Abstract
Both positive and negative regulatory roles have been suggested for the B7 family member PD-L1(B7-H1). PD-L1 is expressed on antigen-presenting cells (APCs), activated T cells, and a variety of tissues, but the functional significance of PD-L1 on each cell type is not yet clear. To dissect the functions of PD-L1 in vivo, we generated PD-L1-deficient (PD-L1–/–) mice. CD4+ and CD8+ T cell responses were markedly enhanced in PD-L1–/– mice compared with wild-type mice in vitro and in vivo. PD-L1–/– dendritic cells stimulated greater wild-type CD4+ T cell responses than wild-type dendritic cells, and PD-L1–/– CD4+ T cells produced more cytokines than wild-type CD4+ T cells in vitro, demonstrating an inhibitory role for PD-L1 on APCs and T cells. In vivo CD8+ T cell responses also were significantly enhanced, indicating that PD-L1 has a role in limiting the expansion or survival of CD8+ T cells. Studies using the myelin oligodendrocyte model of experimental autoimmune encephalomyelitis showed that PD-L1 on T cells and in host tissues limits responses of self-reactive CD4+ T cells in vivo. PD-L1 deficiency converted the 129S4/SvJae strain from a resistant to experimental autoimmune encephalomyelitis-susceptible strain. Transfer of encephalitogenic T cells from wild-type mice into PD-L1–/– recipients led to exacerbated disease. Disease was even more severe in PD-L1–/– recipients of PD-L1–/– T cells. These results demonstrate that PD-L1 on T cells, APCs, and host tissue inhibits naïve and effector T cell responses and plays a critical role in T cell tolerance.
PD-L1 (B7-H1) is a ligand for programmed cell death-1 (PD-1) and does not bind to other CD28 family members (1). PD-1 is expressed on activated but not resting CD4+ and CD8+ T, B, and myeloid cells (2, 3). PD-1–/– mice develop an autoimmune-like phenotype, which is delayed in onset as compared with CTLA-4–/– mice (4, 5). This phenotype demonstrates an important negative regulatory role for PD-1 and suggests a role for PD-1 in regulating B and/or T cell tolerance.
PD-L1 is expressed on resting and up-regulated on activated B, T, myeloid, and dendritic cells (DCs) (1, 6–9). In contrast to B7–1 and B7–2, PD-L1 also is expressed in nonhematopoietic cells (e.g., microvascular endothelial cells), in nonlymphoid organs (e.g., heart and placenta), and in a variety of tumors (6, 8, 10–12). The expression of PD-L1 within nonlymphoid tissues suggests that PD-L1 may regulate self-reactive T or B cells in peripheral tissues and/or may regulate inflammatory responses in the target organs. However, the roles of PD-L1 on T cells, antigen-presenting cells (APCs), and host tissues are not yet clear. Many potential bidirectional interactions occur between PD-L1 and PD-1 because of the broad expression of PD-L1 and the expression of PD-1 on T cells, B cells, and macrophages. Recent studies using anti-PD-L1 mAbs in vivo have suggested a role for PD-L1 in regulating autoimmune diseases (13, 14). However, these studies could not distinguish the importance of PD-L1 expression on T cells, APCs, and host cells. The function of PD-L1 is also unclear because of conflicting results, with some studies suggesting a stimulatory role and others an inhibitory role (1, 6, 11). To determine the obligatory functions of PD-L1 in vivo, we generated PD-L1-deficient (PD-L1–/–) mice. Our results indicate that PD-L1 in the T cell, APC, and host tissue plays a critical role in negatively regulating T cell responses.
Materials and Methods
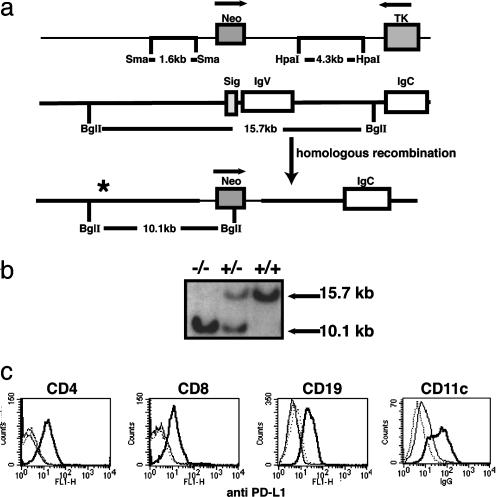
PD-L1–/– Mice. Two targeting vectors were constructed. The first replaced the signal and all of the IgV exon of the PD-L1 gene and was electroporated into a C57BL/6 ES cell line (Fig. 1a). The second replaced the signal and two-thirds of the IgV exon of the PD-L1 gene and was electroporated into the J1 129S4/SvJae (129Sv) ES cell line (Fig. 6, which is published as supporting information on the PNAS web site). Homologous recombinants were identified by Southern blot analysis (Fig. 1b). The genomic probe used for Southern blotting was external to the genomic DNA used in targeting vectors and was generated by PCR with the following primers: 5′-GAGGAGATGGGTGTTGCTAGGAGT-3′, 5′-GAGGAGATGGGTGTTGCTAGGAGT-3′, and 5′-CCCTAGAGACCCCATCTTAATCTACCCTAGAGACCCCATCTTAATCTA-3′. BglI digest yields a 15.7-kb band in wild type and a 10.1-kb band in the targeted allele on C57BL/6 background and a 11.1-kb band in the targeted allele and a 15.7-kb band in wild type on the129Sv background. Three C57BL/6 and 129S4/SvJae ES clones with the PD-L1 homologous recombinant event were microinjected into blastocysts and gave rise to germ-line transmission of the PD-L1 mutation. Mice heterozygous for the PD-L1 mutation were interbred. Genotyping of the mice was performed by PCR. PD-L1 primers to detect the IgV region deleted in PD-L1–/– mice were 5′-CTAACAGGTGATCCGTTTCCTATG-3′ and 5′-GCCGTGATAGTAAACGCTGAA-3′. Sequences of the Neo primers were 5′-ATTGAACAAGATGGATTGCAC-3′ and 5′-CGTCCAGATCATCCTGATC-3′. PCR products were 305 bp and 474 bp for PD-L1 and Neo, respectively. Mice were used on the C57BL/6 background unless otherwise stated. All animals were maintained in the animal facility of Brigham and Women's Hospital under virus Ab-free conditions in accordance with institutional and Institutional Animal Care and Use Committee (www.iacuc.org) standards.
