Abstract
Aims
To address barriers to implementing the “Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)” in medical settings, we adapted the traditional interviewer-administered (IA) ASSIST to an audio-guided computer assisted self-interview (ACASI) format. This study sought to validate the ACASI ASSIST by estimating the concordance, correlation, and agreement of scores generated using the ACASI versus the reference standard IA ASSIST. Secondary aims were to assess feasibility and compare ASSIST self-report to drug testing results.
Design
Participants completed the ACASI and IA ASSIST in a randomly assigned order, followed by drug testing.
Setting
Urban safety-net primary care clinic.
Participants
A total of 393 adult patients.
Measurements
Scores generated by the ACASI and IA ASSIST; drug testing results from saliva and hair samples.
Findings
Concordance between the ACASI and IA ASSIST in identifying moderate-high risk use was 92–99% for each substance class. Correlation was excellent for global scores (ICC=0.94, CI 0.92–0.95) and for substance-specific scores for tobacco (ICC=0.93, CI 0.91–0.94), alcohol (ICC=0.91, CI 0.89–0.93) and illicit drugs (ICC=0.85, CI 0.85–0.90), and good for prescription drugs (ICC=0.68, CI 0.61–0.73). Ninety-four percent of differences in global scores fell within anticipated limits of agreement. Among participants with a positive saliva test, 74% self-reported use on the ACASI ASSIST. The ACASI ASSIST required a median time of 3.7 minutes (range 0.7–15.4), and 21 (5.3%) participants requested assistance.
Conclusions
The computer self-administered Alcohol, Smoking and Substance Involvement Screening Test appears to be a valid alternative to the interviewer-administered approach for identifying substance use in primary care patients.
Keywords: Drugs, alcohol, tobacco, substance abuse, screening, ACASI
Introduction
Alcohol and drug use disorders are among the top ten causes of preventable death in the United States. Screening followed by brief intervention for unhealthy alcohol use in adult primary care patients has a strong evidence base, and is among the most cost-effective preventive health services.(1–4) While the efficacy of this approach for reducing drug use in U.S. populations has not been established,(5–8) in medical practice settings screening for drugs may be justified on clinical grounds. Drug use can have serious implications for the prevention and treatment of other medical conditions,(9) and primary care providers may have the ability to offer patients treatment services including pharmacotherapy and referral to specialty care. Yet integrating screening and interventions for substance use into busy medical settings has proven challenging.(10–14)
Identification of drug and alcohol use could be facilitated by a unified approach that combines screening and assessment for tobacco, alcohol, and drugs, and quickly and reliably gathers enough information to provide a detailed and accurate risk assessment to guide clinical interventions. One such instrument is the “Alcohol, Smoking and Substance Involvement Screening Test (ASSIST),” a structured interview developed by the World Health Organization (WHO) for use in general healthcare settings, and validated in a large multi-site international study.(15–18) However, the ASSIST has not been widely adopted,(10, 19) in part because it takes approximately 5–15 minutes of face-to-face interaction with the patient, includes complex skip patterns, and requires computation of a score by the interviewer.
A patient self-administered version of the ASSIST, which could be completed prior to the medical encounter using a tablet or kiosk computer, has the potential to facilitate its implementation in health care settings. We thus adapted the previously validated interviewer-administered ASSIST to a patient self-administered format using audio-guided computer assisted self-interview (ACASI) technology. ACASI technology supports patients with limited reading ability because relevant text is read aloud in real time, and response options are clearly indicated using symbols. Computerized self-interview questionnaires have proven sensitive for detecting stigmatized behaviors, have comparable validity to traditional interview formats, are easily adapted to multiple languages, and can be integrated into medical settings.(20–26)
A self-administered screening and assessment tool has a number of potential advantages in busy medical practices. Time constraints have been identified as a primary barrier to implementation of substance use screening and interventions in primary care.(13, 27–36) Although well-resourced clinics may be able to utilize medical staff to conduct face-to-face screening, this requires personnel time and training, can threaten fidelity when screening items are not delivered exactly as written,(37, 38) and patients may be less willing to report substance use in a face-to-face interview.(39, 40) Moreover, by occupying patients’ waiting time with an activity that has personal relevance, this approach has the potential to increase patient satisfaction.(41, 42)
A prior study found that the ACASI ASSIST had excellent test-retest reliability in a sample of 101 adult primary care patients.(43) However, the validity of adapting what was designed as an interviewer-administered screening instrument into an ACASI format must be demonstrated before it can be recommended for widespread adoption. To provide that evidence, we sought to validate the ACASI ASSIST by estimating the concordance, correlation, and agreement of results generated using the ACASI versus the reference standard IA ASSIST. Secondary aims were to compare self-reported drug use on the ASSIST to biologic drug testing results, and to examine the feasibility and acceptability of the ACASI ASSIST.
Methods
Recruitment
The study was conducted in the adult primary care clinic of a large municipal hospital in New York City from July 2012 to June 2013. A convenience sample of 399 participants was enrolled. Based on a prior study conducted at this clinical site,(44) this sample was anticipated to include least 50 participants with moderate- to high-risk substance use to inform our comparison of the two instruments. While we did not conduct prior simulations to determine the precision of comparisons between the ACASI and IA ASSIST as a function of prevalence and sample size, 50 individuals with moderate-high risk alcohol or drug use would provide sufficient power to distinguish between good (ICC=0.6) and excellent (ICC=0.8) agreement.
Participants were consecutively recruited using pre-specified paths through the seats of the clinic’s waiting area. Eligible individuals were age 21–65 years, English speaking, and current clinic patients. Individuals over age 65 were excluded because unhealthy drug and alcohol use is less prevalent in this age group (44, 45) and the sample size in our study would not support meaningful analyses. Participants were randomly assigned in counterbalanced order to complete either the computer (ACASI) or interviewer (IA) ASSIST first. All participants completed both instruments in sequence, with one ASSIST version directly following the other. Interviews were conducted anonymously and in a private room. Participants completed the ACASI ASSIST independently using a touch-screen tablet computer, with headphones. Any requests for assistance were tracked by the research assistant (RA) using a standardized form. After completing both versions of the ASSIST, all participants were asked to participate in saliva drug testing, and a randomly selected sample of 39 participants was additionally offered hair testing. The institutional review board of the NYU School of Medicine reviewed and approved all study procedures.
