Abstract
Detection of DENV IgM in a cross-section of febrile patients using ELISA to determine sero-prevalence in the rain forest region in West Africa.
1.0. Introduction
The Centers for Disease Control and Prevention in 2014 estimated that about 50-100 million human dengue virus (DENV) infections occur annually, with nearly 2.5 billion people at risk. Over 30,000 deaths in children worldwide have been attributed to Dengue Hemorrhagic Fever and Dengue Shock Syndrome (Halstead, 2007). Presently, there is no effective antiviral drug against DENV (Harris et al., 2000). Several African countries continue to report dengue outbreaks and/or sporadic cases, while dengue is being diagnosed in travellers returning to Europe and North America from African countries (Amarasinghe et al., 2011). Although dengue exists in the World Health Organization Africa region, surveillance data is poor. It is not officially reported to the World Health Organization from countries in the region, where the burden of dengue is yet to be estimated (World Health Organization, 2009) despite increasing frequency of outbreaks (Nathan and Dayal, 2007). In many African countries, several cases of febrile illnesses are presumptively diagnosed as malaria. A study in Tanzania showed 14 laboratory-confirmed malaria cases, out of 528 patients with tentative malaria diagnosis (Crump et al., 2013). In 2013 and 2014, over 50% of febrile patients visiting the University of Ibadan Health Services (Jaja Clinic) tested negative to malaria parasite count. Between 2011 and 2012 in Abidjan – Cote d'Ivoire, 0.4% patients had IgM antibody (L'Azou et al., 2015). During the 2013 dengue epidemic in Luanda, Angola Ministry of Health reported 811 positive dengue cases (Sharp et al., 2015). In 2014, 3.2% dengue IgM was reported in a study involving 218 children in Ghana (Stoler et al., 2015). Several other reports suggest people in some other West African countries are experiencing high rates of dengue infections (Bhatt et al., 2013). Stoler et al., (2014) highlighted the need for increased dengue surveillance in this part of Africa. Due to the dearth of relevant data on the epidemiology of dengue, we investigated the occurrence of dengue for eight months mainly during the rainy season in Nigeria.
2.0. Materials and methods
2.1. Study design
This cross-sectional study involved febrile patients from various urban centres in the rainforest region of Oyo State, Nigeria. Adeoyo Hospital Yemetu (7°24’14”N, 3°54’22”E) was selected as the study site because of the relatively high patient enrollment. Patients visiting the hospital were from 11 of the 33 Local Government Areas (LGAs) in Oyo State namely: Ibadan South East LGA, Ibadan North West LGA, Ibadan North LGA, Ibadan North East LGA, Egbeda LGA, Ona-Ara LGA, Oluyole LGA, Ido LGA, Ibadan South West LGA, Akinyele LGA and Lagelu LGA. Questionnaire was used to measure location of participants, clinical signs, socioeconomic status, whether or not they had been bitten by day-time-biting mosquitoes or if they took anti-malaria drugs before visiting the hospital. It is a modification of the enrolment questionnaire used by the Uganda People's Defense Forces, African Union Peace keeping troops and the Centres for Disease Control and Prevention USA. Informed consent was obtained and questionnaire interpreted in Yoruba for those who did not understand English Language. In the inclusion criteria, patients of all ages with the following symptoms were considered: temperatures ≥38°C for less than 10 days, headache, rash, fatigue, muscle ache, nausea, vomiting and diarrhea; while patients who tested positive to malaria parasite examination, those who had jaundice, fever of known bacterial and parasitic origin, fractures, complicated malaria according to the World Health Organization's definition were excluded (World Health Organization, 2001). In addition, patients who tested positive to typhoid fever, HIV, measles virus and other known infections were excluded.
2.2. Sample collection and processing
Plasma samples were collected from 274 febrile patients from 2 days old to 90 years of age. This was from April to December 2014. Three milliliter of blood was collected from each patient into sample bottle containing EDTA and transported in triple packaging to the Arbovirus Laboratory of the Department of Virology, University of Ibadan. After centrifugation for 5mins at 3,500 rpm (Microfuge™, Germany), the plasma was carefully transferred using Pasteur pipette in a Delta Series Biosafety Level II cabinet (Labconco Corp. Kansas City, Missouri), and preserved at −60°C until tested.
