Abstract
Toll-like receptors (TLRs) and the downstream adaptor molecule myeloid differentiation factor 88 (MyD88) play an essential role in the innate immune responses. Here, we demonstrate that genetic deficiency of TLR4 or MyD88 is associated with a significant reduction of aortic plaque areas in atherosclerosis-prone apolipoprotein E-deficient mice, despite persistent hypercholesterolemia, implying an important role for the innate immune system in atherogenesis. Apolipoprotein E-deficient mice that also lacked TLR4 or MyD88 demonstrated reduced aortic atherosclerosis that was associated with reductions in circulating levels of proinflammatory cytokines IL-12 or monocyte chemoattractant protein 1, plaque lipid content, numbers of macrophage, and cyclooxygenase 2 immunoreactivity in their plaques. Endothelial-leukocyte adhesion in response to minimally modified low-density lipoprotein was reduced in aortic endothelial cells derived from MyD88-deficient mice. Taken together, our results suggest an important role for TLR4 and MyD88 signaling in atherosclerosis in a hypercholesterolemic mouse model, providing a pathophysiologic link between innate immunity, inflammation, and atherogenesis.
Atherosclerosis is now recognized to be a chronic inflammatory disease characterized by subendothelial accumulation of atherogenic lipoproteins, extracellular matrix, neovessels, calcium, and inflammatory cells. Activation of inflammatory genes in the vessel wall with subsequent adhesion, chemoattraction, subendothelial migration, retention, and activation of inflammatory and immune cells such as monocytes and T cells are believed to play a critical role in the initiation, progression, and destabilization of atherosclerosis (1–6). Increasing interest has focused on the potential role of infectious agents and components of the innate immune system as contributors to atherosclerosis (7), but clinical trials investigating treatment of cardiovascular diseases with antibiotics have produced conflicting results (8–10), perhaps because of inadequate power to detect differences or other shortcomings (11).
Notwithstanding clinical disappointments, experimental evidence has accumulated that suggest that with enhanced understanding, treatment of cardiovascular disease with antibiotics and/or immunization may one day become feasible (12–14). In particular, intriguing data from animal studies suggest the potential importance of Toll-like receptors (TLRs) and other key components of the innate immune system in atherosclerosis-based pathologies (15). TLRs are a family of receptors that activate proinflammatory signaling pathways in response to microbial pathogens or pathogen-associated molecular patterns. Ligand binding to TLRs results in the recruitment of the adaptor molecule myeloid differentiation factor 88 (MyD88) to the Toll/IL-1 receptor domain of the receptor. Intracellular propagation of the signal leads to activation of the transcription factor NF-κB, thereby influencing inflammatory responses (16).
We and others (17, 18) have shown that human and murine lipid-rich atherosclerotic plaques express TLR4, and that TLR4 expression in macrophages is up-regulated by oxidized but not native low-density lipoprotein (LDL). In vitro studies have demonstrated that minimally modified LDL (MM-LDL), a proinflammatory and proatherogenic lipoprotein, is recognized by TLR4 and the coreceptor CD14 on macrophages, and binding of this lipoprotein leads to actin polymerization and spreading of macrophages (19). In human endothelial cells (ECs), the recognition of MM-LDL by TLR4 results in the secretion of IL-8, a chemokine important in monocyte transmigration and retention in the vessel wall (20). However, this effect of MM-LDL was CD14-independent. Patients expressing a polymorphism in the TLR4 gene manifest lipopolysaccharide (LPS) hyporesponsiveness, are protected from carotid artery atherosclerosis and acute coronary events (21–24), and derive greater benefit from risk reduction with statins (25). Collectively, these findings suggest that TLR signaling may play a role in the development of atherosclerotic plaques.
By using a genetic approach, we show here that atherosclerosisprone hypercholesterolemic mice Apoe–/– that also harbor a null mutation in either the adaptor molecule MyD88 or its upstream receptor TLR4 exhibit reduced aortic atherosclerosis, plaque lipid content, plaque macrophage infiltration, and cyclooxygenase (COX)-2 immunoreactivity without significantly altering circulating cholesterol levels or lipoprotein profiles. We further provide evidence suggesting that MyD88 deficiency leads to decreased levels of the circulating proinflammatory molecules IL-12p40 and/or monocyte chemoattractant protein 1 (MCP-1) and impaired endothelial–leukocyte adhesion in response to MM-LDL, which may contribute to the atheroprotective effects of MyD88 deficiency. These results implicate TLR4- and MyD88-mediated signaling as a pivotal link between hypercholesterolemia, inflammation, and atherosclerosis, and may provide a foundation for the development of innovative therapeutic targets for prevention and therapy of atherosclerosis.
Materials and Methods
Generation of Apoe/MyD88 and Apoe/TLR4 Double Knockout Mice. Apoe-deficient C57BL/6 mice were obtained from The Jackson Laboratory. MyD88–/– and TLR4–/– mice were kindly provided by S. Akira (Osaka University, Osaka). Both strains were backcrossed for four generations into the C57BL/6 strain. MyD88–/– and TLR4–/– mice were crossed with Apoe–/– C57BL/6 mice. Heterozygous mice were intercrossed to generate homozygous Apoe–/– mice bearing combinations of MyD88+/+, +/–, –/–, and TLR4+/+ and –/– mice. This process results in a total backcross of five generations onto the C57BL/6 background. Male and female mice were fed a high (0.15%)-cholesterol diet (Harlan Teklad, Madison, WI) for 6 months. All experiments were performed according to Cedars–Sinai Medical Center Institutional Animal Care and Use Committee guidelines.
