro-inflammatory nuclear transcription factor NF-κB(nuclear factor κB), pro-inflammatory cytokines IL-6 (interleukin-6), TNF-α (tumor necrosis factor-α) and MCP-1 (monocyte chemoattractant protein-1) are increased in older adults5,7,53. Resveratrol restored endothelial function in type 2 diabetes by inhibiting TNFα-induced activation of NAD(P)H oxidase and preserving eNOS phosphorilation35. In a human coronary arterial endothelial cell study, resveratrol inhibited TNF-α-induced NF-κB activation and inflammatory gene expression54. Althoug inflammatory parameters were not examined in the present study, it may speculated that antiinflammatory actions of resveratrol participates in the restroration of age-related impared Ach-mediated vasorelaxation.
The increase in adrenomedullary catecholamine biosynthesis may play an important role in the age-related increase in sympathetic nervous activity and is likely a significant factor in the development of hypertension and cardiovascular diseases with age. Catecholamines are formed from their amino acid precursor tyrosine in the brain, chromaffin cells of the adrenal medulla, and sympathetic nerves. TH catalyzes the hydroxylation of tyrosine, producing dopamine, whereas DβH catalyzes the conversion of catecholamines from the adrenal medulla. We and others have shown that the levels of catecholamine biosynthetic enzymes15-18,55 and NPY increase with age in the adrenal medulla19,20,22,56. In present study, resveratrol treatment normalized the levels of DβH and NPY in the adrenal medulla of aged rats. Notably, the levels of TH were not changed by resveratrol administration. TH, DβH and NPY are reliable biomarkers of sympathetic nervous system activity, thus resveratrol may partially normalize elevated sympathetic activity with age. Because elevated plasma catecholamine levels may be contributing to the increased prevalence of hypertension in the elderly, this previously unrecognized therapeutic benefit of resveratrol is especially important in the elderly where the higher prevalence of hypertension is associated with elevated morbidity and mortality1,2.
CONCLUSIONS
This study indicates that age-related dysfunction inendothelium-dependent vasodilation in MAs can bereversed with resveratrol treatment. The two-week treatment with resveratrol tends to increase eNOS level in aorta from aged rats, and these changes are associated with improvement of age-associated endothelial function. Direct vasorelaxation by resveratrol is unchanged across age, suggesting that resveratrol-mediated relaxation is endothelial independent. Resveratrol partially reversed the hyperactivity of adrenomedullary function with age by reducing the elevated adrenomedullary DβH and NPY levels. These resveratrol-mediated responses may have a therapeuticpotential in the treatment of cardiovascular diseases and hypertension, especially in the elderly.
CONFICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs (Grant number F47701 (VA RR&D) and the National Institute of Aging grant T32 AG000196 and presented in part at Experimental Biology 2011, Washington, DC, 9-13 April, 2011 and 65th Annual Scientific Meeting of The Gerontological Society of America, San Diego, 14-18 November, 2012.
References
- 1.Anon. Hypertension. Vol. 23. National High Blood Pressure Education Program Working Group; 1994. National High Blood Pressure Education Program Working Group Report on Hypertension in the Elderly; pp. 275–285. [DOI] [PubMed] [Google Scholar]
- 2.Tuck ML. et al. Armbrecht JA., Coe R., Wongsurawat N. Endocrine function and aging. New York: Springer-Verlag: 1989,. Treatment of hypertension in the elderly; pp. 147–160. [Google Scholar]
- 3.Virmani R., Avolio AP., Mergner WJ., Robinowitz M., Herderick EE., Cornhill JF., Guo SY., Liu TH., Ou DY., O’Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis: comparision between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatta EG., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A., Pacher P., Kaley G., Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egashira K., Inou T., Hirooka Y., Kai H., Sugimachi M., Suzuki S., Kuga T., Urabe Y., Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.CIR.88.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Seals DR., Jablonski KL., Donato AJ. . Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou TC., Yen MH., Li CY., Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.HYP.31.2.643. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A., Ungvari Z., Edwards JG., Kaminski P., Wolin MS., Koller A., Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M., Sanchez M., Minoves N., Salaices M., Balfagón G. Aging increases neuronal nitric oxide release and superoxide anion generation in mesenteric arteries from spontaneously hypertensive rats. J Vasc Res. 2003;40:509–519. doi: 10.1159/000075183. [DOI] [PubMed] [Google Scholar]