Study Instruments: IA ASSIST and ACASI ASSIST
The ASSIST used for both the IA and ACASI versions was based on the WHO ASSIST V3.0; (17) a brief structured interview that covers nine substances (tobacco, alcohol, cannabis, cocaine, stimulants, inhalants, sedatives, hallucinogens, opioids) and assesses lifetime and current use, consequences of use, and failure to stop or cut down. The ASSIST instruments used in our study were adapted to include two additional substance classes: prescription opioids and prescription stimulants. These were added in response to the emergence of prescription drug misuse as a public health concern in the U.S., and were included in the ACASI ASSIST instrument used in our prior test-retest reliability study.(43)
IA ASSIST
The IA ASSIST was delivered by the RA as an interview, following standard procedures for ASSIST administration.(46, 47) Participants were provided with a written response card, and the RA read aloud and verbatim the introduction and all items and responses. For participants who had questions about a prescription medication item, the RA provided a clarification based on language from the ASSIST introduction, which states that medications should be reported if they have been “used for reasons other than prescription, or taken more frequently or at higher doses than prescribed.”
ACASI ASSIST
The ACASI ASSIST items were identical to those of the IA ASSIST, but additionally included clarifying items for each of the prescription drug classes (see Table 1). These items were added because participants frequently misinterpret questions concerning misuse of prescription drug items on self-administered questionnaires,(48) including on an earlier version of the ACASI ASSIST.(49) If the individual’s responses to the clarification items were not consistent with non-medical use of a prescription drug, in the analysis we recoded the response to zero (no use in the past 3 months) and the subsequent ASSIST items for that substance were coded as zero. Recoding was required for 3/10 individuals who reported prescription stimulants, for 12/32 who reported prescription sedatives, and for 19/35 who reported prescription opioids.
Table 1.
Items added to the ASSIST to clarify non-medical use of prescription stimulants, sedatives, and opiates.
| IA ASSIST | ACASI ASSIST | |||||||
|---|---|---|---|---|---|---|---|---|
| Question 1 | Question 2 | Question 1 | Question 2 | Clarifying items for prescription medications | ||||
| Item | Response options | Action | Interpretation of a positive response | |||||
| Prescription opioids (morphine, codeine, fentanyl, oxycodone [OxyContin, Percocet], hydrocodone [Vicodin], methadone, buprenorphine [Suboxone], etc.) | Added | Added | Added | Added | a. Is this a medication that you can buy in the store without a prescription (over the counter)? | Yes/No | If ‘Yes,’ skip items b–d. | Not a prescription medication; not non-medical use. |
| Clarifying items if response is ‘Yes’ | b.Was it prescribed for you? | Yes/No | If ‘Yes,’ administer items c and d. | Is a prescription medication; unknown if non-medical use. | ||||
| Prescription stimulants (Ritalin, Concerta, Dexedrine, Adderall, diet pills, etc.) | Added | Added | Added | Added | c. Do you ever use MORE of your [stimulant/sedative/opiate] medication, that is, take a higher dosage, than is prescribed for you? | Yes/No | Regardless of response, administer item d. | Non-medical use |
| Clarifying items if response is ‘Yes’ | d. Do you ever use your [stimulant/sedative/opiate] medication MORE OFTEN, that is, shorten the time between dosages, than is prescribed for you? | Yes/No | No further follow-up items, return back to ASSIST Question 2. | Non-medical use | ||||
| Prescription sedatives: Same as in ASSIST V3.0 | - | - | - | - | ||||
| Clarifying items if response is ‘Yes’ | ||||||||
The ASSIST items were delivered in their entirety, and written text on the computer screen was identical to the words of the voice instruction. The ACASI ASSIST was created using QDS Software (Nova Research Co).
Measures
Prevalence
Prevalence of lifetime and current (past 3 months) use was based on responses to the IA ASSIST Questions 1 and 2, respectively.
Risk scores
ASSIST global scores and substance specific involvement scores (SSIS) were calculated using standard ASSIST methodology, for both the IA and the ACASI versions.(17) The global score represents the sum of all responses to ASSIST Questions 1–8. With inclusion of the prescription opioids and stimulants categories, the global score has a potential range of 0 to 498. Following the standard approach to scoring the ASSIST, the SSIS is the sum of responses to ASSIST Questions 2–7, for each substance, and has a potential range of 0 to 39. ASSIST scores were further aggregated into two summary categories: ‘prescription drugs’ (prescription opioids, sedatives, and stimulants) and ‘illicit drugs’ (all other drugs, excluding tobacco and alcohol).
Risk level
WHO-recommended cutoffs were applied to SSIS scores for each substance to determine substance-specific levels of risk (low, moderate, or high).(17) In some analyses we collapsed the moderate and high risk levels into a single ‘moderate-high risk’ category because high risk substance use was relatively infrequent in our sample, and in a general medical setting the priority is to distinguish between individuals whose substance use requires clinical intervention (i.e., moderate or high risk use) versus those who do not require intervention.(17) Level of risk for the aggregate ‘prescription drugs’ and ‘illicit drugs’ categories was based on the highest risk level for any substance in that category.
Time required to complete the ACASI ASSIST was automatically recorded by the computer, while time for the IA ASSIST was recorded by the RA using a stopwatch. Following completion of both versions of the ASSIST, the RA administered a structured questionnaire asking participants if they “prefer to be asked these questions by a person (as an interview) or by the computer,” followed by a standard demographic questionnaire.
Biologic tests
Saliva and hair tests were conducted for cannabis, benzodiazepines, cocaine, amphetamines, opioids, and phencyclidine (PCP). Participants reported any medical use of prescription sedatives, opioids, or stimulants, and test results consistent with medical use were classified as ‘negative’ with respect to drug misuse. Saliva testing, which was performed with the Intercept™ immunoassay (OraSureTechnologies), has equivalent accuracy to urine drug screening tests, and a window of detection of up to 3 days for most drugs.(50–52) Hair testing, performed by Omega Laboratories, was conducted in a randomly selected sample of 39 individuals. Two samples had an insufficient quantity for testing, so 37 tests were reported.