Assessment of Atherosclerosis in Aortas and Aortic Sinus. Aortas were excised, adherent (adventitial) fat was removed, and were then fixed in HistoCHOICE (Amrecso, Solon, OH). Whole aortas were opened longitudinally from the aortic arch to the iliac bifurcation, mounted en face, and stained for lipids with Oil red O. Hearts were embedded in OCT compound (Tissue Tek, Sakura, Torrance, CA) and cross sections of the aortic sinus were stained with Oil red O. Lesion areas were quantified with imagepro plus (Media Cybernetics, Silver Spring, MD). Image analysis was performed by a trained observer blinded to the genotype of the mice. To assess macrophage infiltration into atherosclerotic plaques, frozen sections of aortic sinuses were fixed, incubated with 3% hydrogen peroxide, and permeabilized. The sections were incubated with MOMA-2, a macrophage-specific antibody, or control IgG antibody (Serotec). For visualization, 3-amino-9-ethyl-carbazole chromogen (DAKO) was used as a substrate (12). MOMA-2 macrophage immunopositive areas were quantified with imagepro plus.
COX-2 Staining. Sections were blocked in 0.1 M Tris·HCl/0.15 M NaCl/0.5% blocking reagent (TNB blocking buffer) and incubated with primary COX-2 or control IgG antibody (Santa Cruz Biotechnology) diluted in TNB blocking buffer at 4°C overnight (1:5,000 dilution), followed by incubation with streptavidin-horseradish peroxidase complex. The signal was enhanced by using the tyramide signal amplification kit (NEN Life Science Products, Boston) according to the manufacturer's recommendations and sections were counterstained for nuclei with 100 nM SYTOX green (Molecular Probes).
Lipid Profiles. Sera from mice were obtained at the time of killing, after an overnight fast. Total cholesterol concentrations were determined in duplicate by using a colorimetric assay (infinity cholesterol reagent, Sigma Diagnostics, St. Louis). Triglyceride concentrations were determined by using the L-type triglyceride H assay according to the manufacture's instructions (Wako Chemicals USA, Richmond, VA). Lipoprotein profile was determined by using HPLC.
Functional Studies in Isolated Murine Aortic ECs. Thoracic aortas from MyD88–/– and MyD88+/+ mice were cleaned and cut into 3-mm-long rings. These segments were turned inside-out and placed in tissue culture dishes coated with collagen type I (BD Biosciences, Bedford, MA) and incubated in MCDB131 (Invitrogen) supplemented with 20% FBS (Omega Scientific, Tarzana, CA), 1% antibiotic/antimycotic solution (Invitrogen), 1 unit/ml heparin (Sigma), and 60 μg/ml EC growth supplement (Upstate, Charlottesville, VA). The vessel rings were removed once cell outgrowth was observed. The identity of cells was confirmed by staining with von Willebrand factor (DAKO) and by 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-acetylated LDL (Molecular Probes) uptake experiments (purity of ECs > 95%). For adhesion assays ECs were grown to confluency. The cells were stimulated with MM-LDL, native LDL, LPS, or tumor necrosis factor (TNF)-α in culture medium containing 1% lipoprotein-deficient serum for 6 h. After incubation, cells were washed with medium without serum and incubated with human peripheral blood mononuclear cells labeled with 5 μM 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (Molecular Probes) for 1 h. Cells were washed and lysed with 0.5 N NaOH. Fluorescence was measured in a fluorescence microplate reader (excitation, 485 nm; emmission, 528 nm).
Serum Levels of Cytokines and Chemokines. IL-10, IL-12p40, MCP-1, and chemokine KC concentrations in the sera of mice were measured by ELISAs according to the manufacturer's instructions (BD Biosciences for IL-10, IL-12p40, and MCP-1 and R & D Systems for KC).
Statistical Analysis. Data are expressed as mean ± SD. Statistical significance was determined by one-factor ANOVA with Bonferroni correction and Student's t test. P < 0.05 was considered to be statistically significant.
Results
MyD88 Deficiency Does Not Alter Circulating Cholesterol or Lipoprotein Profiles in Apoe–/– Mice. To study the role of MyD88 on the development of atherosclerosis, we generated double knockout mice by intercrossing Apoe–/– and MyD88–/– mice, backcrossed onto the C57BL/6 strain for at least five generations to enhance congenicity and reduce secondary sources of variance. Apoe–/–/MyD88–/– and littermate controls (Apoe–/–/MyD88+/– and Apoe–/–/MyD88+/+) were fed a high-cholesterol diet and were killed at 6 months. To evaluate the effect of MyD88 on plasma lipid concentrations, we measured total serum cholesterol concentrations. No significant differences in total cholesterol or plasma triglyceride concentrations were found between the genotypes at 24 weeks of age (Table 1, which is published as supporting information on the PNAS web site). There was no significant effect of MyD88 deficiency on lipoprotein profiles (data not shown).