- 11.Van der Loo B., Labugger R., Skepper JN., Bachschmid M., Kilo J., Powell JM., Palacios-Callender M., Erusalimsky JD., Quaschning T., Malinski T., Gygi D., Ullrich V., Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esler MD., Turner AG., Kaye DM., Thompson JM., Kingwell BA., Morris M., Lambert GW., Jennings GL., Cox HS., Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 13.Esler M., Hastings J., Lambert G., Kaye D., Jennings G., Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–R916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- 14.Seals DR., Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kedzierski W., Porter JC. Quantitative study of tyrosine hydroxylase mRNA in catecholaminergic neurons and adrenals during development and aging. Brain Res Mol Brain Res. 1990;7:45–51. doi: 10.1016/0169-328X(90)90072-L. [DOI] [PubMed] [Google Scholar]
- 16.Tumer N., Hale C., Lawler J., Strong R. Modulation of tyrosine hydroxylase gene expression in the rat adrenal gland by exercise: effects of age. Brain Res Mol Brain Res. 1992;14:51–56. doi: 10.1016/0169-328X(92)90009-Z. [DOI] [PubMed] [Google Scholar]
- 17.Tumer N., LaRochelle JS. Tyrosine hydroxylase expression in rat adrenal medulla: influence of age and cold. Pharmacol Biochem Behav. 1995;51:775–780. doi: 10.1016/0091-3057(95)00030-Z. [DOI] [PubMed] [Google Scholar]
- 18.Voogt JL., Arbogast LA., Quadri SK., Andrews G. Tyrosine hydroxylase messenger RNA in the hypothalamus, substantia nigra and adrenal medulla of old female rats. Brain Res Mol Brain Res. 1990;8:55–62. doi: 10.1016/0169-328X(90)90009-3. [DOI] [PubMed] [Google Scholar]
- 19.Erdem SR., Broxson CS., Erdem A., Spar DS., Williams RT., Tümer N. The age-related discrepancy in the effect of neuropeptide Y on select catecholamine biosynthetic enzymes in the adrenal medulla and hypothalamus in rats. Neuropharmacology. 2002;43:1280–1288. doi: 10.1016/S0028-3908(02)00293-9. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi H., Yokokawa K., Iwasa A., Yoshida H., Miki N. Age-dependent increase in neuropeptide Y gene expression in rat adrenal gland and specific brain areas. J Neurochem. 1991;57:1840–1847. doi: 10.1111/j.1471-4159.1991.tb06393.x. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg JM., Terenius L., Hökfelt T., Martling CR., Tatemoto K., Mutt V., Polak J., Bloom S., Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- 22.Tumer N., Broxson CS., LaRochelle JS., Scarpace PJ. Induction of tyrosine hydroxylase and neuropeptide Y by carbachol: modulation with age. J Gerontol A Biol Sci Med Sci. 1999;54:B418–B423. doi: 10.1093/gerona/54.10.B418. [DOI] [PubMed] [Google Scholar]
- 23.Jang M., Cai L., Udeani GO., Slowing KV., Thomas CF., Beecher CW., Fong HH., Farnsworth NR., Kinghorn AD., Mehta RG., Moon RC., Pezzuto JM. Cancer chemopreventative activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 24.Vinson JA. Flavonoids in foods as in vitro and in vivo antioxidants. Adv Exp Med Biol. 1998;439:151–164. doi: 10.1007/978-1-4615-5335-9_11. [DOI] [PubMed] [Google Scholar]
- 25.Baur JA., Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 26.Bradamante S., Barenghi L., Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu JM., Wang ZR., Hsieh TC., Bruder JL., Zou JG., Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine. Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Chen CK., Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 29.Naderali EK., Doyle PJ., Williams G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin Sci. 2000;98:537–543. doi: 10.1042/cs0980537. [DOI] [PubMed] [Google Scholar]
- 30.Naderali EK., Smith LH., Doyle JP., Williams G. The mechanism of resveratrol-induced vasorelaxation differs in the mesenteric resistance arteries of lean and obese rats. Clin Sci. 2001;100:55–60. doi: 10.1042/cs1000055. [DOI] [PubMed] [Google Scholar]
- 31.Jager U. Nguyen-Duong H. Relaxant effect of trans resveratrol on isolated porcine coronary arteries. Arzneimittelforschung. 1999;49:207–211. doi: 10.1055/s-0031-1300403. [DOI] [PubMed] [Google Scholar]
- 32.Rakici O., Ugursay K., Coskun B., Aslamaci S., Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J of Cardiol. 2005;105:209–215. doi: 10.1016/j.ijcard.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Bonetti PO., Lerman LO., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 34.Rush JW., Quadrilatero J., Levy AS., Ford RJ. Chronic resveratrol enhances endothelium dependent relaxation but does not alter eNOS level in aorta of spontaneously hypertensive rats. Exp Biol Med. 2007;232:814–822. [PubMed] [Google Scholar]
- 35.Zhang H., Zhang J., Ungvari Z., Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou JG., Wang ZR., Huang YZ., Cao KJ., Wu JM. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med. 2003;11:317–320. doi: 10.3892/ijmm.11.3.317. [DOI] [PubMed] [Google Scholar]