2.3. Serological assay
Sera were tested in 2014 using Dengue IgM (sandwich) ELISA kit commercially obtained from Diagnostic Automation/ Cortez Diagnostics Inc. Calabasas, CA, USA (ISO 13485:2003 and ISO 9001:2008). Assay was performed according to manufacturer's instructions after adding 40μl Rheumatoid Factor absorbent to each tube containing 100 μl of negative control, positive control or diluted samples. ELISA reader with SoftMax™ Pro software v5.4 1999-2009 (MDS Analytical Technologies Inc., USA) was set for bichromatic reading between 450 – 650nm. Negative control (NC) used was diluted negative human serum while positive control (PC) was diluted positive human serum provided in the kit. Expected values for negative control is 0.0 - 0.30 Optical density (OD) units while positive control value is ≥0.50 OD units. Results were interpreted based on these values. Assay is 97.8% specific and 93.5% sensitive.
2.4. Polymerase Chain Reaction detection of other flaviviruses
Conventional Polymerase Chain Reaction (PCR) was used to test for the 3’ non-coding region of yellow fever virus according to Onyango et al., (2004), west nile virus according to Turrell et al., (2005) and Zika virus as used by Faye et al., (2008) in dengue IgM positive samples to rule out cross-reactivity by these flaviviruses.
2.5. Ethical consideration
Approval for this study was obtained from Institutional Review Board of the University of Ibadan/University College Hospital (UI/EC/13/0412) and the Ethics Board of Oyo State Ministry of Health (AD13/479/496).
2.6. Statistical analysis
Data analysis was done using SPSS version 16.0 software (IBM Corp. released 2011, IBM SPSS Statistics for Windows, Armonk, NY, USA). Chi square and Fischer's exact tests were used to determine association between IgM positivity and other variables in a univariate analysis. P value <0.05 was considered statistically significant.
3.0. Results
The number of patients tested monthly were as follows: April (n = 40), May (n = 41), June (n = 30), August (n = 30), September (n = 42), October (n = 33), November (n = 25) and December (n = 33). Of these, 82 (29.9%) were males and 192 (70.1%) females. No sample was collected in July due to strike action by resident doctors and allied health workers. Out of 274 people studied; 64 (23.3%) revealed evidence of recent dengue exposure in which 17 (26.6%) males and 47 (73.4%) females were IgM positive. There was a significant association between IgM antibody level and months with high prevalence (X2=0.000; p < 0.05). Reliability of questionnaire from where clinical and socio-demographic data were obtained was found to be 72.1% with Cronbach alpha reliability statistics. Although 61 (95.3%) of those infected were low income earners and 3 (4.7%) in the middle class, no association was observed between IgM level and socioeconomic status. Eighty-four (30.7%) people took anti-malaria drugs before hospital visitation. No participant had all the clinical signs. Analysis showed no association between any of the clinical signs and recent dengue exposure. One hundred and seven (39.1%) people reported to have been bitten by day-time mosquitoes, 92 (33.5%) were not while 75 (27.4%) were not sure if they had been bitten. Out of the 64 patients exposed to dengue virus, 23 (35.9%) had been bitten by daytime biting mosquitoes, 25 (39.0%) were not bitten and 16 (25%) were not sure if they had been bitten. There was no significant association between those who were bitten, those who were not and those who were not sure if they had been bitten (X2=0.569; p>0.05). The participants in this study were not vaccinated against YFV. No study participant was positive for WNV, YFV and Zika virus by PCR.
