Abstract
Objective
To determine correlations of the EEG frequency spectrum with neuropsychological status in children with idiopathic epilepsy.
Methods
Forty-six children ages 8 to 18 years old with idiopathic epilepsy were retrospectively identified and analyzed for correlations between EEG spectra and neuropsychological status using multivariate linear regression. In addition, the theta/beta ratio, which has been suggested as a clinically useful EEG marker of attention-deficit hyperactivity disorder (ADHD), and an EEG spike count were calculated for each subject.
Results
Neuropsychological status was highly correlated with posterior alpha (8-15 Hz) EEG activity in a complex way, with both positive and negative correlations at lower and higher alpha frequency sub-bands for each cognitive task in a pattern that depends on the specific cognitive task. In addition, the theta/beta ratio was a specific but insensitive indicator of ADHD status in children with epilepsy; most children both with and without epilepsy have normal theta/beta ratios. The spike count showed no correlations with neuropsychological status.
Conclusions
(1) The alpha rhythm may have at least two sub-bands which serve different purposes. (2) The theta/beta ratio is not a sensitive indicator of ADHD status in children with epilepsy. (3) The EEG frequency spectrum correlates more robustly with neuropsychological status than spike count analysis in children with idiopathic epilepsy.
Significance
(1) The role of posterior alpha rhythms in cognition is complex and can be overlooked if EEG spectral resolution is too coarse or if neuropsychological status is assessed too narrowly. (2) ADHD in children with idiopathic epilepsy may involve different mechanisms from those in children without epilepsy. (3) Reliable correlations with neuropsychological status require longer EEG samples when using spike count analysis than when using frequency spectra
Keywords: Quantitative EEG, neuropsychological status, IQ, alpha rhythm, theta/beta ratio, spike count
Introduction
Cognitive impairment is a major complication of the epilepsies (Lin et al., 2012), and there has been longstanding interest in the relationship between neuropsychological status and interictal EEG abnormalities (Fastenau, 2011). The epilepsy-oriented EEG-cognition literature has focused on the interictal spike, and much has been learned, potentially with relevance towards clinical management (Binnie 2003). In contrast, EEG-cognition studies in subjects without epilepsy have focused on other quantitative EEG measures, particularly frequency spectral analysis (Gasser et al., 1983; Benninger et al., 1984; Marosi et al., 1999; Schmid et al., 2002; Thatcher et al, 2005; Polunina and Davydov, 2006). Less is known about the relationship of EEG spectra to cognition in people with epilepsy, in either adults (Wilkus, Dodrill, 1976; Dodrill, Wilkus, 1976) or children (Tedrus et al., 2006).
EEG frequency spectra in normally developing children without epilepsy show relative decreases in delta (1-3 Hz) and theta (4-7 Hz) activity and increases in alpha (8-15 Hz) and beta (16-30 Hz) activity as they grow older (Matousek and Petersen, 1973; Benninger et al., 1984; Gasser et al., 1988a,b; Clarke et al., 2001). In children with attention deficit hyperactivity disorder (ADHD) who do not also have epilepsy, there is an increase in the ratio of theta to beta activity at the vertex (Cz) relative to children who do not have either ADHD or epilepsy. Age-dependent theta/beta thresholds have been defined that are both sensitive (86-90%) and specific (94-98%) in identifying children with ADHD (Monastra et al., 1999; Monastra et al., 2001; Snyder et al., 2008). These studies specifically excluded children with epilepsy. It is not known whether the theta/beta ratio can distinguish children with both ADHD and epilepsy from children who have epilepsy but not ADHD. Given that ADHD is especially prevalent in children with epilepsy (Hermann et al., 2007), this question is clinically relevant.
To address current gaps in knowledge, we explore (1) how EEG spectra vary with respect to cognitive performance in children with idiopathic epilepsy, (2) whether the theta/beta ratio distinguishes children with both ADHD and idiopathic epilepsy from children with epilepsy but not ADHD, and (3) whether spike counts on the same EEG samples show correlations with neuropsychological status.
Methods
Study population
Candidate study subjects were identified retrospectively from a research database consisting of 124 children ages 8-18 with new onset epilepsy (Jackson et al., 2013). Inclusion criteria for this database consisted of a diagnosis of idiopathic epilepsy made within the prior 12 months, normal neurological examination, normal developmental history, normal neuroimaging if obtained for clinical reasons, and no other developmental or neurological disorders. The medical records for these children were reviewed by a board-certified pediatric neurologist to confirm that these children had idiopathic epilepsy. On medical record review, 55 of the 124 subjects also had accessible clinical EEG's performed at the University of Wisconsin within 12 months of neuropsychological testing. The other children either did not have EEG's performed at the University of Wisconsin within this time frame, or their EEG's had been archived and were not readily accessible for quantitative analysis. These 55 subjects were classified by a board-certified pediatric neurologist as having partial-onset epilepsy (PGE score = 1), primary generalized epilepsy (PGE score = 2), or uncertain (PGE score = 1.5).
Institutional review board approval
Approval to conduct this study was received from the health sciences institutional review board of the University of Wisconsin School of Medicine and Public Health. Written informed consent was obtained from legal guardians of children and adolescents in this study. Written informed consent was obtained from subjects 18 years of age and over in this study, and written informed assent was obtained from all children 8-17 years of age.
Neuropsychological testing
All subjects underwent detailed neuropsychological evaluation including Wechsler Abbreviated Scale of Intelligence (WASI) and Continuous Performance Test-II (CPT-II). WASI scores included age-adjusted verbal IQ (VIQ) and performance IQ (PIQ). CPT-II scores included the age-adjusted scores for errors of omission (OM), errors of commission (CO), and reaction time (RT). To determine ADHD status, the Kiddie–Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS-PL) was used (Kaufman et al., 1997). This is a structured diagnostic interview designed to assess current and previous episodes of psychopathology in children and adolescents using DSM-IV criteria (American Psychiatric Association, 2000). The K-SADS-PL has been utilized in several studies of children with epilepsy to identify rates of psychiatric comorbidity including ADHD (Ott et al., 2003; Caplan et al, 2005). The K-SADS-PL is conducted by directly interviewing the parent and the child separately. In the current study, two interviewers were specially trained to administer the clinical interview, and interviews were randomly selected for review to ensure diagnostic consistency. Fifteen percent of subjects were randomly selected for independent review to ensure reliability of diagnoses and reduce rater drift. Interviewers were not blinded to seizure history as this often arose spontaneously during the interview.
The CPT-II measures (OM, CO and RT) were reversed in sign so that higher values of these measures corresponded to fewer errors of omission and commission and faster reaction times. Subjects diagnosed with ADHD were assigned an ADHD score of -1, and those without ADHD were assigned an ADHD score of 0. Assigned in this way, higher numerical scores for OM, COM, RT and ADHD correspond to better cognitive function.
EEG acquisition and analysis
All EEG's were performed using the 10-20 International System for electrode placement (Jasper 1958) and sampled at 256 Hz. Impedances were less than 5 kOhms, with amplifier bandpass of 1.6 to 256 Hz. All of the 55 available EEG's were reviewed by a board-certified pediatric neurologist. We used Nicolet equipment exclusively (Natus-Nicolet, Middleton WI). EEG's were obtained for routine clinical indications, typically to help in the diagnosis and categorization of epilepsy.