Fig. 1.
Generation of PD-L1–/– mice. (a) The structure of the PD-L1-targeting vector is shown (Top), and Neo replaced the signal exon and the IgV region (C57BL/6 construct). (Middle) The genomic organization of the PD-L1 gene (not to scale). Exons are open boxes. Homologous recombination of the PD-L1 gene is represented (Bottom). *, the position of the probe. (b) Southern blot analysis of PD-L1–/– mice. Wild-type DNA yields a 15.7-kb band and the targeted allele yields a 10.1-kb band. (c) Splenocytes were activated for 24 h with anti-CD3 (1 μg/ml) for T cells or LPS (10 μg/ml) for B cells. Flt-3-expanded DCs were isolated and matured overnight on plastic. Cells were stained with anti-PD-L1 FITC and relevant lineage-specific mAb-PE. Graphs are gated on CD4+, CD8+, CD19+, or CD11c+ cells as indicated. PD-L1+/+ mice (thick solid line), PD-L1–/– mice (dotted line), and isotype control (thin solid line).
PD-L1 Expression. PD-L1 expression was detected by flow cytometry. Splenocytes (2 × 106) were activated for 24 h with 1 μg/ml anti-CD3 clone 145–2C11 (Bio Express, West Lebanon, NH) or 10 μg/ml lipopolysaccharide (LPS) (Sigma). Cells activated with anti-CD3 were double-stained with anti-CD4-PE or anti-CD8-PE and a mAb against PD-L1 conjugated to FITC (clone 10F.9G2) (10). LPS-activated blasts were stained with anti-CD19-PE and anti-PD-L1-FITC. Flt-3 ligand-expanded DCs were cultured overnight and double-stained with CD11c-PE and PD-L1 FITC. Ten thousand events were analyzed on a FACS-Calibur (Becton Dickinson). All Abs were obtained from BD Pharmingen unless otherwise stated.
In Vitro Assays. CD4+ or CD8+ T cells were purified (>99%) from mice by positive selection with magnetic-activated cell-sorting separation (MACS) columns (Miltenyi Biotec, Auburn, CA) and stimulated with anti-CD28 (1 μg/ml) and various concentrations of anti-CD3 for 3 days. To assay proliferation, cultures were pulsed with 1 μCi (1 Ci = 37 GBq) per well of [3H]thymidine (New England Nuclear) for the last6hofthe 72-h incubation period. For mixed lymphocyte reaction cultures, mice were injected with Flt-3 ligand (20 μg) every other day for 10 days (15). DCs were purified by positive-selection MACS columns (Miltenyi Biotec) and matured overnight by adherence to plastic. BALB/c CD4+ T cells were purified as described above and cultured with varying concentrations of γ-irradiated (3,300 rads) DCs. Proliferation was assayed as described above for 3 days. Aliquots of supernatants were harvested at various times after initiation of cultures. IL-2, IL-4, IFN-γ, and IL-10 levels were analyzed by ELISA with mAbs and recombinant cytokine standards from BD Pharmingen. Detection limits were: IL-2, 20 pg/ml; IL-4, 40 pg/ml; IFN-γ, 100 pg/ml; and IL-10, 200 pg/ml.
In Vivo CD8+ T Cell Responses. C57BL/6 PD-L1+/+ or PD-L1–/– mice were immunized in the hind footpads with 100 μg of ovalbumin (OVA; Sigma) in complete Freund's adjuvant (CFA; Sigma). Ten days later, draining lymph node (LN) cells were restimulated with EL4-OVA or EL4 cells for 3 days and then double-stained with anti CD8-FITC and Kb-SIINFEKL tetramer-PE (Trudeau Institute, Saranac Lake, NY). To assay IFN-γ production, CD8+ T cells were purified from LN cells by magnetic-activated cell-sorting separation columns and 1 × 105 cells were cultured with 5 × 104 γ-irradiated (20,000 rads) EL4-OVA or EL4 cells in 24-well plates. IFN-γ was assayed at days 1, 2, and 3 as described above.