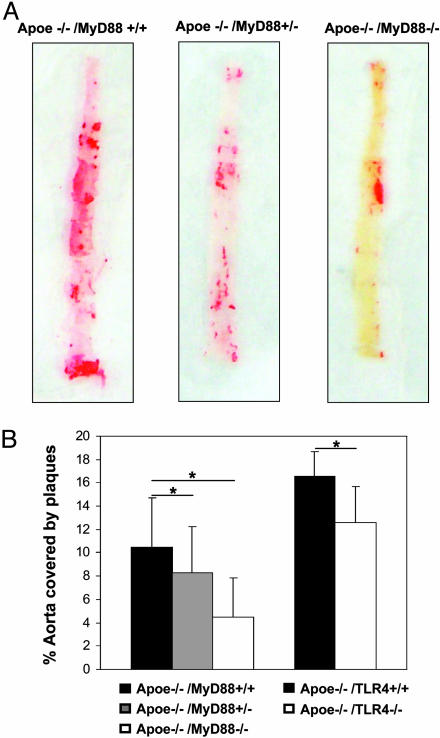
MyD88 Deficiency Reduces the Extent of Aortic Atherosclerosis in Apoe–/– Mice. Apoe–/– mice fed an atherogenic diet develop atherosclerotic lesions with morphological characteristics similar to those seen in humans (26). To evaluate the effect of MyD88 deficiency on the extent of aortic atherosclerosis, we measured the total lesion area by using en face preparation of the aorta and Oil red O staining for lipids (Fig. 1A). Computer-assisted quantitative histomorphometric analysis revealed a 57% reduction in the extent of atherosclerosis in Apoe–/–/MyD88–/– mice. The extent of atherosclerosis in the Apoe–/–/MyD88+/– mice was intermediate between that observed in Apoe–/–/MyD88+/+ and Apoe–/–/MyD88–/– mice, which is consistent with a gene dose–response effect (Fig. 1B Left). The atheroprotective effect of MyD88 deficiency was observed in male as well as in female mice.
Fig. 1.
MyD88 and TLR4 deficiency reduces the extent of aortic atherosclerosis. (A) Aortas of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice fed with a high-cholesterol diet for 6 months were isolated and stained for lipid deposition with Oil red O. Representative specimens from the three groups are shown. (B) Quantification of plaque areas in whole aortas in Apoe–/–/MyD88–/– (Left) and Apoe–/–/TLR4–/– (Right) mice. Aortas of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, Apoe–/–/MyD88+/+, Apoe–/–/TLR4–/–, and Apoe–/–/TLR4+/+ mice stained for lipid deposition with Oil red O. Means and SD (n = 13 for Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, Apoe–/–/MyD88+/+; n = 8 for Apoe–/–/TLR4+/+; and n = 12 for Apoe–/–/TLR4–/–) of plaque areas are shown. Total plaque area in MyD88–/– or TLR4–/– was significantly reduced compared with corresponding wild-type mice (*, P < 0.01).
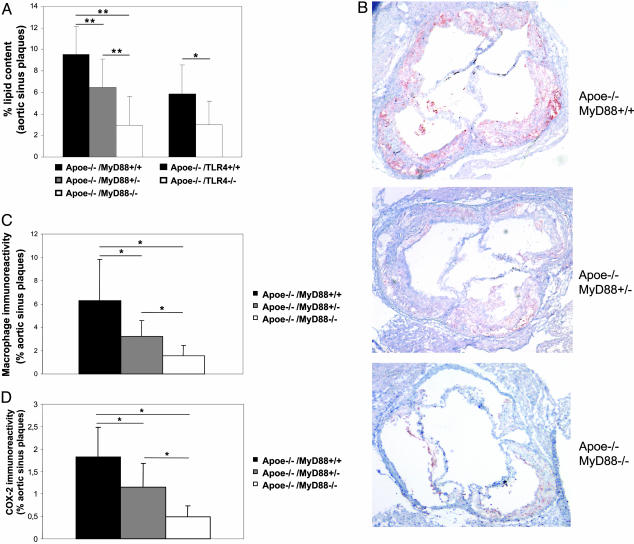
MyD88 Deficiency Is Associated with Reduction in Plaque Lipid Content, Macrophage Infiltration, and COX-2 Immunoreactivity. To examine the effect of MyD88 deficiency on the composition of atherosclerotic plaques, we examined lipid content with Oil red O histochemical staining and the extent of macrophage infiltration with MOMA-2 immunostaining in aortic sinus plaques of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice. Aortic sinus plaques of Apoe–/–/MyD88–/– mice exhibited significantly less lipid and macrophage accumulation than plaques of Apoe–/–/MyD88+/+ mice (69% and 75% reduction, respectively). Intermediate levels were observed in Apoe–/–/MyD88+/– mice (Fig. 2 A Left, and B and C), which was again consistent with a gene dose–response relationship. Quantification of lesion size of aortic sinus plaques revealed a significant reduction of lesion size in Apoe–/–/MyD88–/– compared with Apoe–/–/MyD88+/+ mice (P < 0.05, data not shown). To determine whether reduction in the extent of atherosclerosis produced by MyD88 deficiency is accompanied by decreased expression of the proinflammatory enzyme COX-2, we assessed COX-2 immunostaining in the aortic sinus plaques of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice. Lesions from Apoe–/–/MyD88+/+ mice showed extensive COX-2 immunostaining, which was markedly less in Apoe–/–/MyD88–/– mice (Fig. 2D, and Fig. 6, which is published as supporting information on the PNAS web site; 73% reduction). Intermediate levels of COX-2 immunoreactivity were observed in Apoe–/–/MyD88+/– mice (Figs. 2D and 6).
Fig. 2.
Lipid content, macrophage infiltration, and COX-2 expression in aortic sinus plaques is reduced in Apoe–/–/MyD88–/– mice. (A) Quantification of the lipid content in aortic plaques from Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ (Left) and Apoe–/–/TLR4–/– and Apoe–/–/TLR4+/+ (Right) mice. Shown are means and SD of the percentage of lipid content relative to total plaque areas (n = 10 for Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+; n = 7 for Apoe–/–/TLR4+/+; and n = 8 for Apoe–/–/TLR4–/–). Relative lipid content in Apoe–/–/MyD88–/– or Apoe–/–/TLR4–/– is significantly reduced compared with corresponding wild-type mice (**, P < 0.01; *, P < 0.05). (B) Representative MOMA-2 staining of aortic sinus plaques from Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice. (C) Quantitative analysis of macrophage immunoreactivity in aortic sinus plaques of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice, expressed as a proportion of the total plaque areas (n = 10 in each group). Means and SD are shown (*, P < 0.01). (D) Quantitative analysis of COX-2 immunofluorescent staining in sclerotic plaques of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice (n = 7 in each group). Means and SD are shown (*, P < 0.01).