Statistical analysis
We examined responses for an order effect with paired sample Mann-Whitney U tests comparing ASSIST scores for those who received the ACASI version first (N=191) against those who received the IA version first (N=202). This was done for global scores, SSIS for each substance, and for the combined categories of illicit drugs and of prescription drugs. Scores were examined for both the ACASI and the IA versions of the ASSIST. We found no significant difference in scores based on assignment to the ACASI-first versus IA-first group and proceeded to conduct the remainder of the analyses without regard to order of administration.
To achieve the primary aim of evaluating the ACASI ASSIST by comparison to the IA ASSIST, we conducted analyses of concordance, correlation, and agreement. Concordance of scores indicating low versus moderate-high risk use was examined for each substance, and for the combination categories of illicit drugs and prescription drugs. Concordance indicates whether the ACASI and IA ASSIST made the same classification of individuals whose substance use requires clinical intervention (due to moderate- or high-risk use) and those who do not require intervention (due to low-risk use). We examined the proportion of individuals who had either concordant risk levels or an increased or decreased risk level on the ACASI versus the IA ASSIST. Correlation of results, for low versus moderate-high risk use, was examined using Cohen’s Kappa. Kappa coefficients were computed for all substance classes having prevalence greater than 10% in the study population, and were not calculated for lower prevalence substances because the dependence of Kappa on prevalence can compromise its interpretation in conditions of markedly low prevalence.(53, 54) Exact 95% confidence intervals (CIs) were calculated, and Kappa coefficients were interpreted using standard cutoffs for level of agreement.(55)
We also examined correlation between the ACASI and IA ASSIST scores as continuous variables using Intraclass Correlation Coefficients (ICCs). The measures of concordance and correlation are complementary; concordance demonstrates exact agreement, while a correlation coefficient gives information about the degree of change. ICCs were calculated using a single measurement, absolute agreement definition, 2-way mixed model for the following scores: global score, SSIS for each substance, and the summary ‘illicit drugs’ and ‘prescription drugs’ scores. We additionally computed the ICC for the global score limited to the items that comprise ASSIST V3.0 (i.e. without including the prescription stimulant and prescription opioid items). ICCs were interpreted using recommended guidelines for reliability of clinical instruments.(56)
The Bland and Altman method was used to measure agreement between the ACASI and IA versions of the ASSIST. As opposed to the ICC, which compares two measures that are not ordered a priori with regard to accuracy, the Bland and Altman approach allows comparison of a new approach (ACASI ASSIST) against an established reference standard (IA ASSIST). Providing a measure of agreement in addition to the ICC is desirable because correlation only measures the strength of the linear relationship between two measures, and does not take into account the scale and true value of the item being measured. As a result, two measures that have very poor agreement can still be highly correlated.(57) Employing the standard Bland and Altman approach (58) we computed the limits of agreement for global scores generated for the ACASI versus IA ASSIST. Bland and Altman analyses for the illicit and prescription drug categories were not performed because differences for these scores did not follow a normal distribution.
As a secondary analysis, we compared ASSIST responses to results of saliva and hair testing, to evaluate the accuracy of self-report of current drug use on the ACASI and IA ASSIST. Results of the biologic tests were compared to ASSIST Question 2, which asks about use in the past 3 months. All analyses were conducted using IBM SPSS Statistics 20.
Results
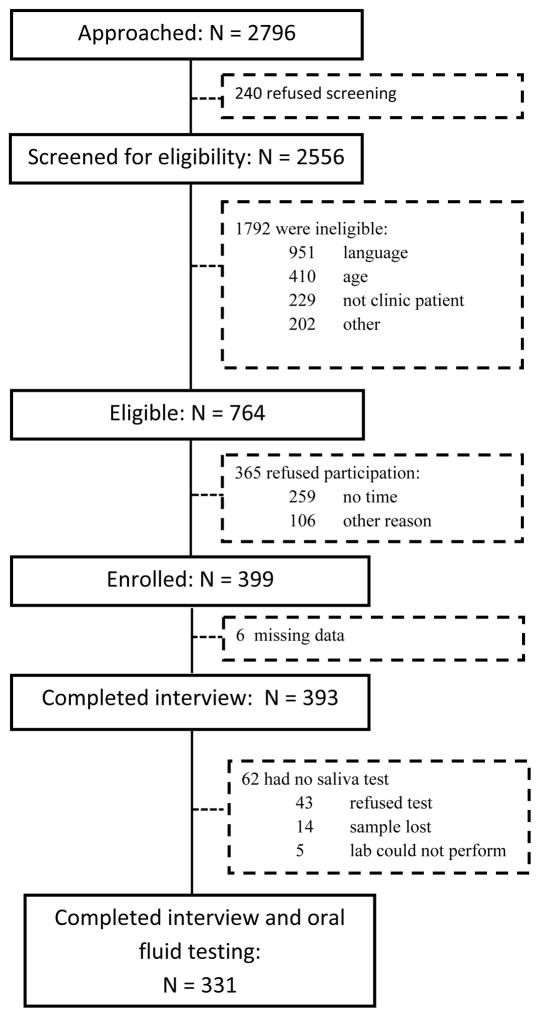
Figure 1 shows recruitment enrollment data. After removing 6 individuals with missing ASSIST data, there were a total of 393 cases for analysis, of which 84% had saliva tests.
Figure 1.
Flowchart of participant recruitment
Participants had diverse demographic characteristics (Table 2). A limited set of demographic characteristics was also collected from eligible individuals who refused to participate. Non-participants were more frequently female (57%) and white (36%), and had a lower average age (42 years). Drug use characteristics of participants, based on responses to the IA ASSIST, are presented in Table 3.
Table 2.