For cytotoxic T lymphocyte assay, mice were immunized as described above, and 10 days later, spleens were removed and splenocytes (30 × 106) were cultured with 2 × 106 EL4-OVA for 4 days (16). EL4 target cells were prepared by incubation with 200 μCi of 51Cr (New England Nuclear) and SIINFEKL peptide (15 μg/ml) (Biosource International, Camarillo, CA) for1hand washed. Effector cells were recovered from splenocyte cultures and plated with SIINFEKL-pulsed 51Cr-labeled EL4 cells as targets (1 × 104) in 150 μl for 5 h. Where indicated, the number of CD8+ T cells was normalized according to SIINFEKL tetramer staining, and 100 μl of supernatant was removed and counted in a γ-counter (Pharmacia). 51Cr-labeled EL4 cells without peptide were used as a control for nonspecific lysis. Maximum release or spontaneous release was measured by incubating EL4 with 2% Triton X-100 (Sigma) or medium alone, respectively. Percent specific lysis was calculated as (mean sample cpm – mean spontaneous release)/(mean total cpm – mean spontaneous release) × 100.
Experimental Autoimmune Encephalomyelitis (EAE). EAE induction and adoptive transfer studies were performed as described (17) with 8-week-old 129Sv mice, four to five mice per group. The mice were clinically scored daily: 0, no disease; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind and forelimb paralysis; 5, moribund state. Proliferation and cytokine production by myelin oligodendrocyte glycoprotein peptide (MOG33–55)-specific T cells were analyzed as described (17). Brains and spinal cords were removed and fixed in 10% formalin. Paraffin-embedded sections were stained with Luxol fast blue-hematoxylin/eosin for light microscopy. Inflammatory foci were counted in the meninges and parenchyma by an unbiased observer (17). For enzyme-linked immunospot (ELISPOT) analysis, mice were immunized with MOG33–55 in CFA, and draining LN cells were restimulated with 100 μg of MOG33–55 for 24 h. Cells were transferred to anti-IFN-γ Ab (BD Pharmingen)-coated ELISPOT plates (Millipore) for 18 h. IFN-γ-producing cells were detected with biotinylated anti-IFN-γ Ab (BD Pharmingen) followed by streptavidin–alkaline phosphatase (Sigma). Spots were visualized by using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Sigma) and enumerated by light microscopy.
Statistics. The Student t test (t test) was used to compare wild-type and knockout groups except in the adoptive transfer studies where a linear regression analysis was performed. The Fisher exact test (F test) was used to compare the significance between the regression coefficients of the various adoptively transferred groups.
Results
PD-L1 Deficiency on the T Cell or the APC Leads to Enhanced IFN-γ Production by CD4+ T Cells in Vitro. To evaluate the functional role of PD-L1 in vivo, we generated mice lacking PD-L1. The PD-L1-targeting vector replaced the PD-L1 signal exon and the Ig-V-like exon with the neomycin drug resistance gene, which should eliminate PD-1 binding to PD-L1 (18) (Fig. 1a). Southern blot analysis confirmed homozygosity for the PD-L1 mutation (Fig. 1b). Because wild-type resting B, T, macrophages, and DCs express low levels of PD-L1, activated cells were evaluated for PD-L1 expression. Activated wild type, but not PD-L1–/– B cells, T cells, macrophages, or DCs, expressed PD-L1 (Fig. 1c). PD-L1–/– mice are viable, born at the expected frequency, and appear normal grossly and histologically. Wild-type and PD-L1–/– mice have comparable numbers of thymocytes, CD4+, CD8+, B220+, Mac1+, DX5+, and regulatory T (CD4+CD25hiCD45RBlo) cells. There was no evidence of spontaneous activation of PD-L1–/– T cells or B cells, as judged by CD62L, CD69, and CD25 expression in 4- to 16-week-old wild-type and PD-L1–/– mice. In addition, the expression of CD80 and CD86 was comparable on wild-type and PD-L1–/– APCs (data not shown).
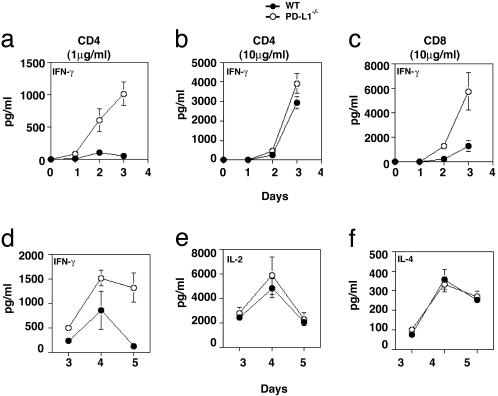
PD-L1 is expressed on T cells and APCs, but the functional significance of PD-L1 on individual cell types is not yet known. To examine the function of PD-L1 on T cells, purified CD4+ and CD8+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28. PD-L1–/– CD4+ T cells produced elevated amounts of IFN-γ as compared with wild-type T cells at a low anti-CD3 concentration (1 μg/ml) (Fig. 2a). However, at a higher anti-CD3 concentration (10 μg/ml) a significant difference did not occur in IFN-γ production (Fig. 2b), consistent with previous data demonstrating that the inhibitory effect of the PD-L:PD-1 pathway on CD4+ T cells was greatest at lower antigen concentrations (11). IFN-γ production by purified PD-L1–/– CD8+ T cells increased significantly as compared with wild-type CD8+ T cells stimulated with anti-CD3 at 10 μg/ml (Fig. 2c). IFN-γ production by PD-L1–/– or wild-type CD8+ T cells was not detected at lower anti-CD3 concentrations. No difference occurred between wild-type and PD-L1–/– CD4+ or CD8+ T cell proliferation (data not shown). Similarly, when purified CD8+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 in the presence of a blocking anti-PD-L1 mAb, IFN-γ production was increased (Fig. 7, which is published as supporting information on the PNAS web site). Because these cultures contain T cells in the absence of APC, these data indicate that, in a T/T interaction, PD-L1 expression on both CD4+ and CD8+ T cells can negatively regulate IFN-γ production.