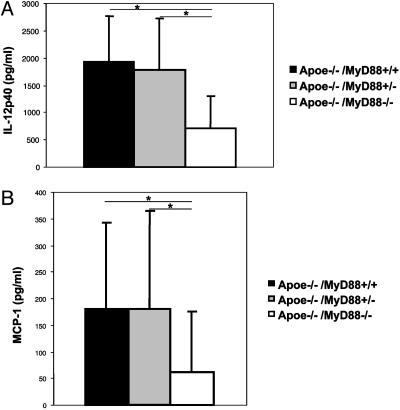
MyD88 Deficiency Is Associated with Reduced Circulating Levels of the Inflammatory Cytokine IL-12 and MCP-1. Although the results above indicate that MyD88 expression has direct effects on the arterial wall, it is possible that MyD88 deficiency may also secondarily inhibit atherogenesis by a general antiinflammatory mechanism. We therefore determined how MyD88 deficiency altered circulating concentrations of key proinflammatory cytokines and chemokines (the T helper 1 cytokine IL-12 and MCP-1) in Apoe–/– mice with or without MyD88 deficiency. Apoe–/–/MyD88–/– mice had markedly reduced serum levels of IL-12p40 and MCP-1 compared with Apoe–/–/MyD88+/+ mice (Fig. 3 A and B). IL-12p40 serum concentrations of Apoe–/–/MyD88+/– mice were comparable with the serum concentrations of wild-type Apoe–/–/MyD88+/+. Serum concentrations of MCP-1 in Apoe–/–/MyD88+/– mice were comparable with those of wild-type Apoe–/–/MyD88+/+ mice. MyD88 deficiency was not associated with any significant changes in the circulating concentrations of the antiinflammatory cytokine IL-10 or the chemokine KC (data not shown). These results appear most consistent with the interpretation that at least part of the suppression of atherogenesis observed in mice with both apolipoprotein E (apoE) and MyD88 deficiency may be mediated by a general reduction in circulating levels of proatherogenic inflammatory molecules.
Fig. 3.
Serum concentration of IL-12p40 and MCP-1 are reduced in Apoe–/–/MyD88–/– mice. (A) IL-12p40 serum concentrations of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice fed with a high-cholesterol diet for 6 months (n = 19 in each group). (B) MCP-1 serum concentrations of Apoe–/–/MyD88–/–, Apoe–/–/MyD88+/–, and Apoe–/–/MyD88+/+ mice (n = 24 in each group). Means and SD are shown (*, P < 0.01).
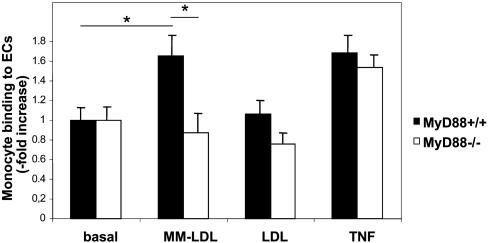
MyD88 Deficiency Is Associated with Reduced Adhesion of Leukocytes to ECs upon Stimulation with MM-LDL. Enhanced leukocyte–EC adhesion constitutes one of the pivotal early events in development of atherosclerotic plaques. To determine the effect of MyD88 deficiency on leukocyte–endothelial adhesion we isolated aortic ECs from MyD88–/– and MyD88+/+ mice and assessed their interaction with leukocytes (human peripheral blood mononuclear cells) upon stimulation with MM-LDL. ECs from the aortas of MyD88+/+ mice showed a dose-dependent increase in leukocyte adhesion when stimulated with MM-LDL, but not with unmodified native LDL (Fig. 4). In contrast, leukocyte adhesion to ECs derived from MyD88-deficient mice did not increase in response to stimulation with either MM-LDL or LDL. However, TNF-α-induced leukocyte adhesion to ECs was not impaired in MyD88–/– ECs and was not different from leukocyte adhesion to MyD88+/+ ECs. These results suggest that impaired leukocyte adhesion to MM-LDL-stimulated MyD88–/– ECs may lead to reduced infiltration of mononuclear phagocytes into developing plaques of Apoe–/–/MyD88–/– mice.
Fig. 4.
ECs from MyD88-deficient mice show reduced MM-LDL-induced adhesion of leukocytes. Adhesion of human peripheral blood mononuclear cells to murine aortic endothelial cells derived from aortas of MyD88–/– or MyD88+/+ mice stimulated with MM-LDL, LDL, or TNF-α. Data from one of three representative experiments with similar results are shown as fold increase in leukocyte adhesion over background (*, P < 0.05).
TLR4 Deficiency Reduces the Extent of Aortic Atherosclerosis in Apoe–/– Mice. To elucidate further the role of MyD88 and upstream receptor(s) on the development of atherosclerosis, we generated double knockout mice by intercrossing Apoe–/– and TLR4–/– mice. Apoe–/–/TLR4–/– and littermate controls (Apoe–/–/TLR4+/+) were fed a high-cholesterol diet and were killed at 6 months. We measured total serum cholesterol and triglyceride concentrations and, as was the case with mice deficient in both Apoe and MyD88, we found no significant differences in total cholesterol or plasma triglyceride concentrations between genotypes (Table 1). We measured the total lesion area of whole aortas after Oil red O staining. Quantitative analysis revealed a 24% reduction in the extent of atherosclerosis in Apoe–/–/TLR4–/– mice (n = 12 for Apoe–/–/TLR4–/– and n = 8for Apoe–/–/TLR4+/+, P < 0.01; Fig. 1B Right).