Selection bias is difficult to avoid in the selection of EEG data for quantitative analysis. To test for the presence of this bias, we selected two sets of 360 seconds of EEG data in bipolar montage for each subject, and compared the results of analysis of both sets of data. The first set consisted of 36 ten-second time windows where only seizures, runs of spikes and polyspikes, and obvious artifact such as electrode pops, muscle artifact and clusters of rapid eye-blinks were excluded. The aim with this data set was to be as inclusive as possible, and to include all data that would normally be felt to be clinically interpretable by a board-certified pediatric neurologist. The first 36 ten-second time windows of EEG data for each subject that met these criteria were saved for analysis. Three EEG's in their entirety were rejected because it was not possible to obtain 360 seconds of EEG data that met these criteria, leaving 52 subjects. We refer to these 52 subjects as the 10s group.
The second EEG data set consisted of 180 two-second time windows of EEG data selected on a more rigorous basis, which also excluded periods of excessive slowing including periods of drowsiness and sleep. Here we scanned through the entire EEG first for each subject, and selected only those parts that represented the “best” or most alert mental states for that subject. Seven EEG's were rejected because it was not possible to obtain 360s of EEG data that met these stricter criteria, leaving 48 subjects. We refer to these 48 subjects as the 2s group. All study subjects in the 2s group are also represented in the 10s group, although the EEG samples in each group are not identical.
The EEG's were analyzed in bipolar montage consisting of Fp1-F7, F7-T3, T3-T5, T5-O1, Fp2-F8, F8-T4, T4-T6, T6-O2, Fp1-F3, F3-C3, C3-P3, P3-O1, Fp2-F4, F4-C4, C4-P4, P4-O2, Fp1-Fp2, Fz-Cz, Cz-Pz, and O1-O2. Each EEG data set was subjected to frequency analysis using the damped-oscillator pseudo-wavelet (Hsu et al., 2010). We used this pseudo-wavelet for convenience. Details are described in Appendix A. For later reference, let S(f, n, i) denote the EEG spectral amplitude at frequency f and channel n for subject i.
Anticonvulsant therapy
To test for the effect of individual anticonvulsant medications on EEG, we included anticonvulsant medications as subject variables. If a subject was on levetiracetam therapy, that subject was assigned an LEV score of 1; otherwise, the subject was assigned an LEV score of 0. Scores were similarly assigned for subjects on valproate (VPA), ethosuximide (ESM), oxcarbazepine (OXC), lamotrigine (LTG), topiramate (TPM) and zonisamide (ZNS). In our 10s group of 52 subjects, 24 subjects were on levetiracetam, 9 were on valproate, 9 on ethosuximide, 7 on oxcarbazepine, 2 on carbamazepine, 2 on lamotrigine, one on topiramate, and 2 on zonisamide, Thirty-three were on monotherapy, 7 were on dual therapy, and 6 were on no therapy at the time of the study. No subjects took either barbiturates or benzodiazepines.
Subjects on carbamazepine, lamotrigine, topiramate, and zonisamide were excluded from regression analysis due to having only 1-2 subjects on each of those medications. Excluding these subjects resulted in 46 subjects in the 10s group and 44 subjects in the 2s group.
Multivariate linear regression of EEG spectral density with subject variables
The 12 subject variables tested were: age of subject, VIQ, PIQ, OM, CO, RT, ADHD, PGE, LEV, VPA, ESM, and OXC. Let x(i, j) represent study variable j for subject i; for instance, x(2,3) would be PIQ for subject 2. Let S(f, n, i) denote the EEG spectral amplitude at frequency f and channel n for subject i. The EEG spectral amplitude S(f, n, i) was normalized, for each channel n and subject i, by dividing by the root mean square of the raw EEG spectral amplitude taken over all frequencies f. Multivariate linear regression was then performed using the following equation:
| (1) |
Here x′(i, j) was x(i, j) normalized, for each subject variable j, by subtracting out the mean and dividing by the standard deviation taken over all subjects i. The linear fitting coefficients c(j, f, n) were found using a least squares fit, and then normalized by dividing by the standard deviation away from zero; this standard deviation was calculated over all subject variables j, frequencies f, and EEG channels n. That this standard deviation spanned the entire space of independent variables (number of subject variables × number of frequency bins × number of EEG channels) corrected for multiple sampling in a consistent way. A value of c(j, f, n) greater than 2 or less than -2 standard deviations was taken to indicate statistically significant positive or negative deviations from zero (p < 0.05).
Theta/beta ratio
The theta/beta ratio was calculated as closely as possible to that reported by Monastra and coworkers (Monastra et al., 1999). EEG for each subject from the referential Cz channel was subjected to Fast Fourier Transform (FFT) and the FFT spectral power was calculated. Following Monastra and coworkers, theta band power was taken to be the area under the curve between 4 and 8 Hz, and the beta band power was taken to be the area under the curve between 13 and 21 Hz. The area under the theta curve was then divided by that under the beta curve. Theta/beta ratios were calculated both for the 10s group and for the 2s group. For the purposes of the theta/beta calculation, we did not exclude subjects on carbamazepine, lamotrigine, topiramate or ethosuximide, because we wished to test the clinical utility of the theta/beta ratio as it might be applied to children with epilepsy in general. Since separate thresholds for the theta/beta ratio for each anticonvulsant are not available, we did not attempt to correct the EEG power spectrum for individual anticonvulsants.
Results
Demographics
Tables 1 and 2 provide a summary of the demographic, cognitive and epilepsy characteristics of the 46 subjects from the 10s group. The subjects ranged in age from 7.6 to 18.1 years with mean of 12.1 and standard deviation of 3.2 years. Twenty-four subjects were female; 22 were male. Of the 46 subjects, 26 had primary generalized epilepsy (57%), 18 had partial-onset epilepsy (39%) and 2 were indeterminate. The charts of the 2 subjects with indeterminate epilepsy classification were further reviewed by a board certified pediatric epileptologist, who confirmed that further epilepsy classification was not possible. The time interval between EEG and neuropsychological testing ranged from 0 to 12 months with mean of 5.4 months and standard deviation of 3.2 months. The time interval between diagnosis of epilepsy and neuropsychological testing ranged from 1 to 14 months with mean of 8.4 months and standard deviation of 3.3 months. Four subjects were originally thought based on chart review to have met the inclusion criterion that neuropsychological testing took place within 12 months of diagnosis, but on later interview of the family, it was found that actual diagnosis took place earlier than documented in the medical charts. One subject was diagnosed 14 months prior to neuropsychological testing and 3 subjects were diagnosed 13 months prior to neuropsychological testing. We elected not to exclude these four subjects in order to maintain consistency in methodology.
Table 1.
Demographics of study population.
| Number of subjects | 46 |
|---|---|
| Number male | 22 (48%) |
| Number female | 24 (52%) |
| Number with primary generalized epilepsy | 26 (57%) |
| Number with partial-onset epilepsy | 18 (39%) |
| Number with epilepsy of undetermined type | 2 (4%) |
| Number on monotherapy | 33 (72%) |
| Number on dual therapy | 7 (15%) |
| Number not on anti-seizure medication | 6 (13%) |
| Number with ADHD | 10 (22%) |
Table 2.