Fig. 2.
PD-L1 deficiency on the T cell and the APC enhanced IFN-γ production by T cells. To evaluate the role of PD-L1 on T cells, purified CD4+ T cells were stimulated with 1 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 (a) and 10 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 (b), and IFN-γ production was analyzed. (c) Purified CD8+ T cells were stimulated as in b, and IFN-γ production was analyzed. To examine the role of PD-L1 on the APC, C57BL/6 PD-L1+/+ or PD-L1–/– DC were compared as stimulators in a mixed lymphocyte reaction with BALB/c CD4+ T cells. DCs were expanded in vivo by Flt-3 ligand, isolated, matured overnight on plastic, irradiated, and cultured with BALB/c CD4+ T cells. IFN-γ (d), IL-2 (e), and IL-4 (f) were assayed by ELISA. These data are representative of three to six independent experiments.
To analyze the role of PD-L1 on DCs, T cell responses to wild-type and PD-L1–/– mature DCs were compared in an allogeneic mixed lymphocyte reaction. Purified BALB/c CD4+ T cells were cultured with irradiated mature dendritic cells from wild-type or PD-L1–/– C57BL/6 mice, and proliferation and cytokine production were measured. BALB/c CD4+ T cells cultured in the presence of C57BL/6 PD-L1–/– DC as stimulators produced markedly increased levels of IFN-γ compared with wild-type C57BL/6 DC stimulators (Fig. 2d), but no differences occurred in IL-2 or IL-4 production (Fig. 2 e and f) or proliferation (data not shown). In additional studies, PD-L1–/– B cell function was examined. No difference occurred between wild-type and PD-L1–/– splenic B cell proliferation to anti-CD40, anti-IgM, or LPS (data not shown). These studies indicate that PD-L1 on both the T cell and DC can regulate activation of naïve T cells, but that PD-L1 does not have an essential role in regulating activation of naïve B cells.
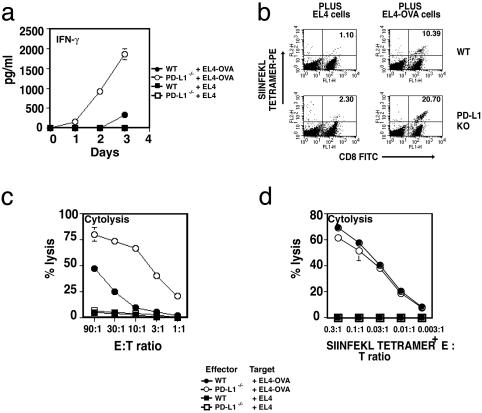
Augmented CD8+ T Cell Responses in PD-L1–/– Mice in Vivo. The severe graft-versus-host-like disease that develops in 2C TCR transgenic PD-1–/– mice, together with the inhibitory effects of transfectants expressing PD-L1 and MHC class I on 2C TCR CD8+ T cell responses, suggests that PD-L1 has an important role in regulating CD8+ T cell responses (4, 19). To examine the obligatory function of PD-L1 for CD8+ T cell responses in vivo, we evaluated CD8+ T cell expansion and cytolytic T lymphocyte activity in PD-L1–/– mice. We immunized PD-L1–/– mice and wild-type mice with OVA in CFA and, 10 days later, isolated CD8+ T cells from LN cells. At day 3, after restimulation with EL4 cells transfected with the OVA gene (EL4-OVA), purified primed CD8+ T cells from PD-L1–/– mice produced five to ten times more IFN-γ (Fig. 3a). Neither wild-type nor PD-L1–/–
Fig. 3.
Augmented CD8+ T cell clonal expansion and cytotoxic T lymphocyte responses in PD-L1–/– mice. (a) Mice were immunized with OVA in CFA, and 10 days later CD8+ T cells from LN cells were purified and restimulated with EL4-OVA or EL4 cells, and IFN-γ production assayed at days 1, 2, and 3. (b) LN cells were restimulated with EL4-OVA or EL4 cells and stained with CD8-FITC and Kb SIINFEKL tetramer-PE. Numbers in the upper right corner represent percent of CD8+ that was tetramer-positive. (c) Mice were immunized with OVA in CFA, and 10 days later splenocytes were restimulated with EL4-OVA cells for 5 days. Effector cells were recovered and plated with 51Cr-labeled SIINFEKL-pulsed EL4 at the indicated effector/target ratios. (d) Experiments were set up as in c, except CD8+ T cell numbers were normalized according to Kb SIINFEKL tetramer staining. These data are representative of four independent experiments.