4.0. Discussion
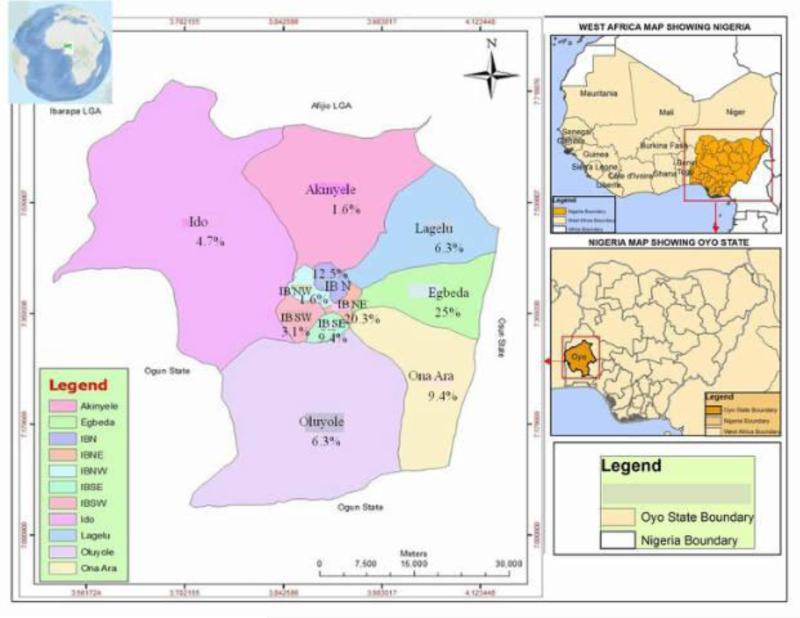
Although several studies on dengue have been reported in Nigeria and parts of west Africa, the present study highlights a high rate of recent dengue infection for eight months during the rainy season in Nigeria (Fig. 1). Most people exposed to dengue in this study are over 18.1 years old (Tables 1 and 2). This is consistent with studies suggesting that adults are more likely to have clinical dengue than young children (Simmons et al., 1931; Graham et al., 1999; Vaughn et al., 2001). A reason for this is because infections in older people are more likely to be due to secondary DENV infections which are associated with greater risks of symptomatic and severe disease (Nisalak et al., 2003; Cummings et al., 2009). However, it is not clear if more than one DENV serotype was circulating in the population during the study period hence the need for further studies to establish the molecular detail. Occurrence of high OD values in older individuals (those ≥18.1 years of age) in this study is an indication that they are more commonly affected (Tables 3). Studies in Latin America where several reports of increased number of adolescents with recent dengue infections have been made (Bhatt et al., 2013; Sharp et al., 2013; Teixeira et al., 2013; Villar et al., 2015) lends credence to the findings in our present study. A cross-sectional survey of febrile patients in Pemba Island, Zanzibar further supports our findings with a sero-prevalence of 15.4% in adults vs. 1.9% in patients under 15 years old (Vairo et al., 2012). However, reports from Asian countries where children under 5 years of age are particularly affected (World Health Organization, 2009) is at variance with the findings in this present study. The reason for this is not clearly understood. However, 15.6% prevalence among children under 5 years of age (Table 1) agrees with a study involving same age group of children in Ilorin, North central Nigeria where high prevalence was reported (Faneye et al., 2014), this underscores the importance of dengue in this age group. Although participants in this study were people visiting a hospital in Ibadan (Fig. 1), it is not certain if they were all bitten by mosquitoes in their abode or outside of their LGAs. Many residents in Ibadan store water in open containers in their houses which provides excellent breeding sites for Aedes species. It is also common sight to find indiscriminate disposal of hollow containers in most parts of Nigeria.
Figure 1.
Dengue prevalence in patients visiting Adeoyo Hospital from 11 LGAs in Ibadan, Nigeria.
Table 1.
Age distribution of patients tested for dengue IgM in Adeoyo Hospital Yemetu - Ibadan.
| Age | Number tested | Number infected | p value |
|---|---|---|---|
| ≤ 1 year | 27 | 2 (3.1%) | |
| 1.1 - 5 years | 35 | 8 (12.5%) | |
| 5.1 - 10 years | 13 | 4 (6.3%) | 0.297 |
| 10.1 - 18 years | 20 | 6 (9.4%) | |
| ≥ 18.1 years | 179 | 44 (68.8%) | |
| Total | 274 | 64 (100%) |
Table 2.