Neuropsychological measures of study population.
| Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|
| Age | 7.6 years | 18.1 | 12.1 | 3.2 |
| VIQ | 79 | 139 | 106 | 15 |
| PIQ | 70 | 138 | 99 | 14 |
| OM | 40.7 | 131.2 | 52.1 | 15 |
| CO | 36.7 | 74.8 | 53.4 | 8.6 |
| RT | 37.9 | 100 | 50.8 | 10.2 |
| Time between EEG and IQ testing | 0 months | 12 | 5.4 | 3.2 |
| Time between diagnosis and IQ testing | 1 months | 14 | 8.4 | 3.3 |
Anticonvulsant therapy vs neuropsychological measures
With anticonvulsant therapy as the covariate, therapy with LEV, VPA, ESM or OXC was not correlated at the 95% confidence level using a linear least squares fit with VIQ, PIQ, OM, CO or ADHD, except for a positive correlation of VPA therapy with ADHD diagnosis (slope of 0.42, 95% confidence interval 0.13 to 0.71).
EEG spectral density
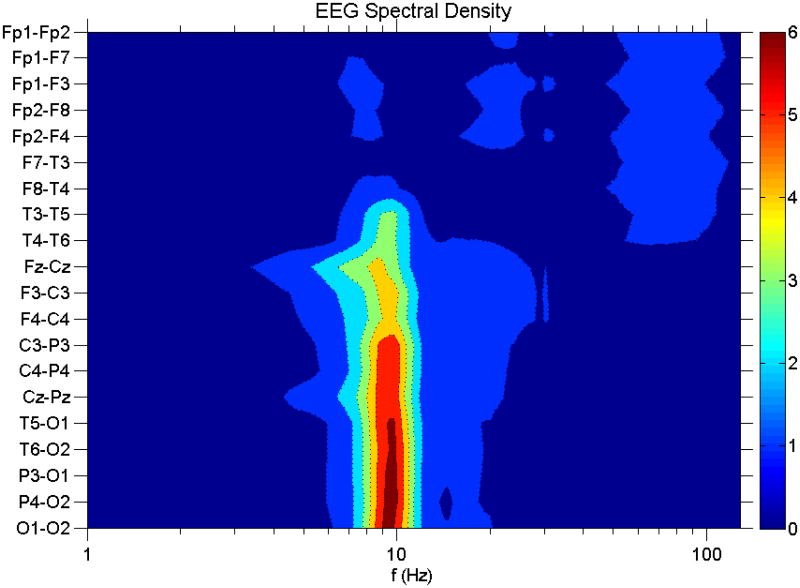
Figure 1 shows the EEG spectral density averaged over all 46 subjects from the 10s group and over all 360 s of EEG data for each subject. The EEG channels are arranged from anterior to posterior to show the natural separation into three topographic regions: frontal, temporal and centroparieto-occipital. Henceforth we will refer to Fp1-F7 and Fp1- F3 as the left frontal channels (LF); Fp2-F8 and Fp2-F4 as the right frontal channels (RF); F7-T3 and T3-T5 as the left temporal channels (LT); F8-T4 and T4-T6 as the right temporal channels (RT); F3-C3, C3-P3, T5-O1 and P3-O2 as the left posterior channels (LP); and F4-C4, C4-P4, T6-O2 and P4-O2 as the right posterior channels (RP). For ease of presentation, EEG frequency spectra from these 6 areas were averaged together prior to normalization; we checked that analysis of individual channels separately yielded similar results.
Figure 1.
EEG spectral density vs frequency. Vertical axis denotes EEG channels, grouped so that the anterior channels are at the top, and the posterior channels are at the bottom. Color intensity is in units of the standard deviation. The frequency scale is logarithmic.
Correlation of EEG spectral density with neuropsychological measures and demographics
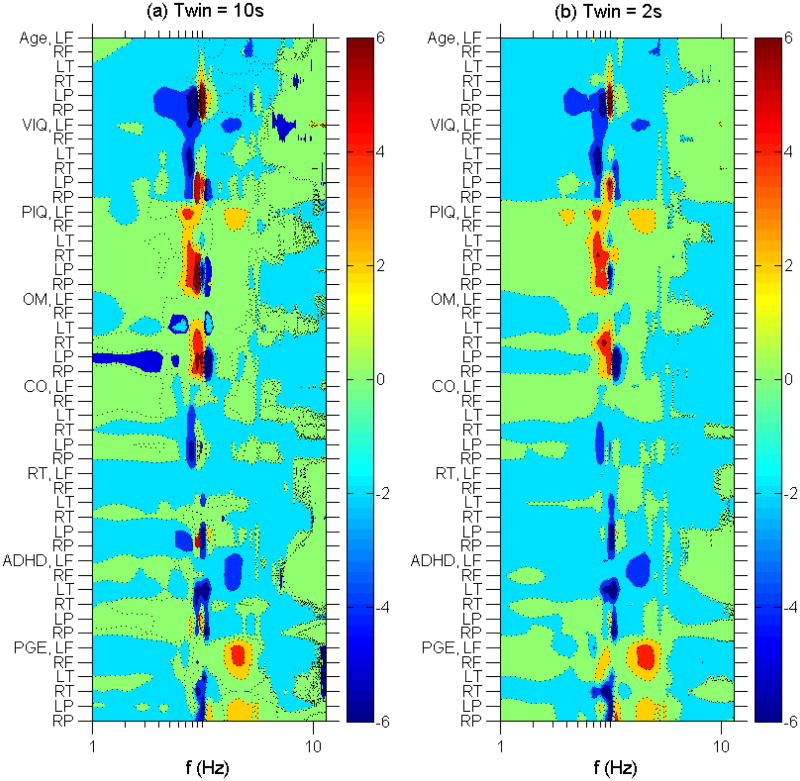
Figures 2 and 3 show the normalized linear regression coefficients c(j, f, n) from Eq. (1) where j indexes the 12 study variables, f indexes the EEG frequencies and n indexes the 6 EEG regions (LF, RF, LT, RT, LP, RP). The major finding is that there were statistically significant correlations of alpha range activity in the posterior head regions with multiple study variables. Moreover, this dependence was complex. With increasing age in our study population, there was a decrease in low alpha activity (7-9 Hz) and an increase in higher alpha (10-12 Hz). Let us refer to this pattern as (−, +); VIQ and, more weakly, CO and RT also showed (−, +) patterns in the alpha range at posterior head regions. In contrast, PIQ and OM strongly showed the opposite pattern, (+, −). Of note, this complex pattern depends on how many and which study variables were linearly regressed together. For example, if each study variable were linearly regressed individually, then age still showed a (−, +) pattern in the alpha range at posterior head regions, but VIQ, PIQ, OM, RT and, more weakly, CO and ADHD all showed a (+, −) pattern. Linearly regressing the study variables age and VIQ together still yielded a (−, +) pattern for age and the opposite (+, −) pattern for VIQ, but including PIQ in addition to age and VIQ yielded a (−, +) pattern for age and VIQ, and the opposite (+, −) pattern for PIQ. That is, the alpha pattern for VIQ flipped in sign when PIQ was added. There were no further flips in sign for VIQ as the remainder of the variables were added to the multivariate linear regression.