CD8+ T cells produced detectable amounts of IFN-γ when stimulated with EL4 cells that did not express OVA. After immunization of PD-L1–/– mice and wild-type mice with OVA and restimulation of LN cells with EL4-OVA or EL4, the expansion of SIINFEKL-specific CD8+ T cells was enumerated with a MHC class I-Kb/SIINFEKL tetramer. The expansion of antigen-specific CD8+ T cells in PD-L1–/– mice was increased compared with wild-type mice (Fig. 3b). In four independent experiments, SIINFEKL-specific CD8+ T cells increased significantly in PD-L1–/– compared with wild-type mice (11.1% ± 6.5 in PD-L1–/– mice vs. 5.1% ± 3.5 in wild-type mice; P = 0.05) (Fig. 8, which is published as supporting information on the PNAS web site). Furthermore, a profound increase occurred in killing of SIINFEKL-pulsed target cells by PD-L1–/– CD8+ T cells in contrast to wild-type controls (Fig. 3c). This increase was specific for SIINFEKL, because little or no nonspecific killing of EL4 cells took place by either PD-L1–/– or wild-type control CD8+ T cells. To distinguish whether the increased cytolysis was due to increased cell number in vivo or increased effector function, CD8+ T cells were normalized according to the antigen-specific (SIINFEKL tetramer) staining. As seen in Fig. 3d when equal numbers of wild-type and PD-L1–/– SIINFEKL-tetramer+ CD8+ T cells were used, no difference in the killing of SIINFEKL-pulsed target cells occurred (Fig. 3d). These results suggest that PD-L1 has an important role in limiting the expansion and/or survival of CD8+ T cells but does not increase the cytolytic activity of the T cell.
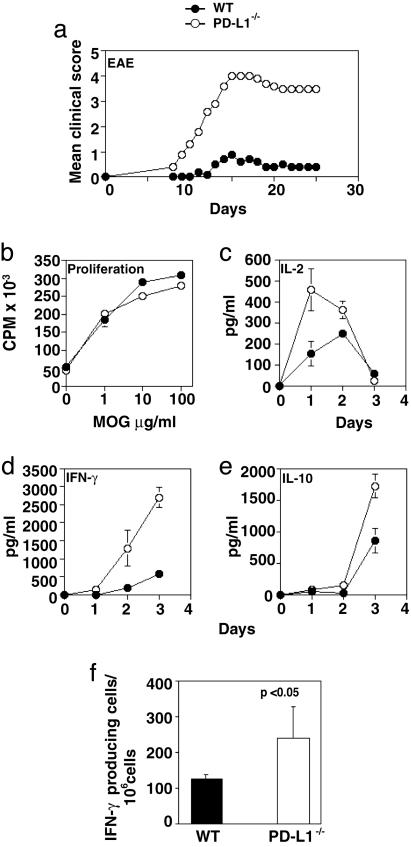
129S4/SvJae PD-L1–/– Mice Become Susceptible to EAE Induction. A role for PD-L1 in regulating self-reactive T cells is beginning to be appreciated (13, 14). PD-L1 could potentially regulate the initial activation of T cells within peripheral lymphoid tissues and/or the reactivation of self-reactive T cells within the target organ. To dissect the relative importance of PD-L1 during the induction and effector phases of autoimmune disease, we investigated EAE in PD-L1–/– mice. EAE, a T cell-mediated inflammatory demyelinating disease, shares many clinical and histological features with multiple sclerosis and is induced by myelin-reactive CD4+ Th1 cells (20). 129S4/SvJae (129Sv) mice are relatively resistant to development of EAE after immunization with MOG35–55. 129Sv wild-type control mice developed a mild and transient disease, consistent with the EAE-resistant 129Sv background. In marked contrast, 129Sv PD-L1–/– mice developed severe clinical EAE with early onset and rapid progression (Fig. 4a and Table 1). PD-L1–/– mice have more inflammatory lesions in the brain and spinal cord than wild-type mice with a significant increase in parenchymal inflammatory foci (Table 1). In three independent experiments, 7 of 14 PD-L1–/– mice died of disease, whereas only 1 of 14 wild-type controls died. On the susceptible C57BL/6 background, PD-L1–/– mice also developed more severe EAE than wild-type controls (Fig. 9, which is published as supporting information on the PNAS web site). Administration of a PD-L1-blocking mAb (10F.9G2) to wild-type C57BL/6 mice during the induction of EAE also led to the rapid onset of severe clinical disease (data not shown).
Fig. 4.
Increased susceptibility to EAE in PD-L1–/– mice. (a) 129Sv PD-L1–/– (○) and PD-L1+/+ (•) mice were immunized with MOG33–55 and mice were scored daily. (b–f) To assess MOG-specific responses, mice were immunized with MOG33–55, and 10 days later draining LN cells were harvested and restimulated with MOG33–55. (b) Proliferation was measured at day 2, and IL-2 (c), IFN-γ (d), and IL-10 (e) were assayed from days 0 to 4 by ELISA. To determine the number of antigen-specific IFN-γ producing cells, draining LN cells were restimulated with MOG33–55 for 24 h, and (f) enzyme-linked immunospot assays were performed. These data are representative of three to four independent experiments.
Table 1. EAE induction by immunization with MOG33–55 peptide in 129Sv PD-L1-deficient mice.
| Clinical EAE*
|
Histological EAE†
|
||||||
|---|---|---|---|---|---|---|---|
| Mice | Incidence | Mortality | Day of onset‡ | Mean maximal score‡ | Meningeal foci‡ | Parenchymal foci‡ | Total foci‡ |
| WT | 10/13 (77%) | 1/14 (7%) | 12 ± 0.6 | 1.6 ± 0.39 | 49.8 ± 13.5 | 29.8 ± 9.0 | 79.6 ± 21.0 |
| PD-L1-/- | 13/14 (92.8%) | 7/14 (50%) | 10.5 ± 0.4 | 3.7 ± 0.52 | 75.8 ± 15.0 | 65.7 ± 15.0 | 141.6 ± 26.2 |
| P < 0.001§ | P = 0.02§ | P = 0.02§ | |||||
Mice were immunized with MOG33-55 peptide and scored daily for 25 days.