Demographics of participants (N=393).
| Characteristic | N (%) |
|---|---|
| Age (years) | |
| Mean, SD | 47, 12 |
| Median | 49 |
| Range | 19–65 |
| Interquartile range | 17 |
| Gender | |
| Female | 190 (48.3) |
| Male | 202 (51.4) |
| Transgender | 1 (0.3) |
| Race/Ethnicity | |
| Black/African American | 176 (45.0) |
| White/Caucasian | 60 (15.3) |
| Hispanic | 112 (28.6) |
| Other | 40 (10.2) |
| Don’t Know/Refused | 3 (0.8) |
| Primary language | |
| English | 316 (80.6) |
| Spanish | 36 (9.2) |
| Other | 40 (10.2) |
| Country of birth | |
| U.S. | 265 (67.4) |
| Other | 128 (32.6) |
| Education (highest level completed) | |
| Less than HS | 69 (17.6) |
| HS grad or GED | 96 (24.4) |
| Some college or trade school | 119 (30.3) |
| College degree (4-year) | 90 (22.9) |
| Other | 19 (4.8) |
| Employment | |
| Employed | 137 (34.9) |
| Unemployed | 103 (26.2) |
| Other | 152 (38.8) |
| Don’t know/Refused | 1 (0.3) |
| Income | |
| <$5,000 | 92 (23.5) |
| $5,000–14,999 | 90 (22.9) |
| $15,000–24,999 | 60 (15.3) |
| $25,000–49,999 | 69 (17.6) |
| ≥ $50,000 | 23 (5.8) |
| Don’t know/Refused | 59 (15.1) |
| Perceived health status* | |
| Very good or excellent | 98 (24.9) |
| Good | 128 (32.6) |
| Fair or poor | 163 (41.4) |
| Don’t know/Refused | 4 (1.0) |
“Would you say your health in general is excellent, very good, good, fair, or poor?”
Table 3.
Prevalence of substance use, based on responses to the interviewer-administered ASSIST, in the 393 participants. Risk categorization is based on standard ASSIST cutoffs.(17)
| Substance | Lifetime use N (%) | Current use N (%) | Low risk N (%) | Moderate risk N (%) | High risk N (%) |
|---|---|---|---|---|---|
| Tobacco | 254 (64.6) | 135 (34.3) | 238 (60.6) | 128 (32.6) | 27 (6.9) |
| Alcohol | 337 (85.8) | 210 (53.4) | 323 (82.2) | 52 (13.2) | 18 (4.6) |
| Any illicit druga | 240 (61.1) | 82 (20.9) | 282 (72.1) | 91 (23.2) | 18 (4.6) |
| Any prescription drugb | 105 (26.7) | 32 (8.1) | 357 (91.1) | 31 (7.9) | 4 (1.0) |
|
| |||||
| Specific drug class | |||||
|
| |||||
| Cannabis | 232 (59.0) | 58 (14.8) | 324 (82.4) | 64 (16.3) | 5 (1.3) |
| Cocainea | 156 (39.7) | 31 (7.9) | 328 (83.7) | 54 (13.8) | 10 (2.6) |
| Hallucinogens | 83 (21.1) | 7 (1.8) | 379 (96.4) | 13 (3.3) | 1 (0.3) |
| Sedatives | 76 (20.1) | 19 (4.8) | 372 (94.7) | 20 (5.1) | 1 (0.3) |
| Heroina | 71 (18.1) | 13 (3.3) | 368 (93.9) | 18 (4.6) | 6 (1.5) |
| Prescription opioids | 47 (12.0) | 14 (3.6) | 377 (95.9) | 13 (3.3) | 3 (0.8) |
| Prescription stimulantsb | 40 (10.2) | 14 (3.6) | 375 (95.7) | 16 (4.1) | 1 (0.3) |
| Methamphetamine | 42 (10.7) | 0 | 388 (98.7) | 5 (1.3) | 0 |
| Inhalants | 39 (9.9) | 3 (0.8) | 384 (97.7) | 9 (2.3) | 0 |
Missing value for 2 participants
Missing value for 1 participant
The median time required to complete the ASSIST was 3.7 minutes (range 0.7–15.4) for the ACASI ASSIST, and 4.4 minutes (range 1.2–19.1) for the IA ASSIST. The majority (85%) of participants said they either preferred the computer to an interviewer, or had no preference. Twenty one (5.3%) participants requested assistance using the ACASI ASSIST, while 47 (12%) requested assistance with the IA ASSIST. For the ACASI ASSIST, 33% of requests were for technical assistance, while the remainder was for difficulty with comprehension of the items or reading. For the IA ASSIST, all requests were for assistance with comprehension, and 47% were to clarify non-medical use of prescription medications.
The ACASI and IA ASSIST results were in agreement for 92–99% of participants with respect to detection of moderate-high risk substance use (Table 4). Where there was lack of concordance between the two measures, more participants reported alcohol and illicit drug use on the ACASI ASSIST, and more participants reported tobacco and prescription drug misuse on the IA ASSIST. Kappa statistics indicated substantial to near-perfect agreement between the ACASI and IA ASSIST instruments.
Table 4.
Concordance of results from the ACASI and IA versions of the ASSIST, for detection of moderate-high risk substance use (N=393).