Figure 2.
Left panel (2a): Contour plot of normalized linear regression coefficients vs subject variables other than anticonvulsant therapy and EEG spectral frequency using 10 s time windows. Vertical axis lists the subject variables broken down into brain regions. Horizontal axis gives the frequency on a logarithmic scale. The color intensity scale gives the normalized linear regression coefficients in standard deviations. Right panel (2b): Same as 2a using 2 s time windows.
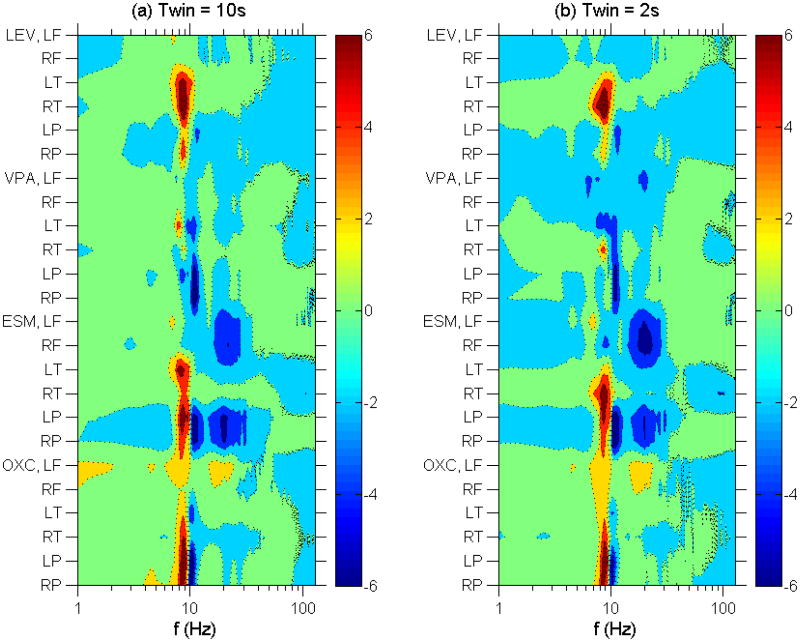
Figure 3.
Left panel (3a): Contour plot of normalized linear regression coefficients vs anticonvulsant therapy and EEG spectral frequency using 10 s time windows. Vertical axis lists the anticonvulsant broken down into brain regions. Horizontal axis gives the frequency on a logarithmic scale. The color intensity scale gives the normalized linear regression coefficients in standard deviations. Right panel (3b): Same as 3a using 2 s time windows.
Also notable are the following findings: (1) with increasing age, there was a decrease in theta frequency activity; (2) with improved PIQ scores, there was an increase in frontal alpha and beta frequency activity; (3) in the absence of an ADHD diagnosis, there was a decrease in frontal beta, decrease in temporal alpha (especial LT alpha), and a complex alternating (+, −, +, −) pattern in the posterior head regions in the theta-alpha range; (4) in the presence of a diagnosis of PGE, there was an increase in frontal and posterior beta, and a decrease in temporal and posterior alpha.
With respect to anticonvulsant therapy, Fig. 3 shows that (1) LEV therapy was correlated with a (+,0) pattern in the alpha range in the temporal head regions and, more weakly, with a (+, −) pattern in the alpha range in the posterior head regions; (2) VPA therapy was correlated with a weak (+, −) pattern in the alpha range in the temporal head regions and a (0, −) pattern in the alpha range in the posterior head regions; (3) ESM therapy was correlated with decreased frontal and posterior beta activity, a (+,0) pattern in the alpha range in the temporal head region and a (+, −) pattern in the alpha range in the posterior head regions; and (4) OXC therapy was correlated with a (+, −) pattern in the alpha range in the posterior head regions, with a weak trend towards increased frontal delta, alpha and beta activity.
Comparing Figs. 2a and 3a with Figs. 2b and 3b, we find that EEG obtained using 10s time windows gave similar results to that obtained using the (more restrictive) 2s time windows.
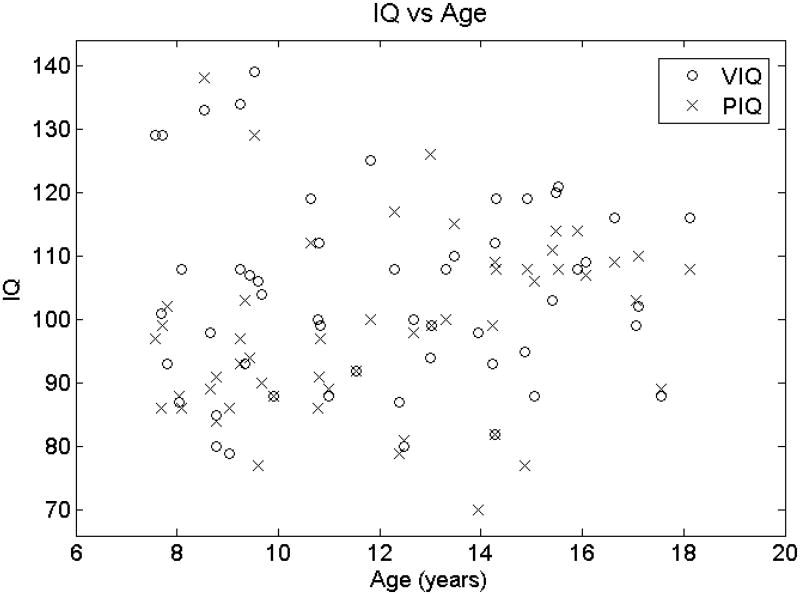
Given that increasing age correlated with a (−, +) pattern in the alpha range in the posterior head regions, it may be surprising that higher PIQ correlated with the opposite (+, −) alpha pattern in the same head region. If the younger subjects in this study had higher PIQ's than the older subjects, then that would explain the reversal of alpha pattern in the posterior head regions. However, Fig. 4 shows that PIQ did not increase with age in our study population, and so the PIQ alpha pattern reversal cannot be explained as an age bias.
Figure 4.
VIQ (open circles) and PIQ (X) vs age (horizontal axis). A linear least squares fit of VIQ as a function of age yields a slope of -0.23 IQ points per year with a 95% confidence range of (−1.64,1.19) IQ points per year. A linear least squares fit of PIQ as a function of age yields a slope of 0.97 IQ points per year with a 95% confidence range of (−0.30,2.25) IQ points per year. Therefore, younger children do not have higher PIQ's.