Mice were immunized with MOG33-55 peptide and killed at day 11 for histology.
Data represent mean ± SE.
P values are compared with wild type (Student's t test).
To investigate T cell responses, we immunized mice with MOG35–55, restimulated draining LN cells with MOG35–55 in vitro, and measured T cell proliferation and cytokine production. Draining LN cells were obtained 10 days after immunization. Although the proliferative response to MOG35–55 was comparable between PD-L1–/– and wild-type cells (Fig. 4b), MOG-specific PD-L1–/– LN cells rapidly produced markedly increased levels of IL-2 and IFN-γ (Fig. 4 c and d). In three independent experiments, PD-L1–/– mice had two to three times the number of IFN-γ-producing cells per 106 cells compared with wild-type controls (P < 0.05) (Fig. 4f). Increased levels of IFN-γ may contribute to exacerbated disease in the PD-L1–/– mice. On day 3 after restimulation, PD-L1–/– LN cells produced relatively higher levels of IL-10 than wild-type LN cells (Fig. 4e). The elevated IL-10 production in PD-L1–/– mice may represent a counterregulatory response to the high levels of IFN-γ production. These data suggest that PD-L1 serves to limit the number of myelin-reactive IFN-γ-producing CD4+ T cells in EAE.
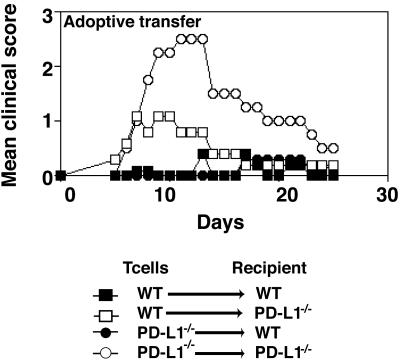
PD-L1–/– Mice Develop Severe EAE After Adoptive Transfer of MOG-Specific T Cells. To evaluate whether PD-L1 has a role on the T cell or in the recipient during the effector phase of EAE, 129Sv wild-type or PD-L1–/– MOG35–55-specific T cells were transferred into wild-type or PD-L1–/– 129Sv recipients. Wild-type MOG35–55-specific cells adoptively transferred into wild-type recipients induced a very mild and transient clinical disease (Fig. 5). In contrast, wild-type MOG35–55-specific T cells transferred into PD-L1–/– recipients induced more severe clinical disease, indicating a role for PD-L1 in the recipient. The transfer of PD-L1–/– T cells into wild-type recipients led to mild clinical disease, similar to that observed in the group receiving wild-type T cells. However, when PD-L1–/– T cells were adoptively transferred to PD-L1–/– recipients, a rapid onset of severe EAE occurred (F test P = 0.01) (Fig. 5). These findings indicate that PD-L1 on effector T cells and on cells in the recipient (which could include APC and vascular endothelial cells in the CNS) limits tissue injury and disease progression.
Fig. 5.
Effector phase of EAE is exacerbated in the absence of PD-L1. Mice were immunized with MOG33–55. LN cells were harvested 10 days later, restimulated with MOG33–55 for 4 days, and transferred into PD-L1+/+ or PD-L1–/– recipients as indicated. These data are representative of four independent experiments.
Discussion
The phenotype of the PD-L1–/– mouse demonstrates that PD-L1 has a critical negative regulatory role in vivo. The profound increase in IFN-γ production by CD8+ T cells in PD-L1–/– mice illustrates the down-regulatory role of PD-L1 in CD8+ T cell responses. The role of PD-L1 in limiting naïve and effector CD4+ T cell responses and the importance of PD-L1 in controlling the responses of self-reactive T cells are highlighted by the severe clinical EAE that develops after immunization of PD-L1–/–mice with MOG35–55 or after the adoptive transfer of MOG35–55-specific T cells into PD-L1–/– recipients. PD-L1 deficiency converted the 129Sv strain from a resistant to an EAE-susceptible strain. Transfer of wild-type or PD-L1–/– encephalitogenic T cells into PD-L1–/– recipients demonstrated a critical role for PD-L1 in limiting pathogenic effector T cell responses and revealed that PD-L1 on both the T cell and in the recipient control encephalitogenic T cell responses.
Cell surface expression of PD-L1 has been detected on many human carcinomas and some T cell tumors (8, 11, 21). In addition, tumor-associated DCs express higher levels of PD-L1. Furthermore, tumor cells that express PD-L1 grow in wild-type mice but are suppressed in PD-1–/– mice (21). Together these findings suggest that PD-L1:PD-1-mediated inhibitory signals give tumors a selective advantage for growth by inhibiting CD8+ T cell responses. The enhanced CD8+ T cell responses in PD-L1–/– mice, together with PD-L1 expression on tumors, suggest that PD-L1 on tumors may limit CD8+ T cell clonal expansion and thereby attenuate tumor-specific responses.
PD-1/PD-L1 interactions also may be important for controlling antiviral CD8+ T cell responses. After infection with adenovirus, PD-1–/– mice exhibited increased proliferation of effector T cells in the liver and enhanced clearance of the virus (22). The enhanced CD8+ T cell expansion in PD-L1–/– mice implicate PD-L1 engagement of PD-1 in regulating antiviral immunity. Chronic viral infections are often associated with suppressed T cell responses. It is possible that PD-1/PD-L1 interactions contribute to the functional inactivation of virus-specific CD8+ T cells during chronic viral infection. Taken together, these findings suggest that blockade of the PD-L1/PD-1 pathway may provide a means to boost antitumor and antiviral immunity.