| Substance Use Variable | Individuals in risk category N (%) | Concordant risk level N (%) | Higher on ACASI N | Lower on ACASI N | Kappa (95% CI) | |
|---|---|---|---|---|---|---|
| ACASI ASSIST | IA ASSIST | |||||
| Tobacco | 367 (93.4) | 11 | 15 | 0.861(0.810–0.912) | ||
| Low risk | 242 (61.6) | 238 (60.6) | ||||
| Mod or high risk | 151 (38.4)) | 155 (39.4) | ||||
| Alcohol | 361 (91.9) | 18 | 14 | 0.728(0.640–0.816) | ||
| Low risk | 319 (81.2) | 323 (82.2) | ||||
| Mod or high risk | 74 (18.8) | 70 (17.8) | ||||
| Illicit Drugs | 364 (92.6) | 21 | 8 | 0.822(0.759–0.885) | ||
| Low risk | 271 (69.0) | 284 (72.3) | ||||
| Mod or high risk | 122 (31.0) | 109 (27.7) | ||||
| Prescription Drugs | 364 (92.6) | 10 | 19 | -- | ||
| Low risk | 367 (93.4) | 358 (91.1) | ||||
| Mod or high risk | 26 (6.6) | 35 (8.9) | ||||
|
| ||||||
| Cannabis | 367 (93.3) | 20 | 6 | 0.7880.710–0.866) | ||
| Low risk | 310 (78.9) | 324 (82.4) | ||||
| Mod or high risk | 83 (21.1) | 69 (17.6) | ||||
| Cocaine | 367 (93.4) | 17 | 9 | 0.769(0.685–0.853) | ||
| Low risk | 321 (81.7) | 321 (81.7) | ||||
| Mod or high risk | 72 (18.3) | 72 (18.3) | ||||
| Heroin | 382 (97.2) | 9 | 2 | -- | ||
| Low risk | 362 (92.1) | 369 (93.9) | ||||
| Mod or high risk | 31 (7.9) | 24 (6.1) | ||||
| Hallucinogens | 380 (96.7) | 9 | 4 | -- | ||
| Low risk | 374 (95.2) | 379 (96.4) | ||||
| Mod or high risk | 19 (4.8) | 14 (3.6) | ||||
| Inhalants | 389 (99.0) | 2 | 2 | -- | ||
| Low risk | 384 (97.7) | 384 (97.7) | ||||
| Mod or high risk | 9 (2.3) | 9 (2.3) | ||||
| Methamphetamine | 388 (98.7) | 3 | 2 | -- | ||
| Low risk | 387 (98.5) | 388 (98.7) | ||||
| Mod or high risk | 6 (1.5) | 5 (1.3) | ||||
| Sedatives | 374 (95.2) | 7 | 12 | -- | ||
| Low risk | 377 (95.9) | 372 (94.7) | ||||
| Mod or high risk | 16 (4.1) | 21 (5.3) | ||||
| Opioids | 380 (96.7) | 6 | 7 | -- | ||
| Low risk | 378 (96.2) | 377 (96.0) | ||||
| Mod or high risk | 15 (3.8) | 16 (4.0) | ||||
| Stimulants | 378 (96.2) | 2 | 13 | -- | ||
| Low risk | 387 (98.5) | 376 (95.7) | ||||
| Mod or high risk | 6 (1.5) | 17 (4.3) | ||||
Correlation of ACASI and IA ASSIST scores was excellent for the global ASSIST score and the substance specific scores for tobacco, alcohol, and the combined class of illicit drugs, and lower for prescription drugs (Table 5). We additionally examined the correlation between global scores when the items were restricted to those included in ASSIST V3.0, and found similar results to those derived using the modified ASSIST: mean score was 31 for the ACASI and 30 for the IA ASSIST; ICC 0.929 (95% CI 0.914 to 0.942).
Table 5.
Correlation between ACASI and IA ASSIST, based on the intraclass correlation coefficient (ICC), N=393.
| Substance Use Variable | ACASI | Interviewer | ICC |
|---|---|---|---|
| Mean score ± SD | Mean score ± SD | ||
| Global ASSIST score | 31.6 ± 32.6 | 31.3 ± 32.7 | 0.937 (.924–.948) |
| Tobacco | 6.5 ± 9.3 | 7.0 ± 9.9 | 0.927 (.912–.940) |
| Alcohol | 6.1 ± 8.3 | 5.8 ± 8.3 | 0.912 (.893–.927) |
| Illicit Drugs | 6.9 ± 13.4 | 6.3 ± 12.8 | 0.854 (.854–.900) |
| Cannabis | 2.6 ± 5.8 | 2.5 ± 5.8 | 0.858 (.829–.882) |
| Cocaine | 2.4 ± 6.2 | 2.2 ± 6.1 | 0.861 (.833–.884) |
| Heroin | 1.0 ± 4.3 | 1.0 ± 4.4 | 0.924 (.908–.937) |
| Hallucinogens | 0.5 ± 2.4 | 0.4 ± 2.2 | 0.748 (.700–.788) |
| Inhalants | 0.2 ± 1.4 | 0.2 ± 1.5 | 0.903 (.883–.920) |
| Methamphetamine | 0.1 ± 0.8 | 0.1 ± 0.9 | 0.461 (.379–.535) |
| Rx Drugs | 3 ± 9 | 2 ± 6 | 0.676 (.613–.729) |
| Sedatives | 0.3 ± 2.3 | 0.6 ± 3.0 | 0.728 (.676–.772) |
| Opioids | 0.4 ± 3.0 | 0.6 ± 3.2 | 0.939 (.926–.950) |
| Stimulants | 0.1 ± 1.0 | 0.4 ± 2.4 | 0.258 (.164–.347) |
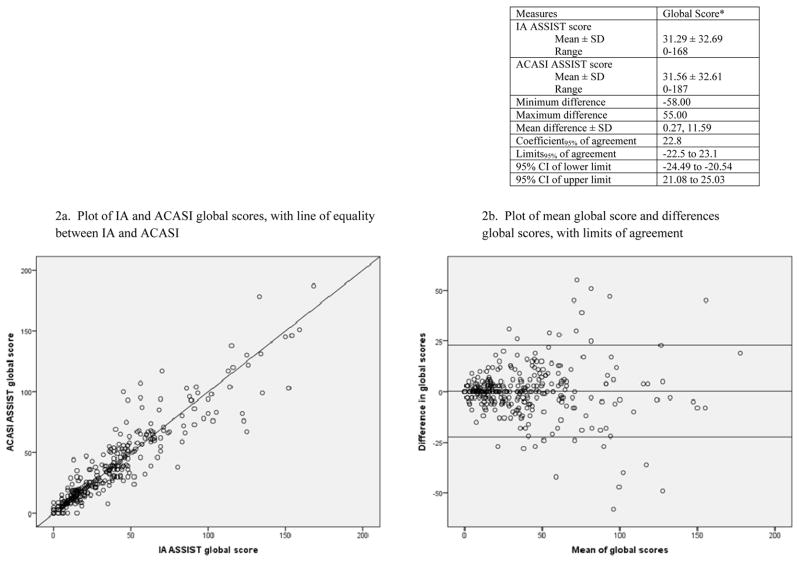
Analysis of agreement using the Bland and Altman approach compared ACASI and IA ASSIST global scores (Figure 2). Twenty-four individuals had scores that fell outside the limits of agreement. Four of them reported illicit drug use on the ACASI but not on the IA ASSIST, reflecting a similar pattern to the concordance results (Table 4), in which reporting of illicit drug use was higher on the ACASI instrument.
Figure 2.
Bland and Altman analysis of differences, for ACASI ASSIST and IA ASSIST global scores.