Monthly distribution of dengue in different age groups of people visiting Adeoyo Hospital Yemetu – Ibadan.
| Age group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | ≤1 year | 1.1-5 years | 5.1-10 years | 10.1-18 years | ≥18.1 years | p value | Monthly prevalence | p value |
| April | 3.10% | 4.70% | 1.60% | 4.70% | 10.90% | 40.00% | ||
| May | 0.00% | 0.00% | 1.60% | 0.00% | 7.80% | 14.60% | ||
| June | 0.00% | 1.60% | 1.60% | 0.00% | 14.10% | 36.60% | ||
| July | Nil* | Nil* | Nil* | Nil* | Nil* | p=0.524 | Nil * | p=0.000 |
| August | 0.00% | 4.70% | 0.00% | 1.60% | 12.50% | 40.00% | ||
| September | 0.00% | 0.00% | 0.00% | 1.60% | 6.30% | 11.90% | ||
| October | 0.00% | 1.60% | 0.00% | 1.60% | 12.50% | 30.30% | ||
| November | 0.00% | 0.00% | 0.00% | 0.00% | 4.70% | 12.00% | ||
| December | 0.00% | 0.00% | 1.60% | 0.00% | 0.00% | 3.00% | ||
The reason for no report of dengue in July was because nobody was sampled during the period.
Table 3.
Distribution of Optical densities of dengue IgM ELISA across age groups
| Age | Negative (≤0.30 OD) | Weakly reactive (0.31 - 0.49 OD) | Moderately reactive (0.50 - 0.99 OD) | Highly reactive (>1.0 - 3.55 OD) | p value |
|---|---|---|---|---|---|
| ≤ 1 year | 18 (6.6%) | 7 (2.6%) | 2 (0.7%) | 0 (0.0%) | |
| 1.1 - 5 years | 14 (5.1%) | 13 (4.7%) | 7 (2.6%) | 1(0.4%) | |
| 5.1 - 10 years | 3 (1.1%) | 6 (2.2%) | 2 (0.7%) | 2 (0.7%) | p=0.126 |
| 10.1 - 18 years | 8 (2.9%) | 6 (2.2%) | 5 (1.8%) | 1 (0.4%) | |
| ≥ 18.1 years | 90 (32.8%) | 45 (16.1%) | 39 (14.2%) | 5 (1.8%) | |
| Total | 133 (48.5%) | 77 (28.1%) | 55 (20.1%) | 9 (3.3%) |
More DENV exposure occurred during the rainy season from April to October as demonstrated by higher seropositivity and IgM level (Tables 2 and 4). In two separate studies on recent dengue infection in different ecological zones in Nigeria, dengue was found to occur more in the rainforest region (Fagbami et al., 1977; Baba et al., 2009). This is due to abundant vegetation cover that is available in tropical rain forests region and the high relative humidity. This present study found monthly peaks in dengue prevalence during the rainy season in April and August (Tables 2 and 4). A study in Selangor, Malaysia showed positive correlation between dengue outbreak and rainfall pattern with increase in number of breeding sites (Li et al., 1985). With reports of dengue outbreaks in other parts of Africa and importation of dengue into Europe from west Africa (Huhtamo et al., 2008; Institut de Veille Sanitaire, 2009; Raut et al., 2015) it is noteworthy that the incidence of dengue in this part of west Africa is very high. YFV, WNV and Zika virus were not detected in this present study but other flaviviruses such as Wesselsbron virus, Banzi, Uganda Z have been isolated in Nigeria and may cross-react in this assay. Fig 5 shows no association between clinical signs and sero-positivity which reinforces the need not to rely absolutely on clinical symptoms in the diagnosis of dengue in this part of the world as it will confound diagnosis.
Table 4.