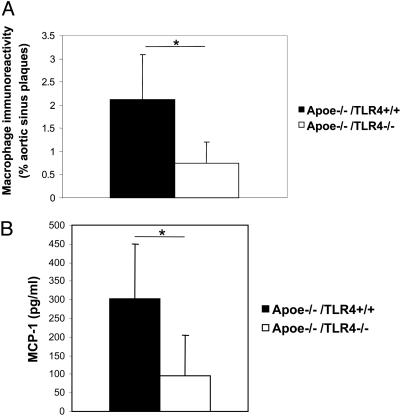
TLR4 Deficiency Is Associated with Reduction in Plaque Lipid Content, Macrophage Infiltration, and MCP-1 Serum Concentration. To further explore the characteristics of atherosclerosis in Apoe–/–/TLR4–/– mice, we quantified lipid content and macrophage infiltration in aortic sinus plaques (Figs. 2A Right panel and 5A). The lipid content of aortic sinus plaques was significant reduced in Apoe–/–/TLR4–/– mice (55% reduction). Macrophage infiltration in Apoe–/–/TLR4–/– mice was also significantly reduced by 65% compared with control mice. Furthermore, MCP-1 serum concentrations were significantly reduced in Apoe–/–/TLR4–/– mice (Fig. 5B). In contrast to Apoe–/–/MyD88–/– mice, there was no significant differences in IL-12p40 serum concentrations between Apoe–/–/TLR4–/– and Apoe–/–/TLR4+/+ mice (data not shown).
Fig. 5.
TLR4 deficiency reduces the extent of macrophage infiltration and MCP-1 secretion. (A) Quantitative analysis of macrophage immunoreactivity in aortic sinus plaques of Apoe–/–/TLR4–/– and Apoe–/–/TLR4+/+ mice, expressed as a proportion of the total plaque areas (n = 7 per group). Means and SD are shown (*, P < 0.01). (B) MCP-1 serum concentrations of Apoe–/–/TLR4–/– and Apoe–/–/TLR4+/+ mice (n = 8 for Apoe–/–/TLR4+/+ and n = 14 for Apoe–/–/TLR4–/–). Means and SD are shown (*, P < 0.01).
Discussion
Accumulating evidence implicates a fundamental link between the immune system and atherosclerosis, but thus far this evidence has been mostly indirect. Here, we demonstrate that genetic deficiency of either MyD88 or TLR4 reduces aortic atherosclerosis in apoE-deficient mice. Furthermore, MyD88 or TLR4 deficiency was associated with alterations in plaque composition that suggest greater structural stability; namely, reduction in lipid and macrophage content, and markedly decreased expression of the proinflammatory enzyme, COX-2. Cell culture studies with ECs derived from mice with or without genetic deficiency in MyD88 showed that leukocyte–EC adhesion was markedly hindered by genetic deficiency of MyD88. Finally, we show that the atheroprotective effect of MyD88 or TLR4 deficiency is not secondary due to altered serum cholesterol or lipoproteins. Our findings of reduced circulating proinflammatory cytokine IL-12 and MCP-1 suggest that the atheroprotective effects of MyD88 and TLR4 deficiency may result in part from reduced systemic inflammation. Collectively, our results directly indicate that innate immune signaling has both systemic and local effects that together markedly inhibit development of atherosclerosis and promote a more stable plaque phenotype.
MyD88 is thought to transduce signals of all of the TLRs (except for TLR3), as well as the IL-1 and IL-18 receptors. Previous studies (27) using IL-1β-deficient mice on an Apoe–/– background found a modest 30% decrease in the extent of atherosclerosis, which is considerably less than the nearly 60% reduction observed in our study with mice deficient in both MyD88 and Apoe (Fig. 1). This finding suggests that MyD88 signaling mediates downstream proatherogenic effects in addition to those depending on intact IL-1β signaling. Our results with Apoe–/–/TLR4–/– mice, which have intact IL-1β signaling, are consistent with this interpretation. As anticipated, in comparison with Apoe–/–/MyD88–/– mice, Apoe–/–/TLR4–/– mice display a more moderate but significant reduction in atherosclerotic lesions (57% vs. 24%, respectively) and a sustained reduction in macrophage infiltration and MCP-1 serum concentration. However, this moderate reduction was not unexpected. Atherosclerosis is multifactorial, dynamic, and highly variable temporally and spatially within the vasculature. Whereas there are many important contributors, there is no single culprit cause that accounts for the overwhelming variance in either the prevalence or extent of the disease. Our results are consistent with these notions, and demonstrate that TLR4 and MyD88 signaling is one contributor, but also directly show that innate immune mechanisms are important contributors to the pathobiology of the disease rather than being secondarily associated with it. In addition, other TLRs using MyD88 may also be involved in atherogenesis. Recently, several bacterial or viral microorganisms have been postulated to be involved in atherogenesis, which contain ligands for TLR2, for instance (6, 28, 29).
MyD88 deficiency has been associated with a shift toward a T helper type 2 response (30–32). In accord with this concept, we observed that MyD88 deficiency was associated with reduced serum concentrations of IL-12p40. We also examined COX-2, IL-12, and MCP-1, because all are implicated in atherogenesis and are downstream targets of TLR4 and MyD88 signaling (33–35). Because IL-12 is directly linked to progression of atherosclerosis in mice, reduced IL-12p40 levels observed in MyD88-but not in TLR4-deficient mice could, in part, account for the further reduction of atherosclerosis we observed in Apoe–/–/MyD88–/– mice (36, 37). TLR4 or MyD88 deficiency was also associated with a marked reduction in circulating levels of MCP-1, a key proatherogenic chemokine (38, 39). MCP-1 deficiency may also have contributed to reduced atherosclerosis in Apoe–/–/MyD88–/– and Apoe–/–/TLR4–/– mice, because recent work has shown that genetic deficiency of MCP-1 (or its receptor CCR2) is associated with reduced macrophage infiltration and atherosclerosis in Apoe or LDL receptor-deficient mice (38, 39).