The increased susceptibility of PD-L1–/– mice to EAE demonstrates the important role for PD-L1 in regulating the responses of self-reactive CD4+ T cells in vivo. Because EAE is induced by self-reactive CD4+ Th1 effector cells, the increase in IFN-γ-producing cells seen in PD-L1–/– mice may contribute to the severity of disease. During EAE PD-L1 is expressed in the brain on vascular endothelial cells and infiltrating mononuclear cells (14, 23). Up-regulation of PD-L1 during Th1-driven inflammation might serve as a negative feedback mechanism for controlling responses in the target organ and limit pathogenic T cell responses in the brain. Furthermore, PD-1 mRNA is highly expressed in CD4+CD25+ T regulatory cells (Treg) which have been shown to regulate EAE (24, 25). Thus, the increased MOG-specific CD4+ T cell responses in PD-L1–/– mice might reflect multiple negative regulatory functions for PD-L1: (i) limiting expansion and/or Th1 differentiation of naïve CD4+ T cells, (ii) negatively regulating reactivation of MOG-specific effector CD4+ T cells in the target organ, and (iii) limiting expansion of antigen-specific T cells through engagement of PD-1 on Tregs. These potential roles for PD-L1 are not mutually exclusive.
In contrast to our analysis of PD-L1–/– mice, a recent study using Abs to PD-1 ligands in EAE suggested that PD-L1 was not important in regulating EAE (14). In that study, the anti-PD-L1 mAb was only administered until 10 days after immunization. Because in PDL1–/– mice PD-L1 is absent throughout the induction and effector phases of the immune response, the Ab administration may not have been sufficient to have blocked PD-L1/PD-1 interactions throughout the response. In addition, the anti-PD-L1 Ab (MIH6) used in these studies may not be an optimal blocking Ab. Indeed, when we administer a different anti-PD-L1 mAb (10F.9G2), which we have characterized as a potent blocker of PD-1/PD-L1 interactions (10), to wild-type C57BL/6 mice during the induction of EAE, the onset of severe clinical disease is rapid (data not shown). Additional studies are needed to reconcile differences between lack of effect of certain anti-PD-L1 mAbs during the induction of EAE and the enhanced EAE induction in PD-L1–/– mice.
The phenotype of the PD-L1–/– mouse implies that PD-L1 has unique functions from PD-L2. Increased T cell responses in PD-L1–/– mice indicate that the presence of PD-L2 is unable to compensate for the lack of PD-L1 in regulating T cell responses. This finding could be related to distinct expression patterns, timing, or sites of action of PD-1 ligands. We have observed PD-L1, but not PD-L2, on T cells and endothelial cells (12, 23). Because both PD-L1 and PD-1 are expressed on T cells, PD-L1/PD-1 interactions on T cells may negatively regulate T/T interactions. Furthermore, PD-L1 is extensively expressed in nonlymphoid tissues, whereas PD-L2 expression is more restricted. During EAE induction, PD-L1, but not PD-L2, is expressed on the vascular endothelium in the brain (14, 23). Therefore, the elimination of PD-L1 may remove a critical negative regulatory signal for controlling encephalitogenic T cell interactions with CNS endothelial cells at the blood–brain barrier.
The function of PD-L1 also may reflect its interaction not only with PD-1 but also with a yet-to-be-identified receptor. It has been suggested that PD-L1 interacts with a second receptor on activated T cells and that this receptor positively regulates T cell expansion, IL-10, and apoptosis (26). The phenotype of PD-L1–/– mice indicates that the down-regulatory effects of PD-L1 predominate but do not rule out a costimulatory function for PD-L1. By comparison, B7–1/B7–2–/– mice primarily exhibit defects in T cell activation (CD28-mediated) rather than defects in negative regulation (CTLA-4-mediated), likely because of the constitutive expression of CD28 and the later up-regulation of CTLA-4. Similarly, engagement of PD-L1 by PD-1 may be the initial and dominant event occurring in vivo.
The demonstration of a key role for PD-L1 in the effector phase of EAE has important therapeutic implications. PD-L1 agonists may provide a novel therapeutic approach for ameliorating human autoimmune diseases and transplant rejection. Likewise, blockade of PD-L1 may provide a new means for enhancing antimicrobial vaccine efficacy and antitumor immunity.
Supplementary Material
Acknowledgments
We thank L. Du, A. Kopelman, J. Burgess, and B. Chang for technical support and L. Vijayakrishnan for help with statistical analysis of the data. This work was supported by grants from the National Institutes of Health (to V.K.K., G.J.F., and A.H.S.), the National Multiple Sclerosis Society (to A.H.S.), and the Wellcome Trust and the Leukemia and Lymphoma Society (to Y.E.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DC, dendritic cell; APC, antigen-presenting cell; LPS, lipopolysaccharide; CFA, complete Freund's adjuvant; OVA, ovalbumin; MOG33–55, myelin oligodendrocyte glycoprotein peptide; EAE, experimental autoimmune encephalomyelitis; LN, lymph node.