Theta/beta ratio vs ADHD diagnosis
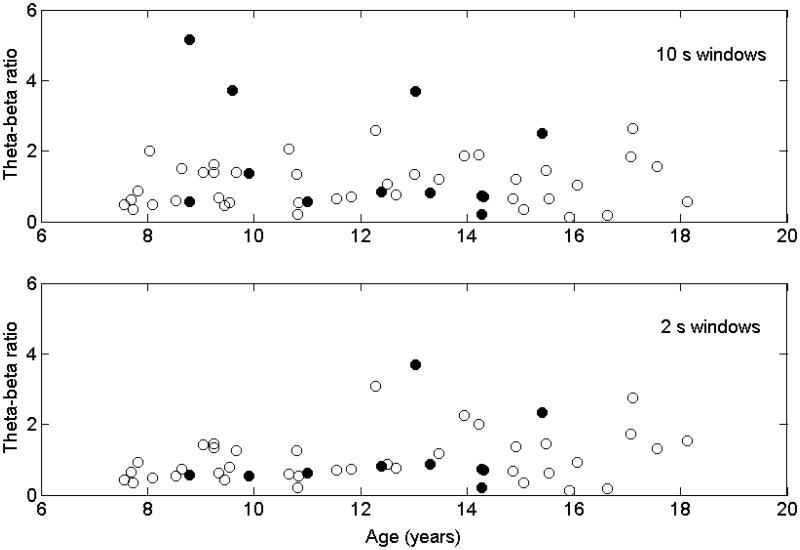
Figure 5 shows the theta/beta ratio vs age using either the 10s EEG windows or the (more restrictive) 2s windows. The suggested theta/beta thresholds, above which a subject may have ADHD, is 5.03 for children 6-11 years old, 3.31 for children 12-15 years old and 2.36 for young adults 16-20 years old (Monastra et al., 1999). Using these thresholds for the 10s group, the sensitivity of the theta/beta ratio for diagnosis of ADHD was 17%, specificity was 98%, false positive rate was 33% and false negative rate was 20%. For the more restrictive 2s group, sensitivity was 10% and specificity was 100%, false positive rate was 0% and false negative rate was 19%.
Figure 5.
Theta-beta ratio (vertical axis) vs age (horizontal axis) and ADHD diagnosis. Solid black circles denote subjects with diagnosis of ADHD. Open circles denote those without ADHD diagnosis. Top box are theta-beta ratios calculated using 10 s EEG time windows with less restrictive inclusion criteria; bottom box are theta-beta ratios calculated using 2 s EEG time windows with more restrictive inclusion criteria.
Spike count
Although not the central focus of this study, we also compared neuropsychological status with EEG spike count – a classic dependent variable in the epilepsy EEG-cognition literature (Fastenau, 2011). The 52 files from the original 10s group were visually examined for spikes. Fifteen files contained spikes; 37 files did not. Of the EEG files that contained spikes, the number of spikes ranged from 1 to 30 with mean of 8.3 and standard deviation of 7.2. There was no statistically significant correlation of spike count with any variable with the exception of valproate use. Of subjects not on valproate, the mean spike count was 2.9 (standard deviation 5.8). Of subjects on valproate, the mean spike count was 0.22 (standard deviation 0.44). The difference in spike count between those on valproate and those not on valproate is statistically significant (p = 0.0052). We did not separately analyze the spike count from the 2s group, as there were fewer spikes in this group.
Discussion
Quantitative analyses of EEG spectra and comparision with IQ in adults and children without epilepsy have shown variable findings within multiple frequency bands (Gasser et al., 1983; Anokhin, Vogel, 1996; Marosi et al., 1999; Schmid et al., 2002; Thatcher et al, 2005; Polunina, Davydov, 2006). Increased activity in the alpha range in the posterior head region with higher IQ and with faster processing times have been the most consistent finding. In a study of 18 adults without epilepsy, reaction times were found to be faster in those with higher alpha frequencies (Surwilo, 1961; Klimesch, 1999).
In adults with epilepsy, subjects with higher posterior alpha frequencies (8.8 to 11 Hz) perform better on neuropsychological measures than subjects with lower posterior frequencies (5.1 to 8.5 Hz) (Dodrill, Wilkus, 1976). In children with epilepsy associated with centrotemporal spikes, higher absolute delta power correlated negatively with performance IQ at the central, temporal, parietal and occipital channels bilaterally, and higher absolute theta correlated negatively with performance IQ in all channels except right central (Tedrus et al., 2006). No correlation was found between EEG band power and verbal or full scale IQ's. The authors argued that their results pointed to a maturational disturbance in children with epilepsy with centrotemporal spikes that affect performance IQ but not verbal IQ (Tedrus et al., 2006). No correlation with alpha power was found, in contradistinction to findings from multiple other groups who studied only subjects without epilepsy (Gasser et al., 1983; Marosi et al., 1999; Schmid et al., 2002; Polunina and Davydov, 2006).
Our results are broadly consistent with prior work (excepting that of Tedrus et al., 2006) in finding highly statistically significant correlation of alpha activity with age and neuropsychological status; however, the pattern of correlations as found using multivariate linear regression is complex. There is a (−, +) EEG correlation pattern in the alpha range with increasing age and higher VIQ. There is also trend towards a (−, +) pattern in the alpha range with faster reaction times and fewer errors of commission. These findings are expected and concordant with current literature. On the other hand, there is a (+, −) pattern in the alpha range with higher PIQ and fewer errors of omission. Although unexpected, this finding is not discordant with current literature, as most studies of the alpha band use frequency bins that are too coarse to discern these subtle shifts in sub-band power (e.g., see Dodrill, Wilkus, 1976; Schmid et al., 2002; Thatcher et al., 2005; Tedrus et al., 2006).
Why higher PIQ and fewer errors of omission should both show a (+, −) pattern in the alpha range is not clear. In 58 adults with ADHD given a random letter cancellation test, more errors of omission were made on the left visual field than the right, and those who made more errors on the left had lower PIQ scores (Sandson et al., 2000). One may therefore conjecture that a lower alpha range oscillation is involved in improving performance on tests of PIQ and in reducing errors of omission, and that this oscillation is based in the right hemisphere.
That higher VIQ and fewer errors of commission both show a (−, +) pattern in the alpha range suggests that they may collocalize to the left hemisphere. In a crossover study where 17 healthy adults received high frequency repetitive transcranial magnetic stimulation (rTMS) vs sham stimulation over the left dorsolateral prefrontal cortex on separate days, subjects made fewer CPT-II errors of commission when they received rTMS compared to sham stimulation (Hwang et al., 2010).
These results suggest there may be at least two alpha sub-bands which perform different functions. A closer examination of Figure 2 shows that VIQ may be associated with a (−, +, −) pattern in the alpha range, and absence of ADHD diagnosis is associated with an even more complex (+, −, +, −) pattern in the alpha range. That there may be alpha sub-bands which play different roles has been proposed before; Klimesch has proposed that there are at least 3 alpha sub-bands serving at least 2 different functions (Klimesch, 1999).
The alpha rhythm was the first EEG rhythm to be described (Berger, 1929). Hypotheses regarding the alpha rhythm include: (1) an electrophysiological marker of an “idling” state of the brain (Pfurtscheller et al., 1996), (2) suppression of unattended sensory information (Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006), especially in the suppression of task-irrelevant information (Klimesch et al., 2007; Jokisch, Jensen, 2007; Jensen, Mazaheri, 2010), and (3) retention and processing of task-relevant information (Palva, Palva 2007; Johnson et al., 2011). The fascinating and complex roles that alpha range rhythms play in cognitive function have been reviewed (Palva, Palva, 2007; Klimesch, 2012). In future studies, we suggest that finer frequency bins be used through the alpha band, on the order of 1 Hz or less.