Increased expression of COX-2 in atherosclerotic lesions together with pharmacologic and direct genetic evidence suggest that COX-2 promotes atherosclerotic lesion formation (40, 41, 42–45). Consistent with these data, we found that MyD88 deficiency resulted in a marked reduction in COX-2 immunoreactivity in atherosclerotic plaques. This result might at least partially account for the inhibition of atherosclerosis we observed in our Apoe–/–/MyD88–/– mice.
One of the key early events in the process of atherogenesis is enhanced leukocyte–EC adhesion. We found that MyD88-deficient ECs demonstrated an impaired ability to support leukocyte adhesion in response to MM-LDL stimulation but not to TNF-α stimulation. Previous data (46) suggested that ECs from C57BL/6J mice exhibited substantial induction of proinflammatory molecules in response to MM-LDL, whereas ECs from C3H/HeJ mice did not. Together, these data suggest that MyD88 might regulate expression and/or function of cellular adhesion molecules on ECs, mononuclear phagocytes, or both. However, the possibility that MyD88 and/or TLR4 might also affect processes such as monocyte to macrophage conversion, or survival or retention of mononuclear phagocytes within the arterial wall cannot be excluded. Further studies will be required to directly test the validity of these hypotheses.
The evidence for involvement of NF-κB in the development of atherosclerosis is substantial, albeit largely indirect (47). However, a recent study by Kanters et al. (48) reported that macrophage-specific inhibition of the NF-κB pathway leads to more severe atherosclerosis in mice, perhaps due to a reduction in IL-10 production by macrophages. However, in our study, there were no differences in IL-10 expression in Apoe–/–/MyD88–/– mice compared with littermate controls. Intriguingly, Kanters et al. (49) more recently reported that bone marrow transplants of p50-deficient donor mice into proatherogenic LDL receptor-null recipient mice leads to a decrease of atherosclerosis. Clearly, the involvement of NF-κB in atherosclerosis is complex, and further studies will be necessary to more clearly elucidate mechanistic details.
Our results contrast with those previously reported by Wright et al. (50), which suggested there was no difference in the extent of atherosclerosis between Apoe–/– and Apoe–/–/lpsd mice, a strain generated by backcrossing Apoe–/– and the LPS-hyporesponsive strain C57BL/10ScN. However, whereas our study directly measured atherosclerosis, Wright et al. (50) estimated atherosclerotic burden from cholesteryl ester content in whole aortas. Additionally, Apoe–/–/lpsd mice only partially reflect a TLR4–/– genotype because they also lack two additional genes with unknown function (51). Furthermore, a different LPS-hyporesponsive strain C3H/HeJ that has nonfunctional TLR4 because of a spontaneous mutation (52), is resistant to development of atherosclerosis (46), a finding that is consistent with our results.
We conclude that genetic deficiency of MyD88, and to a lesser extent, that of TLR4, reduces aortic atherosclerosis in apoE-deficient mice and is associated with alterations in plaque composition that suggest greater stability. Our work represents an important step in establishing a link between TLR4 and MyD88 signaling and hypercholesterolemia and atherosclerosis. With the advent of small-molecule antagonists (53), it may soon be feasible to evaluate the efficacy of therapeutic inhibition of TLR signaling in treating atherosclerosis-based pathologies.
Supplementary Material
Acknowledgments
We thank Stephanie Vogel (University of Maryland, Baltimore) for providing repurified LPS, Joseph Witztum (University of California at San Diego, La Jolla) for providing MM-LDL and LDL, and Maria Abreu for critical reading of the manuscript. This work was supported by National Institutes of Health Grants HL 66436 (to M.A.) and HL51980 and HL58555 (to T.B.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TLR, Toll-like receptor; MCP-1, monocyte chemoattractant protein 1; COX, cyclooxygenase; apoE, apolipoprotein E; MyD88, myeloid differentiation factor 88; LDL, low-density lipoprotein; MM-LDL, minimally modified LDL; LPS, lipopolysaccharide; EC, endothelial cell; TNF, tumor necrosis factor.