Among the 331 individuals with completed saliva tests, 19 (5.7%) tested positive for at least one drug. Of those with a positive saliva test, 12 participants reported use on both versions of the ASSIST, and 2 participants reported use on the ACASI ASSIST but not the IA ASSIST. No participants with positive saliva tests reported current drug use on the IA ASSIST but not the ACASI ASSIST. For the 37 participants who participated in hair testing, 5 (14%) tested positive for at least one drug. Among those with a positive hair test, there were no differences in reporting of current drug use on the ACASI versus IA ASSIST. Hair test results were congruent with the ASSIST results in 33 cases (89%). Of those that were incongruent, one test was positive for cocaine and opioids in an individual who reported only current use of opioids on the ASSIST, and 3 tests were positive in individuals who reported no current drug use on the ASSIST.
Discussion
In this large sample of primary care patients, we found high levels of concordance, correlation, and agreement between responses to the ACASI ASSIST and the IA ASSIST. The ACASI ASSIST was well accepted and feasible for self-administration, with just 5% of participants requesting assistance to complete it. The ACASI ASSIST appears to be a valid alternative to the traditional IA ASSIST for identifying moderate-high risk substance use in primary care patients.
Although the overall concordance between the two modalities was high, we observed slightly more reporting of alcohol and illicit drug use on the ACASI ASSIST. This finding is consistent with multiple prior studies showing that self-administered instruments generate higher rates of reporting of stigmatized behaviors.(39, 40, 59)
Limitations
Our study does have limitations. Although our analyses did not reveal an order effect, repeat administration of a similar instrument has the potential to bias responses. Despite the overall high prevalence of substance use in our sample, few participants reported use of certain drug classes queried by the ASSIST, and relatively few had high-risk use of any substance. This limited our ability to draw some comparisons between the ACASI and IA ASSIST versions.
We examined the ACASI ASSIST only in comparison to the IA ASSIST. Supporting this approach is the fact that the IA ASSIST has been previously validated in a large multi-site study, and demonstrated sufficiently high sensitivity and specificity to be considered a reference standard measure.(18) Yet because both the IA and ACASI versions of the ASSIST rely on self-reported information, we are left with some uncertainty about which instrument best captures the truth about participants’ drug use. Self-report measures have consistently shown good accuracy in research,(60–63) but are nonetheless dependent on accurate and truthful disclosure. The biologic tests for drug use generally supported the self-reported responses to the ACASI ASSIST, but are limited by the relatively brief window of detection for saliva tests, the small proportion of participants who could be offered hair testing, and the lack of additional measures of alcohol use.
Generalizability of our findings to other clinical settings is limited by having conducted the study at a single adult primary care clinic site, and restricting eligibility to English speakers under the age of 65 years. The diversity of our sample, which had good representation of individuals with low levels of formal education, can be considered a strength, since a computer self-administered approach may be more challenging in populations with limited literacy and computer skills.(21, 24, 64) However, it is possible that substance use screening using an ACASI approach would be less acceptable in other patient populations, such as highly educated or elderly patients. The feasibility and validity of the ACASI ASSIST in other languages would need to be assessed before non-English versions can be recommended for use in clinical practice.
Conclusion
The ACASI ASSIST can be recommended for use in primary care settings as an alternative to the traditional interviewer-administered instrument. An ACASI ASSIST could be completed prior to the medical visit, either in the waiting area or at home via an internet portal, and have its results incorporated into the electronic health record at the point of care. This approach has the potential to ease barriers to implementation of substance use screening in health care settings.
While the ASSIST instrument combines screening and assessment, in some clinical settings it may be attractive to use the ACASI ASSIST only as an assessment tool, reserved for patients who have positive responses on an initial brief screen. Validated tools exist for accomplishing alcohol and drug screening in as few as two questions.(65–68). However, because as many as one-third of patients may be expected to have a positive screening result (66), an efficient approach to assessment is still essential. The ACASI ASSIST could provide this assessment efficiently and with enough detail and accuracy to guide clinical interventions to address unhealthy substance use in primary care patients.
Footnotes
Declaration of interest: The authors have no conflicts of interest to declare.
References
- 1.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006 Jul;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013 Aug 6;159(3):210–18. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 3.Solberg LI, Maciosek MV, Edwards NM. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am J Prev Med. 2008 Feb;34(2):143–52. doi: 10.1016/j.amepre.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004 Apr 06;140(7):557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 5.Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, et al. Brief Intervention for Problem Drug Use in Safety-Net Primary Care Settings A Randomized Clinical Trial. Jama-J Am Med Assoc. 2014 Aug 6;312(5):492–501. doi: 10.1001/jama.2014.7860. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saitz R, Palfai TPA, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and Brief Intervention for Drug Use in Primary Care The ASPIRE Randomized Clinical Trial. Jama-J Am Med Assoc. 2014 Aug 6;312(5):502–13. doi: 10.1001/jama.2014.7862. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. Journal of addiction medicine. 2010 Sep;4(3):123–30. doi: 10.1097/ADM.0b013e3181db6b67. Epub 2010/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Preventive Services Task Force. Screening for Illicit Drug Use: US Preventive Services Task Force Recommendation Statement. 2008 [cited 2015 March 30]. Available from: http://www.webcitation.org/6WjMUXPGw.
- 9.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009 Apr 28;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 11.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug and alcohol dependence. 2009 Jan 1;99(1–3):280–95. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amico EJ, Paddock SM, Burnam A, Kung F-Y. Identification of and guidance for problem drinking by general medical providers: results from a national survey. Med Care. 2005 Mar;43(3):229–36. doi: 10.1097/00005650-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Friedmann PD, McCullough D, Saitz R. Screening and intervention for illicit drug abuse: a national survey of primary care physicians and psychiatrists. Archives of internal medicine. 2001 Jan 22;161(2):248–51. doi: 10.1001/archinte.161.2.248. [DOI] [PubMed] [Google Scholar]
- 14.Saitz R, Mulvey KP, Plough A, Samet JH. Physician unawareness of serious substance abuse. The American journal of drug and alcohol abuse. 1997 Aug;23(3):343–54. doi: 10.3109/00952999709016881. [DOI] [PubMed] [Google Scholar]
- 15.McNeely JLJD, Grossman E. Other Drug Use. In: Saitz R, editor. Addressing Unhealthy Alcohol Use in Primary Care. New York: Springer; 2013. pp. 171–88. [Google Scholar]
- 16.Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addictive behaviors. 2011 Dec;36(12):1111–9. doi: 10.1016/j.addbeh.2011.07.007. Epub 2011/08/09. eng. [DOI] [PubMed] [Google Scholar]
- 17.Humeniuk R. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and pilot brief intervention: A technical report of phase II findings of the WHO ASSIST Project2008. 2010 Feb 5; Available from: http://www.webcitation.org/6WjNmTi22.