As our study is relatively small, we investigated whether there is a bias towards higher PIQ in the younger subjects. It has been previously observed that the alpha rhythm shifts to higher frequencies as a child matures (Matousek and Petersen, 1973; Benninger et al., 1984; Gasser et al., 1988a,b; Clarke et al., 2001; Schmid, et al., 2002), which holds true in our study population as well (see Fig. 2). Thus if there were a bias towards higher PIQ in younger children, that would explain the better cognitive performance in subjects with lower frequency alpha rhythms. However, there is not a bias towards higher PIQ in younger children in our population (see Fig. 4). Therefore, we conclude that the subjects who perform better on these cognitive tests on average have lower frequency alpha rhythms than their peers across the age range tested.
It may be surprising that the neuropsychological measures used in this study do not correlate with gamma range EEG activity. Gamma activity is known to increase with conscious cortical activity (see review in Uhlhaas et al., 2011). However, our study involves resting state EEG where no particular cognitive activity is requested of the subjects, perhaps explaining the lack of correlation of neuropsychological performance with gamma activity. To some extent, the same conclusion may hold for beta activity, which is expected to increase in the frontal region with cognitive activity (e.g., see Güntekin, Tulay, 2014). In a sense, it is remarkable that any property of the resting state EEG correlates with cognitive performance as robustly as the alpha rhythm.
Statistical significance of correlations
In interpreting the statistical significance of the influence of the 12 study variables on EEG spectral density, it is important to keep in mind that the linear regression coefficients for these study variables were normalized with respect to each other. Statistically significant correlations were identified when certain coefficients were found to be much larger than the others, i.e., when they were more than 2 standard deviations above the mean. We do not mean to imply that the 12 study variables (age of subject, VIQ, PIQ, OM, CO, RT, ADHD, PGE, LEV, VPA, ESM, and OXC) are enough to predict the EEG spectral density for each brain region and at each EEG frequency. In fact, these study variables are not enough to predict the EEG spectral density; the R square values when including all 12 study variables range from 0.07 to 0.65 (mean 0.27, std 0.08) for the 10s group and from 0.05 to 0.61 (mean 0.30, std 0.10) for the 2s group. Not surprisingly, there are other factors not accounted for by our study variables that determine EEG spectral density. Because these factors may be different for each brain region and at each spectral frequency, the R square statistic is not the most appropriate statistic to evaluate when we wish to compare the relative influence of the 12 study variables on EEG spectral density across the different brain regions and EEG frequencies.
Theta/beta ratio vs ADHD diagnosis
In prior work (Monastra et al., 1999; Monastra et al., 2001; Snyder et al., 2008), the theta/beta ratio was found to be highly sensitive (86-90%) and specific (94-98%) for ADHD. In our population of children with epilepsy, however, the sensitivity is 17% and specificity is 98% in the 10s group and 10% and 100% respectively in the 2s group. Thus the theta/beta ratio appears to be highly specific for a diagnosis of ADHD but not very sensitive.
Our study protocol differs from that of prior work in this field, in that our EEG's are clinical EEG's collected in the resting state from children, with no cognitive task required of our study subjects during the EEG. In contrast, study subjects in all but one of the prior work relating EEG measures to ADHD diagnosis generally required periods of standardized cognitive activity. For example, Monastra and coworkers (Monastra et al., 1999) required their study subjects to stare at a monitor light for 90 s and then to perform tasks of reading, listening and drawing, also for 90 s for each task. A minimum of fifteen 2-second time windows of artifact-free EEG were selected for each task for each study subject, for a minimum of 120 s of EEG data per subject. A total of 482 subjects were recruited from 8 centers, of which 275 were male, 207 were female, 397 have ADHD, 85 do not have ADHD, and ages ranged from 6 to 30 years. However, Snyder and coworkers (Snyder et al., 2008) were able to replicate the work of Monastra and coworkers using a prospective, blinded protocol in which the only behavioral tasks were the eyes-closed resting condition and the eyes open and fixated on a spot. Thus the absence of standardized cognitive tasks during the EEG in our study is not likely to explain the failure of the theta/beta ratio to distinguish children with ADHD from those without.
Other possible reasons why the theta/beta ratio failed to distinguish study subjects with ADHD from those without ADHD in our study include: (1) the smaller sample size of our study, (2) possible differences in the selection of artifact-free EEG data, (3) the use of anticonvulsant drugs by most of our study subjects, and (4) the difference in study populations, namely, that our subjects all have a diagnosis of idiopathic epilepsy, while people with epilepsy and other neurological disorders were specifically excluded in the other studies in this field (Mann et al., 1992; Janzen et al., 1995; Chabot et al., 1996; Monastra et al., 1999; Monastra et al., 2001; Snyder et al., 2008).
While our sample size is smaller, Figure 5 shows that most of the subjects with ADHD in our study have theta/beta ratios solidly in the normal range, whether EEG samples were selected using 10s time windows or the more restrictive 2s time windows. Regarding the presence of anticonvulsant therapy in our study population, we did not apply multivariate linear regression to remove the effect of anticonvulsant therapy before calculating the theta/beta ratio because in clinical practice separate theta/beta thresholds distinct for each anticonvulsant are not available, and so the practical question would be whether the currently available thresholds are reliable regardless of anticonvulsant therapy. Nonetheless, Figure 3 shows that only ethosuximide is likely to affect the theta/beta ratio, tending to increase this ratio (because of a decrease in beta activity when on this drug). However, if the theta/beta ratios were elevated by ethosuximide in children who do not have ADHD, that would raise the false positive rate, and if in children with ADHD, that would raise the sensitivity of diagnosis. Removing the effect of ethosuximide using multivariate linear regression would then decrease the false positive rate and/or lower the sensitivity, but it would not improve the sensitivity of the theta/beta ratio in diagnosing ADHD.
That the theta/beta ratios for most children with both ADHD and idiopathic epilepsy are in the normal range raises the possibility that the mechanism of ADHD may be different in children with idiopathic epilepsy. A larger study with a control population of children without epilepsy would be necessary to exclude other possible explanations, but our findings suggest caution in using the theta/beta ratio as an aid in the diagnosis of ADHD in children with epilepsy.
Spike count
It is generally found that interictal epileptiform activity can cause transient cognitive impairment (TCI) for the duration of the epileptiform discharge (Schwab, 1939; Schwab, 1941; Goode et al., 1970), especially if the discharge lasts for 3 seconds or more, if it is generalized, or if the cognitive task is complex (Wilkus, Dodrill 1976; Dodrill, Wilkus, 1976; Aarts et al., 1984; Binnie et al., 1991; Holmes, Lenck-Santini, 2006; Fastenau, 2011). Some studies, however, find no correlation between interictal spikes and neuropsychological status (e.g., Fonseca et al., 2007).