Note. While this manuscript was being prepared, Bjorkbacka et al. (54) reported that Apoe–/–/MyD88–/– mice develop less atherosclerosis than Apoe–/–/MyD88+/+ littermate controls, which is consistent with our findings here, but also found that Apoe–/–/CD14–/– mice exhibited similar atherosclerosis compared with Apoe–/–/CD14+/+ controls (54). They interpreted these latter findings as being consistent with the interpretation that the TLR4/CD14 complex plays no role in plaque development. CD14 is a coreceptor that recruits and facilitates LPS binding to the TLR4 receptor (55). However, CD14 is not a signaling receptor and does not interact directly with MyD88. Furthermore, TLR4 can interact with its cognate ligands and transduce signals independently of CD14 as well (20, 56). Therefore, CD14-null mice cannot be used as a surrogate for TLR4-null mice. In our study, we directly tested the involvement of TLR4 and found that loss of TLR4 results in decreased plaque formation. Our findings thus corroborate, clarify, and extend the work of Bjorkbacka et al. (54). Collectively, the most likely interpretation is that TLR4 signaling contributes to atherosclerotic plaque development, but that this process does not involve the participation of CD14. Alternatively, CD14-independent TLR4 signaling might mediate proatherogenic influences. This finding is consistent with in vitro data showing that ox-PAPC (a major bioactive component of MM-LDL) may initially bind to a glycosylphosphatidylinositol-anchored molecule other than CD14, and then to TLR4 to activate ECs (20). Unraveling these complexities will be an important goal for future studies.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115–126. [DOI] [PubMed] [Google Scholar]
- 2.Glass, C. K. & Witztum, J. L. (2001) Cell 104, 503–516. [DOI] [PubMed] [Google Scholar]
- 3.Shah, P. K. (2003) J. Am. Coll. Cardiol. 41, 15S–22S. [DOI] [PubMed] [Google Scholar]
- 4.Libby, P. (2002) Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- 5.Hansson, G. K., Libby, P., Schonbeck, U. & Yan, Z. Q. (2002) Circ. Res. 91, 281–291. [DOI] [PubMed] [Google Scholar]
- 6.Binder, C. J., Chang, M. K., Shaw, P. X., Miller, Y. I., Hartvigsen, K., Dewan, A. & Witztum, J. L. (2002) Nat. Med. 8, 1218–1226. [DOI] [PubMed] [Google Scholar]
- 7.Kol, A. & Libby, P. (1998) Trends Cardiovasc. Med. 8, 191–199. [DOI] [PubMed] [Google Scholar]
- 8.Sander, D., Winbeck, K., Klingelhofer, J., Etgen, T. & Conrad, B. (2004) Circulation 109, 1010–1015. [DOI] [PubMed] [Google Scholar]
- 9.Zahn, R., Schneider, S., Frilling, B., Seidl, K., Tebbe, U., Weber, M., Gottwik, M., Altmann, E., Seidel, F., Rox, J., et al. (2003) Circulation 107, 1253–1259. [DOI] [PubMed] [Google Scholar]
- 10.Sander, D., Winbeck, K., Klingelhofer, J., Etgen, T. & Conrad, B. (2002) Circulation 106, 2428–2433. [DOI] [PubMed] [Google Scholar]
- 11.Grayston, J. T. (2003) Circulation 107, 1228–1230. [DOI] [PubMed] [Google Scholar]
- 12.Fredrikson, G. N., Soderberg, I., Lindholm, M., Dimayuga, P., Chyu, K. Y., Shah, P. K. & Nilsson, J. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 879–884. [DOI] [PubMed] [Google Scholar]
- 13.Binder, C. J., Horkko, S., Dewan, A., Chang, M. K., Kieu, E. P., Goodyear, C. S., Shaw, P. X., Palinski, W., Witztum, J. L. & Silverman, G. J. (2003) Nat. Med. 9, 736–743. [DOI] [PubMed] [Google Scholar]
- 14.Hansson, G. K. (2002) Circulation 106, 1599–1601. [DOI] [PubMed] [Google Scholar]
- 15.de Kleijn, D. & Pasterkamp, G. (2003) Cardiovasc. Res. 60, 58–67. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, G. & Ghosh, S. (2001) J. Clin. Invest. 107, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, X. H., Shah, P. K., Faure, E., Equils, O., Thomas, L., Fishbein, M. C., Luthringer, D., Xu, X. P., Rajavashisth, T. B., Yano, J., et al. (2001) Circulation 104, 3103–3108. [DOI] [PubMed] [Google Scholar]
- 18.Edfeldt, K., Swedenborg, J., Hansson, G. K. & Yan, Z. Q. (2002) Circulation 105, 1158–1161. [PubMed] [Google Scholar]
- 19.Miller, Y. I., Viriyakosol, S., Binder, C. J., Feramisco, J. R., Kirkland, T. N. & Witztum, J. L. (2003) J. Biol. Chem. 278, 1561–1568. [DOI] [PubMed] [Google Scholar]
- 20.Walton, K. A., Hsieh, X., Gharavi, N., Wang, S., Wang, G., Yeh, M., Cole, A. L. & Berliner, J. A. (2003) J. Biol. Chem. 278, 29661–29666. [DOI] [PubMed] [Google Scholar]
- 21.Arbour, N. C., Lorenz, E., Schutte, B. C., Zabner, J., Kline, J. N., Jones, M., Frees, K., Watt, J. L. & Schwartz, D. A. (2000) Nat. Genet. 25, 187–191. [DOI] [PubMed] [Google Scholar]
- 22.Kiechl, S., Lorenz, E., Reindl, M., Wiedermann, C. J., Oberhollenzer, F., Bonora, E., Willeit, J. & Schwartz, D. A. (2002) N. Engl. J. Med. 347, 185–192. [DOI] [PubMed] [Google Scholar]
- 23.Kiechl, S., Wiedermann, C. J. & Willeit, J. (2003) Ann. Med. 35, 164–171. [DOI] [PubMed] [Google Scholar]
- 24.Ameziane, N., Beillat, T., Verpillat, P., Chollet-Martin, S., Aumont, M. C., Seknadji, P., Lamotte, M., Lebret, D., Ollivier, V. & De Prost, D. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 61–64. [DOI] [PubMed] [Google Scholar]