- 18.Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012 May;107(5):957–66. doi: 10.1111/j.1360-0443.2011.03740.x. Epub 2011/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.National Institute on Drug Abuse (NIDA) Screening for Drug Use in Medical Settings. National Institutes of Health; 2010. [cited 2015 March 30]. Available from: http://www.webcitation.org/6WjPYIB76. [Google Scholar]
- 20.Bertollo DN, Alexander MJ, Shinn M, Aybar JB. Innovations: clinical computing: an audio computer-assisted self-interviewing system for research and screening in public mental health settings. Psychiatric services. 2007 Jun;58(6):743–5. doi: 10.1176/ps.2007.58.6.743. [DOI] [PubMed] [Google Scholar]
- 21.Butler SF, Budman SH, Goldman RJ, Newman FL, Beckley KE, Trottier D, et al. Initial validation of a computer-administered Addiction Severity Index: the ASI-MV. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2001 Mar;15(1):4–12. doi: 10.1037/0893-164x.15.1.4. Epub 2001/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 22.Harris SK, Csemy L, Sherritt L, Starostova O, Van Hook S, Johnson J, et al. Computer-facilitated substance use screening and brief advice for teens in primary care: an international trial. Pediatrics. 2012 Jun;129(6):1072–82. doi: 10.1542/peds.2011-1624. Epub 2012/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy MK, Bijur PE, Rosenbloom D, Bernstein SL, Gallagher EJ. Feasibility of a computer-assisted alcohol SBIRT program in an urban emergency department: patient and research staff perspectives. Addiction science & clinical practice. 2013 Jan 16;8(1):2. doi: 10.1186/1940-0640-8-2. Epub 2013/01/18. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satre D, Wolfe W, Eisendrath S, Weisner C. Computerized screening for alcohol and drug use among adults seeking outpatient psychiatric services. Psychiatric services. 2008 Apr;59(4):441–4. doi: 10.1176/appi.ps.59.4.441. Epub 2008/04/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schackman BR, Dastur Z, Rubin DS, Berger J, Camhi E, Netherland J, et al. Feasibility of using audio computer-assisted self-interview (ACASI) screening in routine HIV care. AIDS Care. 2009 Aug;21(8):992–9. doi: 10.1080/09540120802657506. [DOI] [PubMed] [Google Scholar]
- 26.Rogers SM, Willis G, Al-Tayyib A, Villarroel MA, Turner CF, Ganapathi L, et al. Audio computer assisted interviewing to measure HIV risk behaviours in a clinic population. Sexually transmitted infections. 2005 Dec;81(6):501–7. doi: 10.1136/sti.2004.014266. Epub 2005/12/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterling S, Kline-Simon AH, Wibbelsman C, Wong A, Weisner C. Screening for adolescent alcohol and drug use in pediatric health-care settings: predictors and implications for practice and policy. Addiction science & clinical practice. 2012;7(1):13. doi: 10.1186/1940-0640-7-13. Epub 2012/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CASA. Missed Opportunity: National Survey of Primary Care Physicians and Patients on Substance Abuse. New York: The National Center on Addiction and Substance Abuse at Columbia University; 2000. [Google Scholar]
- 29.Anderson P. Overview of interventions to enhance primary-care provider management of patients with substance-use disorders. Drug and alcohol review. 2009 Sep;28(5):567–74. doi: 10.1111/j.1465-3362.2009.00113.x. Epub 2009/09/10. eng. [DOI] [PubMed] [Google Scholar]
- 30.Friedmann PD, McCullough D, Chin MH, Saitz R. Screening and intervention for alcohol problems. A national survey of primary care physicians and psychiatrists. J Gen Intern Med. 2000 Feb;15(2):84–91. doi: 10.1046/j.1525-1497.2000.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick KA, Cochran NE, Back AL, Merrill JO, Williams EC, Bradley KA. How primary care providers talk to patients about alcohol: a qualitative study. J Gen Intern Med. 2006 Sep;21(9):966–72. doi: 10.1111/j.1525-1497.2006.00490.x. Epub 2006/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spandorfer JM, Israel Y, Turner BJ. Primary care physicians’ views on screening and management of alcohol abuse: inconsistencies with national guidelines. The Journal of family practice. 1999 Nov;48(11):899–902. [PubMed] [Google Scholar]
- 33.Anderson P, Laurant M, Kaner E, Wensing M, Grol R. Engaging general practitioners in the management of hazardous and harmful alcohol consumption: results of a meta-analysis. J Stud Alcohol. 2004 Mar;65(2):191–9. doi: 10.15288/jsa.2004.65.191. [DOI] [PubMed] [Google Scholar]
- 34.Johnson M, Jackson R, Guillaume L, Meier P, Goyder E. Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health (Oxf) 2011 Sep;33(3):412–21. doi: 10.1093/pubmed/fdq095. [DOI] [PubMed] [Google Scholar]
- 35.Aira M, Kauhanen J, Larivaara P, Rautio P. Factors influencing inquiry about patients’ alcohol consumption by primary health care physicians: qualitative semi-structured interview study. Fam Pract. 2003 Jun;20(3):270–5. doi: 10.1093/fampra/cmg307. Epub 2003/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 36.Yoast RA, Wilford BB, Hayashi SW. Encouraging physicians to screen for and intervene in substance use disorders: obstacles and strategies for change. J Addict Dis. 2008;27(3):77–97. doi: 10.1080/10550880802122687. [DOI] [PubMed] [Google Scholar]
- 37.Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, et al. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med. 2011 Mar;26(3):299–306. doi: 10.1007/s11606-010-1509-4. Epub 2010/09/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, et al. Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study. J Gen Intern Med. 2015 Mar 3; doi: 10.1007/s11606-015-3248-z. Epub 2015/03/04. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourangeau R, Smith TW. Asking sensitive questions - The impact of data collection mode, question format, and question context. Public Opin Quart. 1996 Sum;60(2):275–304. [Google Scholar]
- 40.Wight RG, Rotheram-Borus MJ, Klosinski L, Ramos B, Calabro M, Smith R. Screening for transmission behaviors among HIV-infected adults. Aids Educ Prev. 2000 Oct;12(5):431–41. English. [PubMed] [Google Scholar]