Although we found highly statistically significant correlations of EEG spectral structure with multiple measures of cognitive function, spike count analysis in our study failed to show statistically significant correlations with any subject variable other than that subjects on valproate had fewer spikes. The suppressive effect of valproate on spikes is well known (Bruni et al., 1980). The lack of correlation of spike count with neuropsychological status is not surprising given that the EEG sample of 360 s per subject is rather short, and is not likely to be a reliable measure of the true spike rate. Other studies involving spike counts used entire EEG files of at least 20 minutes per subject (e.g., see Wilkus, Dodrill, 1976). Our results therefore suggest that EEG spectral analysis is more sensitive than spike count analysis when searching for quantitative correlations between EEG and neuropsychological status in children with epilepsy.
Volume conduction effects
Volume conduction may cause electrophysiological frequencies present in one part of the brain to appear as though it were also present in neighboring parts of the brain. Estimates of the spatial lengthscale of volume conduction effects using scalp EEG coherence shows that volume conduction effects drop by 50% for EEG electrodes separated by 3-4 cm (Srinavasan et al., 1998; Nunez et al., 1999). Volume conduction effects can be partially removed by the use of nearest neighbor bipolar montages (as we use), and even more effectively by the use of spline-Laplacians (Nunez et al., 1999). Volume conduction effects are most acutely problematic when high spatial resolution is required. In our study, however, we do not attempt high spatial resolution. Rather, we used large brain regions (frontal, temporal and centro-parieto-occipital) which were chosen based on a natural segregation of brain regions, each with its own characteristic spectrum (see Figure 1).
Limitations
Limitations of this study include: (1) the lack of control subjects who do not have epilepsy, (2) an EEG sampling frequency of 256 Hz which precludes complete characterization of the gamma band, and which does not allow analysis of yet higher frequency oscillations that have attracted recent interest among epilepsy researchers (Zijlmans et al., 2012), (3) the rather sparse spatial distribution of EEG electrode placements, (4) the lack of sophisticated pre-processing such as ICA removal of eyeblink and muscle artifact, (5) the time interval of up to one year between the time of the EEG and neuropsychological testing, and (6) the lack of a cognitive task required of subjects during the EEG. We do not have control subjects in this study because of its retrospective nature. The sampling rate and sparse electrode placement are due our reliance on the retrieval of EEG's performed on these subjects for purely clinical reasons. We did not attempt ICA removal of eyeblink and muscle artifact from EEG because we wished to see if our methods are robust enough to yield correlations of EEG with neuropsychological measures even without such sophisticated (and difficult to validate) techniques for cleaning up the EEG. It may be of interest to perform simultaneous EEG and neuropsychological testing, and to require specific cognitive tasks during EEG, to see if there are correlations that appear during cognitive activity that do not appear in a random sample. On the other hand, that we do find highly statistically significant correlations even when the EEG's were not performed at the same time as neuropsychological testing and when no cognitive tasks are required of study subjects suggests that our results are particularly robust.
Conclusion
We confirm findings from prior studies that alpha range EEG activity is highly correlated with cognitive performance, but we find that this pattern of correlation is more complex than previously appreciated. It will be of interest to see if these findings are replicated in larger studies, and whether they also hold in normally developing children without epilepsy. Distinguishing features of our study include the finer frequency resolution of our multivariate linear regression analysis, and the restriction of our study sample to children with new onset epilepsy, before the effects of years of drug treatment and seizures have accumulated. Repeating this analysis later in the course of epilepsy may yield additional information on how EEG-cognition associations evolve over time.
Highlights.
The posterior alpha rhythm has a strong but complex relationship to cognitive performance in children with idiopathic epilepsy.
The theta/beta ratio is an insensitive marker of ADHD in children with idiopathic epilepsy.
In children with idiopathic epilepsy, the EEG frequency spectrum correlates more robustly with neuropsychological status than spike count analysis.
Acknowledgments
We appreciate support from NIH 3R01-NS044351 and the Mathias Koch Memorial Fund.
Appendix A
The damped-oscillator oscillator detector (DOOD) is a pseudo-wavelet method for detecting oscillations in time series (Hsu et al., 2010). A set of mathematical harmonic oscillators is defined, each with its own resonant frequency and damping constant. Time series data such as the voltage output from an EEG channel are fed into each mathematical oscillator as a driving force. If there is an oscillatory component in the EEG data that is resonant with the resonant frequency of the mathematical oscillator, then the oscillator will gain in energy. If one defines a set of such mathematical oscillators such that the resonant frequencies span a frequency range of interest, one can monitor the energies of each mathematical oscillator to determine the frequency components that contribute to the EEG time series. The damping constant allows us to time-resolve when an EEG oscillatory component starts and stops; if the EEG oscillation stops, then the mathematical oscillator resonant with that oscillation loses energy on a time scale inversely related to the damping constant.
Through the uncertainty principle, the damping constant also defines the bandwidth of each mathematical oscillator. A large damping constant gives better time resolution but a wider bandwidth, i.e., poorer frequency resolution. A small damping constant gives a smaller bandwidth but poorer time resolution. For a finite set of frequencies, one would want the bands to overlap, so as obtain complete coverage of the intended frequency range. For this study, we chose the pseudo-wavelet frequencies to be linearly spaced from 0.5 to 128 Hz in steps of 0.25 Hz, except for a gap between 55 and 65 Hz to avoid the 60 Hz environmental artifact. The total number of pseudo-wavelets is then 471. The damping constant of each pseudo-wavelet was chosen to be g = 0.25 Hz, because that is the frequency spacing between adjacent pseudo-wavelets. The instantaneous (time-resolved) spectral density is then time-averaged over the 360 s of EEG available for each EEG sample. We use the velocity version of this pseudo-wavelet, which simply means that instead of feeding the EEG output directly into the pseudo-wavelets, a finite difference time derivative of the EEG data is taken first. We have shown that the velocity version of the damped-oscillator oscillator detector is convenient and reliable (Hsu et al., 2010). Compared to conventional Fast Fourier Transform, it is capable of superior time-resolution and somewhat “cleaner” spectra, as the DOOD method is less sensitive to random, non-oscillatory noise.