Monthly distribution of Optical densities of dengue IgM ELISA in study participants
| Age | Negative (≤0.30 OD) | Weakly reactive (0.31 - 0.49 OD) | Moderately reactive (0.50 - 0.99 OD) | Highly reactive (>1.0 - 3.55 OD) |
|---|---|---|---|---|
| April | 11 (4.0%) | 13 (4.7%) | 14 (5.1%) | 2 (0.7%) |
| May | 21 (7.7%) | 14 (5.1%) | 6 (2.2%) | 0 (0.0%) |
| June | 10 (3.6%) | 8 (2.9%) | 10 (3.6%) | 2 (0.7%) |
| July | Nil* | Nil* | Nil* | Nil* |
| August | 13 (4.8%) | 6 (2.2%) | 7 (2.6%) | 4 (1.5%) |
| September | 26 (9.5%) | 11 (4.0%) | 5 (1.8%) | 0 (0.0%) |
| October | 16 (5.8%) | 7 (2.6%) | 9 (3.3%) | 1 (0.36%) |
| November | 13 (4.7%) | 9 (3.3%) | 3 (1.1%) | 0 (0.0%) |
| December | 23 (8.3%) | 9 (3.3%) | 1 (0.4%) | 0 (0.0%) |
| Total | 133 (48.5%) | 77 (28.1%) | 55 (20.1%) | 9 (3.3%) |
The reason for no report of dengue in July was because nobody was sampled during the period.
Transmission of DENV in several urban LGAs (Fig. 1) is an indication that it is maintained in urban-human populations. Many of these areas are forested, with stagnant water and indiscriminate hollow containers that litter everywhere providing excellent breeding sites for increased vector activity. Mosquito-DENV-Human cycle has been reportedly found in nearly all urban and peri-urban environments throughout the tropics and subtropics (Rossi et al., 2012). Dengue in the tropical rain forest region has increased due to uncontrolled urbanization, lack of effective and sustainable vector control programs (Guzman et al., 2010). Although the serotype(s) involved in this epidemic is/are not known, all DENV serotypes involved in urban dengue cycle are transmitted by domestic and peridomestic Ae. aegypti aegypti and Ae. albopictus (Simmons et al., 1931; Sabin, 1952; Rosen et al., 1954; Halstead et al., 1964; Halstead, 1997). These Aedes species circulate steadily in Ibadan, south-west Nigeria (Onoja et al., 2016) and have been reported with other Aedes species in the rainforest region of south-east Nigeria (Anosike et al., 2007). Our findings extend the information on monthly dengue epidemiology in Nigeria and the observation of unrecognized recent dengue infection serves as an early warning of possible dengue outbreaks, as mosquito control efforts are not sustained to the peril of the inhabitants. A previous study investigating DENV serotypes in Aedes aegypti from different ecological zones in Nigeria lends credence to this assertion, with the detection of all serotypes in both male and female mosquitoes (Baba et al., 2009).
In conclusion, endemicity of dengue in the rain forest region of Nigeria has been brought to the fore. Hitherto, it was undiagnosed in most hospitals due to lack of routine screening. Blind treatment of dengue and other arboviral infections as malaria or typhoid fever was the practice. With this study, it has become more evident that routine dengue testing should be done for febrile illnesses. Active surveillance for DENV and other arboviruses is necessary to identify periods of increased virus transmission and to determine primary, secondary or tertiary DENV infections. Improved sanitary measures such as removing hollow containers from gutters and waterways will enhance environmental control strategy by dislodging or destroying mosquito larvae and controlling the vector.
Table 5.
Pattern of clinical signs among study participants
| Clinical signs | No. of participants presenting with clinical signs | No. of participants exposed to dengue virus | p = value |
|---|---|---|---|
| Head ache | 22 (24.7%) | 23(35.9%) | 0.586 |
| Joint pain | 7 (20%) | 5 (7.8%) | 0.352 |
| Body pain | 12 (33.3%) | 9(14.1%) | 0.129 |
| Back ache | 2 (20%) | 0 (0.0%) | 0.309 |
| Vomiting | 16 (31.4%) | 0 (0.0%) | 0.257 |
| Rash | 1 (10%) | 0 (0.0%) | 0.280 |
| Fatigue | 15 (28.3%) | 0 (0.0%) | 0.891 |
Highlights.