- 25.Boekholdt, S. M., Agema, W. R., Peters, R. J., Zwinderman, A. H., van der Wall, E. E., Reitsma, P. H., Kastelein, J. J. & Jukema, J. W. (2003) Circulation 107, 2416–2421. [DOI] [PubMed] [Google Scholar]
- 26.Breslow, J. L. (1996) Science 272, 685–688. [DOI] [PubMed] [Google Scholar]
- 27.Kirii, H., Niwa, T., Yamada, Y., Wada, H., Saito, K., Iwakura, Y., Asano, M., Moriwaki, H. & Seishima, M. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 656–660. [DOI] [PubMed] [Google Scholar]
- 28.Libby, P., Ridker, P. M. & Maseri, A. (2002) Circulation 105, 1135–1143. [DOI] [PubMed] [Google Scholar]
- 29.Muhlestein, J. B. & Anderson, J. L. (2003) Cardiol. Clin. 21, 333–362. [DOI] [PubMed] [Google Scholar]
- 30.Schnare, M., Barton, G. M., Holt, A. C., Takeda, K., Akira, S. & Medzhitov, R. (2001) Nat. Immunol. 2, 947–950. [DOI] [PubMed] [Google Scholar]
- 31.Muraille, E., De Trez, C., Brait, M., De Baetselier, P., Leo, O. & Carlier, Y. (2003) J. Immunol. 170, 4237–4241. [DOI] [PubMed] [Google Scholar]
- 32.Kaisho, T., Hoshino, K., Iwabe, T., Takeuchi, O., Yasui, T. & Akira, S. (2002) Int. Immunol. 14, 695–700. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- 34.Rhee, S. H. & Hwang, D. (2000) J. Biol. Chem. 275, 34035–34040. [DOI] [PubMed] [Google Scholar]
- 35.Kawai, T., Takeuchi, O., Fujita, T., Inoue, J., Muhlradt, P. F., Sato, S., Hoshino, K. & Akira, S. (2001) J. Immunol. 167, 5887–5894. [DOI] [PubMed] [Google Scholar]
- 36.Lee, T. S., Yen, H. C., Pan, C. C. & Chau, L. Y. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 734–742. [DOI] [PubMed] [Google Scholar]
- 37.Davenport, P. & Tipping, P. G. (2003) Am. J. Pathol. 163, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boring, L., Gosling, J., Cleary, M. & Charo, I. F. (1998) Nature 394, 894–897. [DOI] [PubMed] [Google Scholar]
- 39.Gu, L., Okada, Y., Clinton, S. K., Gerard, C., Sukhova, G. K., Libby, P. & Rollins, B. J. (1998) Mol. Cell 2, 275–281. [DOI] [PubMed] [Google Scholar]
- 40.Schonbeck, U., Sukhova, G. K., Graber, P., Coulter, S. & Libby, P. (1999) Am. J. Pathol. 155, 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burleigh, M. E., Babaev, V. R., Oates, J. A., Harris, R. C., Gautam, S., Riendeau, D., Marnett, L. J., Morrow, J. D., Fazio, S. & Linton, M. F. (2002) Circulation 105, 1816–1823. [DOI] [PubMed] [Google Scholar]
- 42.Rott, D., Zhu, J., Burnett, M. S., Zhou, Y. F., Zalles-Ganley, A., Ogunmakinwa, J. & Epstein, S. E. (2003) J. Am. Coll. Cardiol. 41, 1812–1819. [DOI] [PubMed] [Google Scholar]
- 43.Belton, O. A., Duffy, A., Toomey, S. & Fitzgerald, D. J. (2003) Circulation 108, 3017–3023. [DOI] [PubMed] [Google Scholar]
- 44.Olesen, M., Kwong, E., Meztli, A., Kontny, F., Seljeflot, I., Arnesen, H., Lyngdorf, L. & Falk, E. (2002) Scand. Cardiovasc. J. 36, 362–367. [DOI] [PubMed] [Google Scholar]
- 45.Verma, S., Raj, S. R., Shewchuk, L., Mather, K. J. & Anderson, T. J. (2001) Circulation 104, 2879–2882. [DOI] [PubMed] [Google Scholar]
- 46.Shi, W., Wang, N. J., Shih, D. M., Sun, V. Z., Wang, X. & Lusis, A. J. (2000) Circ. Res. 86, 1078–1084. [DOI] [PubMed] [Google Scholar]
- 47.Collins, T. & Cybulsky, M. I. (2001) J. Clin. Invest. 107, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanters, E., Pasparakis, M., Gijbels, M. J., Vergouwe, M. N., Partouns-Hendriks, I., Fijneman, R. J., Clausen, B. E., Forster, I., Kockx, M. M., Rajewsky, K., et al. (2003) J. Clin. Invest. 112, 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanters, E., Gijbels, M. J., van der Made, I., Vergouwe, M. N., Heeringa, P., Kraal, G., Hofker, M. H. & de Winther, M. P. (2004) Blood 103, 934–940. [DOI] [PubMed] [Google Scholar]
- 50.Wright, S. D., Burton, C., Hernandez, M., Hassing, H., Montenegro, J., Mundt, S., Patel, S., Card, D. J., Hermanowski-Vosatka, A., Bergstrom, J. D., et al. (2000) J. Exp. Med. 191, 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qureshi, S. T., Lariviere, L., Leveque, G., Clermont, S., Moore, K. J., Gros, P. & Malo, D. (1999) J. Exp. Med. 189, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Huffel, C. V., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 53.Bartfai, T., Behrens, M. M., Gaidarova, S., Pemberton, J., Shivanyuk, A. & Rebek, J., Jr. (2003) Proc. Natl. Acad. Sci. USA 100, 7971–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjorkbacka, H., Kunjathoor, V. V., Moore, K. J., Koehn, S., Ordija, C. M., Lee, M. A., Means, T., Halmen, K., Luster, A. D., Golenbock, D. T. & Freeman, M. W. (2004) Nat. Med. 10, 416–421. [DOI] [PubMed] [Google Scholar]
- 55.Wright, S. D., Ramos, R. A., Tobias, P. S., Ulevitch, R. J. & Mathison, J. C. (1990) Science 249, 1431–1433. [DOI] [PubMed] [Google Scholar]
- 56.Means, T. K., Wang, S., Lien, E., Yoshimura, A., Golenbock, D. T. & Fenton, M. J. (1999) J. Immunol. 163, 3920–3927. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.