Footnotes
Conflict of Interest: None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts JHP, Binnie CD, Smith AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107:293–308. doi: 10.1093/brain/107.1.293. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th, Text Revision. 2000. [Google Scholar]
- Anokhin A, Vogel F. EEG alpha rhythm frequency and intelligence in normal adults. Intelligence. 1996;23:1–14. [Google Scholar]
- Benninger C, Matthis P, Scheffner D. EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalogr Clin Neurophysiol. 1984;57:1–12. doi: 10.1016/0013-4694(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber das elektroenkephalogramm des menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Binnie CD, Channon S, Marston DL. Behavioral correlates of interictal spikes. Adv Neurology. 1991;55:113–126. [PubMed] [Google Scholar]
- Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–730. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- Bruni J, Wilder BJ, Bauman AW, Willmore LJ. Clinical efficacy and long-term effects of valproic acid therapy on spike-and-wave discharges. Neurology. 1980;30:42–46. doi: 10.1212/wnl.30.1.42. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46:720–730. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- Chabot RA, Merkin H, Wood L, Davenport TL, Serfontein G. Sensitivity and specificity of QEEG in children with attention deficit or specific developmental learning disorders. Clin Electroencephalogr. 1996;27:26–34. doi: 10.1177/155005949602700105. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Wilkus RJ. Neuropsychological correlates of the electroencephalogram in epileptics: II. The waking posterior rhythm and its interaction with epileptiform activity. Epilepsia. 1976;17:101–109. doi: 10.1111/j.1528-1157.1976.tb03388.x. [DOI] [PubMed] [Google Scholar]
- Fastenau PS. Transient cognitive impairment: impact of interictal epileptiform discharges on neuropsychological functioning and implications for clinical care and research. In: Helmstaedter C, Hermann B, Lassonde M, Kahane P, Arzimanoglou A, editors. Neuropsychology in the Care of People with Epilepsy. Surrey, UK: John Libbey Eurotext; pp. 2011pp. 69–91. [Google Scholar]
- Fonseca LC, Tedrus GMAS, De Camargo Pacheo EM, Berretta MF, Campregher AA, Costa DM. Benign childhood epilepsy with centrotemporal spikes. Arq Neuropsiquiatr. 2007;65:569–575. doi: 10.1590/s0004-282x2007000400004. [DOI] [PubMed] [Google Scholar]
- Gasser T, Von Lucadou-Muller I, Verleger R, Bächer P. Correlating EEG and IQ: A new look at an old problem using computerized EEG parameters. Electroencephalogr Clin Neurophysiol. 1983;55:493–504. doi: 10.1016/0013-4694(83)90160-8. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bächer P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol. 1988a;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gasser T, Jennen-Steinmetz C, Sroka L, Verleger R, Mocks J. Development of the EEG of school-age children and adolescents. II. Topography. Electroencephalogr Clin Neurophysiol. 1988b;69:100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Goode DJ, Penry JK, Dreifuss FE. Effects of paroxysmal spike-wave on continuous visual-motor performance. Epilepsia. 1970;11:241–254. doi: 10.1111/j.1528-1157.1970.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Tulay E. Event related bet and gamma oscillatory responses during perception of affective pictures. Brain Res. 2014;1588:45–56. doi: 10.1016/j.brainres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, McMillan A, Seidenberg M. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Kim SH, Park CS, Bang SA, Kim SE. Acute high-frequency rTMS of the left dorsolateral prefrontal cortex and attentional control in healthy young men. Brain Res. 2010;1329:152–8. doi: 10.1016/j.brainres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Hsu D, Hsu M, Grabenstatter HL, Worrell GA, Sutula TP. Time-frequency analysis using damped-oscillator pseudo-wavelets: application to electrophysiological recordings. J Neurosci Meth. 2010;194:179–192. doi: 10.1016/j.jneumeth.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Dabbs K, Walker NM, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr. 2013;162:1047–1053. doi: 10.1016/j.jpeds.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen T, Graap K, Stephanson S, Marshall W, Fitzsimmons G. Differences in baseline EEG measures for ADD and normally achieving preadolescent males. Biofeedback Self-Regul. 1995;20:65–82. doi: 10.1007/BF01712767. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalograph Clin Neurophysiol. 1958;10:370–375. [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Sutterer DW, Acheson DJ, Lewis-Peacock JA, Postle BR. Increased alpha-band power during the retention of shapes and shape-location associations in visual short-term memory. Front Psychology. 2011;2:128. doi: 10.3389/fpsyg.2011.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and schizophrenia for school-age childrent – Present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adol Psychiatr. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C, Lubar J, Zimmer A, Miller C, Muenchen R. Quantitative analysis of EEG in boys with attention deficit hyperactivity disorder: controlled study with clinical implications. Pediatr Neurol. 1992;8:30–36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- Marosi E, Rodriguez H, Harmony T, Yanez G, Rodriguez M, Bernal J, Fernandez T, Silva J, Reyes A, Guerrero V. Broad band spectral EEG parameters correlated with different IQ measurements. Int J Neurosci. 1999;97:17–27. doi: 10.3109/00207459908994300. [DOI] [PubMed] [Google Scholar]
- Matousek P, Petersen I. Frequency analysis of the EEG in normal children and in normal adolescents. In: Kellaway P, Petersen I, editors. Automation of clinical electroencephalography. New York: Raven Press; 1973. pp. 75–102. [Google Scholar]
- Monastra VJ, Lubar JF, Linden J, Van Deusen P, Green G, Wing W, Phillips A, Fenger TN. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology. 1999;13:424–433. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15:136–144. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein Rb, Shi Z, Carpenter MR, Srinivasan R, Tucker DM, Doran SM, Cadusch PJ, Wijesinqhe RS. EEG coherency II: experimental comparisons of multiple measures. Clin Neurophysiol. 1999;110:469–486. doi: 10.1016/s1388-2457(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Ott D, Siddarth P, Gurbani S, Koh S, Tournay A, Shields WD, Caplan R. Behavioral disorders in pediatric epilepsy: unmet psychiatric need. Epilepsia. 2003;44:591–597. doi: 10.1046/j.1528-1157.2003.25002.x. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization in the alpha band – an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Polunina AG, Davydov DM. EEG correlates of Wechsler Adult Intelligence Scale. Int J Neuroscience. 2006;116:1231–1248. doi: 10.1080/00207450600550287. [DOI] [PubMed] [Google Scholar]
- Sandson TA, Bachna KJ, Morin MD. Right hemisphere dysfunction in ADHD: visual hemispatial inattention and clinical subtype. J Learn Disabil. 2000;33:83–90. doi: 10.1177/002221940003300111. [DOI] [PubMed] [Google Scholar]
- Schmid RG, Tirsch WS, Scherb H. Correlation between spectral EEG parameters and intelligence test variables in school-age children. Clin Neurophysiol. 2002;113:1647–1656. doi: 10.1016/s1388-2457(02)00212-2. [DOI] [PubMed] [Google Scholar]
- Schwab RS. Method of measuring consciousness in attacks of petit mal epilepsy. Arch Neurol Psychiatr. 1939;41:215–217. [Google Scholar]
- Schwab RS. The influence of visual and auditory stimuli on the electroencephalographic tracing of petit mal. Am J Psychiatr. 1941;9:1301–1312. [Google Scholar]
- Snyder SM, Quintana H, Sexson SB, Knott P, Haque AFM, Reynolds DA. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatr Res. 2008;159:346–358. doi: 10.1016/j.psychres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng. 1998;45:814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Surwillo WW. Frequency of the alpha rhythm, reaction time and age. Nature. 1961;191:823–824. [Google Scholar]
- Tedrus GMAS, Fonseca LC, Tonelotto JMF, Costa RM, Chiodi MG. Benign childhood epilepsy with centrotemporal spikes: quantitative EEG and the Wechsler intelligence scale for children (WISC-III) Clin EEG Neurosci. 2006;37:193–197. doi: 10.1177/155005940603700306. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North D, Biver C. EEG and intelligence: relations between EEG coherence, EEG phase delay and power. Clin Neurophysiol. 2005;116:2129–2141. doi: 10.1016/j.clinph.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High (>60 Hz) gamma-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol. 2011;105:14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Wilkus RJ, Dodrill CB. Neuropsychological correlates of the electroencephalogram in epileptics: I. Topographic distribution and average rate of epileptiform activity. Epilepsia. 1976;17:89–100. doi: 10.1111/j.1528-1157.1976.tb03387.x. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–178. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]