- 41.Maister DH. The psychology of waiting lines: Harvard Business School. 1984. [Google Scholar]
- 42.Meyer C. While customers wait, add value. Harvard business review. 2001 Jul-Aug;79(7):24, 6. Epub 2001/07/13. eng. [PubMed] [Google Scholar]
- 43.McNeely J, Strauss SM, Wright S, Rotrosen J, Khan R, Lee JD, et al. Test-retest reliability of a self-administered Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary care patients. Journal of substance abuse treatment. 2014 Feb 10;10(14):00025–7. doi: 10.1016/j.jsat.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JD, Delbanco B, Wu E, Gourevitch MN. Substance use prevalence and screening instrument comparisons in urban primary care. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2011 Jul;32(3):128–34. doi: 10.1080/08897077.2011.562732. Epub 2011/06/11. eng. [DOI] [PubMed] [Google Scholar]
- 45.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-46, HHS Publication No.(SMA) 13–4795. [Google Scholar]
- 46.Humeniuk R. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): manual for use in primary care. Geneva: World Health Organization; 2010. [Google Scholar]
- 47.ASSIST Train the Trainer Online Course. World Health Organization Collaborating Centre for Research in the Treatment of Drug and Alcohol Problems; [cited 2014 August 21, 2014]. Available from: http://www.webcitation.org/6Wf0lDPzM. [Google Scholar]
- 48.McNeely J, Halkitis PN, Horton A, Khan R, Gourevitch MN. How patients understand the term “nonmedical use” of prescription drugs: insights from cognitive interviews. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2014;35(1):12–20. doi: 10.1080/08897077.2013.789463. Epub 2014/03/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear SE, Shedlin M, Gilberti B, Fiellin M, McNeely J. Feasibility and Acceptability of an Audio Computer-Assisted Self-Interview Version of the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) in Primary Care Patients. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2015 Jul;9:0. doi: 10.1080/08897077.2015.1062460. Epub 2015/07/15. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008 Apr;10(4):607–12. doi: 10.1080/14622200801978680. Epub 2008/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 51.Heltsley R, DePriest A, Black DL, Robert T, Marshall L, Meadors VM, et al. Oral fluid drug testing of chronic pain patients. I. Positive prevalence rates of licit and illicit drugs. Journal of analytical toxicology. 2011 Oct;35(8):529–40. doi: 10.1093/anatox/35.8.529. Epub 2011/10/19. eng. [DOI] [PubMed] [Google Scholar]
- 52.Cone EJ, Presley L, Lehrer M, Seiter W, Smith M, Kardos KW, et al. Oral fluid testing for drugs of abuse: positive prevalence rates by Intercept immunoassay screening and GC-MS-MS confirmation and suggested cutoff concentrations. Journal of analytical toxicology. 2002 Nov-Dec;26(8):541–6. doi: 10.1093/jat/26.8.541. Epub 2002/12/28. eng. [DOI] [PubMed] [Google Scholar]
- 53.Spitznagel EL, Helzer JE. A Proposed Solution to the Base Rate Problem in the Kappa-Statistic. Archives of general psychiatry. 1985;42(7):725–8. doi: 10.1001/archpsyc.1985.01790300093012. English. [DOI] [PubMed] [Google Scholar]
- 54.Thompson WD, Walter SD. A reappraisal of the kappa coefficient. Journal of clinical epidemiology. 1988;41(10):949–58. doi: 10.1016/0895-4356(88)90031-5. Epub 1988/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 55.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 56.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6(4):284–90. [Google Scholar]
- 57.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–10. Epub 1986/02/08. eng. [PubMed] [Google Scholar]
- 58.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical methods in medical research. 1999 Jun;8(2):135–60. doi: 10.1177/096228029900800204. Epub 1999/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Dubowitz H, Hudson-Martin E, Lane W. Comparison of 3 data collection methods for gathering sensitive and less sensitive information. Ambul Pediatr. 2008 Jul-Aug;8(4):255–60. doi: 10.1016/j.ambp.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 60.Babor TFBJ, Del Boca FK. Validity of self-reports in applied research on addictive behaviors: fact or fiction? Behavioral Assessment. 1990;(12):5–31. [Google Scholar]
- 61.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003 Dec;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 62.Hser YI. Self-reported drug use: results of selected empirical investigations of validity. NIDA Res Monogr. 1997;167:320–43. [PubMed] [Google Scholar]
- 63.Secades-Villa R, Fernandez-Hermida JR. The validity of self-reports in a follow-up study with drug addicts. Addict Behav. 2003 Aug;28(6):1175–82. doi: 10.1016/s0306-4603(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 64.Reichmann WM, Losina E, Seage GR, Arbelaez C, Safren SA, Katz JN, et al. Does modality of survey administration impact data quality: audio computer assisted self interview (ACASI) versus self-administered pen and paper? PloS one. 2010;5(1) doi: 10.1371/journal.pone.0008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009 Jul;24(7):783–8. doi: 10.1007/s11606-009-0928-6. Epub 2009/02/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Archives of internal medicine. 2010 Jul 12;170(13):1155–60. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNeely J, Cleland CM, Strauss SM, Palamar JJ, Rotrosen J, Saitz R. Validation of Self-Administered Single-Item Screening Questions (SISQs) for Unhealthy Alcohol and Drug Use in Primary Care Patients. J Gen Intern Med. 2015 May 19; doi: 10.1007/s11606-015-3391-6. Epub 2015/05/20. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNeely J, Strauss SM, Saitz R, Cleland CM, Palamar JJ, Rotrosen J, et al. A brief patient self-administered substance use screening tool for primary care: two-site validation study of the Substance Use Brief Screen (SUBS) The American journal of medicine. 2015 Mar 10; doi: 10.1016/j.amjmed.2015.02.007. Epub 2015/03/15. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]