References
- 1.Freeman, G. J., Long, A. J., Iwai, Y., Bourque, K., Chernova, T., Nishimura, H., Fitz, L. J., Malenkovich, N., Okazaki, T., Byrne, M. C., et al. (2000) J. Exp. Med. 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agata, Y., Kawasaki, A., Nishimura, H., Ishida, Y., Tsubata, T., Yagita, H. & Honjo, T. (1996) Int. Immunol. 8, 765–772. [DOI] [PubMed] [Google Scholar]
- 3.Ishida, M., Iwai, Y., Tanaka, Y., Okazaki, T., Freeman, G., Minato, N. & Honjo, T. (2002) Immunol. Lett. 84, 57–62. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. (1999) Immunity 11, 141–151. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura, H., Okazaki, T., Tanaka, Y., Nakatani, K., Hara, M., Matsumori, A., Sasayama, S., Mizoguchi, A., Hiai, H., Minato, N. & Honjo, T. (2001) Science 291, 319–322. [DOI] [PubMed] [Google Scholar]
- 6.Dong, H., Zhu, G., Tamada, K. & Chen, L. (1999) Nat. Med. 5, 1365–1369. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki, T., Akiba, H., Iwai, H., Matsuda, H., Aoki, M., Tanno, Y., Shin, T., Tsuchiya, H., Pardoll, D. M., Okumura, K., et al. (2002) J. Immunol. 169, 5538–5545. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. A., Dorfman, D. M., Ma, F., Sullivan, E. L., Munoz, O., Wood, C. R., Greenfield, E. A. & Freeman, G. J. (2003) J. Immunol. 170, 1257–1266. [DOI] [PubMed] [Google Scholar]
- 9.Dong, H., Strome, S. E., Matteson, E. L., Moder, K. G., Flies, D. B., Zhu, G., Tamura, H., Driscoll, C. L. & Chen, L. (2003) J. Clin. Invest. 111, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppihimer, M. J., Gunn, J., Freeman, G. J., Greenfield, E. A., Chernova, T., Erickson, J. & Leonard, J. P. (2002) Microcirculation 9, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchman, Y., Wood, C. R., Chernova, T., Chaudhary, D., Borde, M., Chernova, I., Iwai, Y., Long, A. J., Brown, J. A., Nunes, R., et al. (2001) Nat. Immunol. 2, 261–268. [DOI] [PubMed] [Google Scholar]
- 12.Rodig, N., Ryan, T., Allen, J. A., Pang, H., Grabie, N., Chernova, T., Greenfield, E. A., Liang, S. C., Sharpe, A. H., Lichtman, A. H. & Freeman, G. J. (2003) Eur. J. Immunol. 33, 3117–3126. [DOI] [PubMed] [Google Scholar]
- 13.Ansari, M. J., Salama, A. D., Chitnis, T., Smith, R. N., Yagita, H., Akiba, H., Yamazaki, T., Azuma, M., Iwai, H., Khoury, S. J., et al. (2003) J. Exp. Med. 198, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salama, A. D., Chitnis, T., Imitola, J., Akiba, H., Tushima, F., Azuma, M., Yagita, H., Sayegh, M. H. & Khoury, S. J. (2003) J. Exp. Med. 198, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dascher, C. C., Hiromatsu, K., Xiong, X., Sugita, M., Buhlmann, J. E., Dodge, I. L., Lee, S. Y., Roura-Mir, C., Watts, G. F., Roy, C. J., et al. (2002) J. Immunol. 169, 6951–6958. [DOI] [PubMed] [Google Scholar]
- 16.Moore, M. W., Carbone, F. R. & Bevan, M. J. (1988) Cell 54, 777–785. [DOI] [PubMed] [Google Scholar]
- 17.Chang, T. T., Jabs, C., Sobel, R. A., Kuchroo, V. K. & Sharpe, A. H. (1999) J. Exp. Med. 190, 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, S., Bajorath, J., Flies, D. B., Dong, H., Honjo, T. & Chen, L. (2003) J. Exp. Med. 197, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter, L., Fouser, L. A., Jussif, J., Fitz, L., Deng, B., Wood, C. R., Collins, M., Honjo, T., Freeman, G. J. & Carreno, B. M. (2002) Eur. J. Immunol. 32, 634–643. [DOI] [PubMed] [Google Scholar]
- 20.Kuchroo, V. K., Anderson, A. C., Waldner, H., Munder, M., Bettelli, E. & Nicholson, L. B. (2002) Annu. Rev. Immunol. 20, 101–123. [DOI] [PubMed] [Google Scholar]
- 21.Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T. & Minato, N. (2002) Proc. Natl. Acad. Sci. USA 99, 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai, Y., Terawaki, S., Ikegawa, M., Okazaki, T. & Honjo, T. (2003) J. Exp. Med. 198, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, S. C., Latchman, Y. E., Buhlmann, J. E., Tomczak, M. F., Horwitz, B. H., Freeman, G. & Sharpe, A. H. (2003) Eur. J. Immunol. 33, 2706–2716. [DOI] [PubMed] [Google Scholar]
- 24.Lechner, O. J., Lauber, A., Franzke, A., Sarukhan, H., von Boehmer, H. & Buer, J. (2001) Curr. Biol. 8, 587–592. [DOI] [PubMed] [Google Scholar]
- 25.Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. (2002) J. Immunol. 169, 4712–4716. [DOI] [PubMed] [Google Scholar]
- 26.Dong, H., Strome, S. E., Salomao, D. R., Tamura, H., Hirano, F., Flies, D. B., Roche, P. C., Lu, J., Zhu, G., Tamada, K., et al. (2002) Nat. Med. 8, 793–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.