- 37.Soylemez S., Gurdal H., Sepici A., Akar F. The effect of long-term resveratrol treatment to estrogen in aortae from male and female rats: Role of nitric oxide and superoxide. Vascular Pharmacol. 2008;49:97–105. doi: 10.1016/j.vph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Soylemez S., Sepici A., Akar F. Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats. Cardiovasc Drugs Ther. 2009;23:449–458. doi: 10.1007/s10557-009-6198-z. [DOI] [PubMed] [Google Scholar]
- 39.Erdos B., Miller AW., Busija DW. Impaired endothelium-mediated relaxation in isolated cerebral arteries from insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;282:H2060–H2065. doi: 10.1152/ajpheart.01124.2001. [DOI] [PubMed] [Google Scholar]
- 40.Novakovic A., Gojkovic-Bukarica L., Peric M., Nezic D., Djukanovic B., Markovic-Lipkovski J., Heinle H. The mechanism of edothelium-idependent relaxation iduced by the wine polyphenol resveratrol in human internal mammary artery. J Pharmacol Sci. 2006;101:85–90. doi: 10.1254/jphs.FP0050863. [DOI] [PubMed] [Google Scholar]
- 41.Gojkovic-Bukarica L., Novakovic A., Kanjuh V., Bumbasirevic M., Lesic A., Heinle H. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J Pharmacol Sci. 2008;108:124–130. doi: 10.1254/jphs.08128FP. [DOI] [PubMed] [Google Scholar]
- 42.Nagaoka T., Hein TW., Yoshida A., Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: Role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007;48:4232–4239. doi: 10.1167/iovs.07-0094. [DOI] [PubMed] [Google Scholar]
- 43.Novakovic A., Gojkovic-Bukarica L., Kanjuh V., Heinle H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol and Toxicol. 2006;99:360–364. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- 44.Andriambeloson E., Stoclet JC., Andriantsitohaina R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J Cardiovasc Pharmacol. 1999;33:248–254. doi: 10.1097/00005344-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Beretz A., Stierle A., Anton R., Cazenave JP. Role of cyclic AMP in the inhibition of human platelet aggregation by quercetin, a flavonoid that potentiates the effects of prostacyclin. Biochem Pharmacol. 1982;31:3597–3600. doi: 10.1016/0006-2952(82)90581-0. [DOI] [PubMed] [Google Scholar]
- 46.Smith AR., Visioli F., Hagen TM. Plasma membrane-associated endothelial nitric oxide synthase and activity in aging rat aortic vascular endothelia markedly decline with age. Arch Biochem Biophys. 2006;454:100–105. doi: 10.1016/j.abb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Van der Loo B., Bachschmid M., Labugger R., Schildknecht S., Kilo J., Hahn R., Palacios-Callender M., Lüscher TF. Expression and activity patterns of nitric oxide synthases and antioxidant enzymes reveal a substantial heterogeneity between cardiac and vascular aging in the rat. Biogerontology. 2005;6:325–334. doi: 10.1007/s10522-005-4807-1. [DOI] [PubMed] [Google Scholar]
- 48.Bruder JL., Hsieh TC., Lerea KM., Olson SC., Wu JM. Induced cytoskeletal changes in bovine pulmonary artery endothelial cells by resveratrol and the accompanying modified responses to arterial shear stress. BMC Cell Biol. 2001;2:1–11. doi: 10.1186/1471-2121-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh TC., Juan G., Darzynkiewicz Z., Wu JM. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. 1999;59:2596–2601. [PubMed] [Google Scholar]
- 50.Wallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.CIR.0000029925.18593.5C. [DOI] [PubMed] [Google Scholar]
- 51.Muller-Delp JM., Gurovich AN., Christou DD., Leeuwenburgh C. Redox balance in the aging microcirculation: New friends, new foes, and new clinical directions. Microcirculation. 2011;19:19–28. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H., Förstermann U. Resveratrol: A multifunctional compund improving endothelial function. Cardiovasc Drugs Ther. 2009;23:425–429. doi: 10.1007/s10557-009-6209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungvari Z., Orosz Z., Labinskyy N., Rivera A., Xiangmin Z., Smith K., Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 54.Csiszar A., Smith K., Labinskyy N., Orosz Z., Rivera A., Ungvari Z. Resveratrol attenuates TNF-α induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 55.Banerji TK., Parkening TA., Collins TJ. Adrenomedullary catecholaminergic activity increases with age in male laboratory rodents. J Gerontol. 1984;39:264–268. doi: 10.1093/geronj/39.3.264. [DOI] [PubMed] [Google Scholar]
- 56.Erdos B., Erdem SR., Erdem A., Broxson CS., Tümer N. Effect of age on angiotensin II-mediated downregulation of adrenomedullary catecholamine biosynthetic enzymes. Exp Gerontol. 2008;43:806–809. doi: 10.1016/j.exger.2008.04.012. [DOI] [PubMed] [Google Scholar]