Prevalence of 23.4% recent dengue virus exposure was found in 8 months.
Highest monthly prevalence of 40% dengue exposure occurred in April and August.
People in 11 Local Government Areas were exposed to dengue virus.
Routine dengue testing is recommended for febrile illnesses in Nigeria.
Acknowledgement
We thank Dr. Georgina N. Odaibo for her expert opinion on the serological assay. We extend our appreciation to the Medical Laboratory Scientists in Adeoyo Hospital who carried out malaria and widal tests on febrile patients samples before our study commenced.
Funding
This work was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Centre, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not represent the views of the funding organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
We declare that there is no conflict of interest.
References
- Adedayo F, Nioma I, Olanrewaju MB, Adeyinka A, Ebele A. Serological evidence of recent dengue virus infection among febrile children in a semi-arid zone. Am. J. Infect. Dis. 2013;9:7–10. [Google Scholar]
- Amarasinghe A, Kuritsky JN, Letson GN, Margolis HS. Dengue Virus Infection in Africa. Emerg. Infect. Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anosike JC, Nwoke EBB, Okere AN, Oku EE, Asor JE, Emmy-Egbe IO, Adimike DA. Epidemiology of tree-hole breeding mosquitoes in the tropical rainforest of Imo State, South-East Nigeria. Ann. Agric. Environ. Med. 2007;14:31–38. [PubMed] [Google Scholar]
- Baba MM, Vorndam AV, Adeniji JA, Diop O, Olaleye DO. Dengue Virus Infections in Patients Suspected of Malaria/Typhoid in Nigeria. J. of Am. Sc. 2009;5:129–134. [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Dengue and Climate. 2010 http://www.cdc.gov/dengue/entamologyEcology/climate html.
- Centers for Disease Control and Prevention Dengue homepage. 2014 www.cdc.gov/dengue/clinicalLab/realTime.html.
- Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am. J. Trop. Med. Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- Cummings DAT, Iamsirithaworn S, Lessler JT, McDermott A, Prasanthong R, Nisalak A, Jarman RG, Burke DS, Gibbons RV. The Impact of the Demographic Transition on Dengue in Thailand: Insights from a Statistical Analysis and Mathematical Modeling. PLoS Med. 2009;6(9):e1000139. doi: 10.1371/journal.pmed.1000139. doi:10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E, Videa E, Perez L, Sandoval E, Tellez Y, Perez ML, Cuadara R, Rocha J, Idiaquez W, Alonso RE, Delgado MA, Campo LA, Acevedo F, Gonzalez A, Amador JJ, Balmaseda A. Clinical, epidemiologic and virologic features of dengue in the 1998 epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl. Trop. Dis. 2013;7:2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbami AH, Monath TP, Fabiyi A. Dengue virus infections in Nigeria: a survey for antibodies in monkeys and humans. Trans. R. Soc. Trop. Med. Hyg. 1977;71:60–65. doi: 10.1016/0035-9203(77)90210-3. [DOI] [PubMed] [Google Scholar]
- Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Amadou, Sall A. One-step RT-PCR for detection of Zika virus. Journal of Clinical Virology. 2008;43:96–101. doi: 10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Focks DA, Haile DH, Daniels E, Mount GA. Dynamic life table model of a container-inhabiting mosquito, Aedes aegypti (L) (Diptera: Culicidae). Simulation, Results and Validation. J. Med. Entomol. 1993;30:1018–1028. doi: 10.1093/jmedent/30.6.1018. [DOI] [PubMed] [Google Scholar]
- Gould EA, de Lamballerie X, Zanotto PM, Holmes EC. Origins, evolution, and vector/host co-adaptations within the genus Flavivirus. Adv. Virus Res. 2003;59:277–314. doi: 10.1016/s0065-3527(03)59008-x. [DOI] [PubMed] [Google Scholar]
- Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Erlin S, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. Am. J. Trop. Med. Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat. Rev. Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Epidemiology of dengue and dengue hemorrhagic fever. In: Gubler D, Kuno G, editors. Dengue and dengue hemorrhagic fever. CAB International; Wallingford (UK): 1997. pp. 23–44. [Google Scholar]
- Halstead SB. Epidemiology of dengue and dengue hemorrhagic fever. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. CABI International; Oxon: 1997. pp. 23–44. [Google Scholar]
- Halstead SB, Sukhavachana P, Nisalak A. In vitro recovery of dengue viruses from naturally infected human beings and arthropods. Nature. 1964;202:931–932. doi: 10.1038/202931a0. [DOI] [PubMed] [Google Scholar]
- Hubalek Z, Halouzka J, Juricova Z, Prikazsky Z, Zakova J, Sebesta O. Surveillance of mosquito-borne viruses in Breclav after the flood of 1997. Epidemiol. Mikrobiol. Imunol. 1999;48:91–96. [PubMed] [Google Scholar]
- Huhtamo E, Uzcategui NY, Siikamaki H, Saarinen A, Piiparinen H, Vaheri A, Vapalahti O. Molecular epidemiology of dengue virus strains from Finnishtravelers. Emerg. Infect. Dis. 2008;14:80–83. doi: 10.3201/eid1401.070865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut de Veille Sanitaire, France. Bulletin HebdomadairebInternational . French: 2009. No151 Available from: http://www.invs.sante.fr/international/bhi_120808.pdf. [Google Scholar]
- Li CF, Lim TW, Han LL, Fang R. Rainfall, abundance of Aedes aegypti and dengue infection in Selangor, Malaysia. Southeast Asian J. of Trop. Med. and Pub. Health. 1985;16:560–568. [PubMed] [Google Scholar]
- Nathan MB, Dayal-Drager R. Recent epidemiological trends, the global strategy and public health advances in dengue.. Working paper 3.1 in: Report of the Scientific Working Group meeting on Dengue; Geneva. 1–5 October 2006; Geneva: World Health Organization, Special Programme for Research and Training in Tropical Diseases; 2007. pp. 29–-34. [Google Scholar]
- Olaniran OJ. Changing patterns of rain-days in Nigeria. GeoJournal. 1990;22:99–107. [Google Scholar]
- Olaniran OJ. Evidence of climatic change in Nigeria based on annual series of rainfall of different daily amounts, 1919–1985. Climatic Change. 1992;19:319–341. [Google Scholar]
- Olaniran OJ. The 55th Inaugural Lecture. University of Ilorin; Nigeria: 2002. Rainfall Anomalies in Nigeria: The Contemporary Understanding. p. 55. [Google Scholar]
- Onoja AB, Adeniji JA, Opayele AV. Yellow fever vaccination in Nigeria: Focus on Oyo State. Highland Medical Research Journal. 2015;16(2) In press. [Google Scholar]
- Onyango CO, Grobbelaar AA, Gibson GVF, Sang RC, Sow A, Swanepoel R, Burt FJ. Yellow Fever Outbreak, Southern Sudan, 2003. Emerg. Infect. Dis. 2004;10:1668–1670. doi: 10.3201/eid1009.030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W, Lloyd L. Planning social mobilization and communication for dengue fever prevention and control: a step-by-step guide. World Health Organization; Geneva: 2004. [Google Scholar]
- Raut CG, Rao NM, Sinha DP, Hanumaiah H, Manjunatha MJ. Chikungunya, Dengue, and Malaria Co-Infection after Travel to Nigeria, India. Emerg. Infect. Dis. 2015;21:908–910. doi: 10.3201/eid2105.141804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk ofdengue in Europe. Acta Trop. 2014;129:1–14. doi: 10.1016/j.actatropica.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rosen L, Rozeboom LE, Sweet BH, Sabin AB. The transmission of dengue by Aedes polynesiensis Marks. Am. J. Trop. Med. Hyg. 1954;3:878–882. doi: 10.4269/ajtmh.1954.3.878. [DOI] [PubMed] [Google Scholar]
- Rossi SL, Nasar F, Cardosa J, Mayer SV, Tesh RB, Hanley KA, Weaver SC, Vasilakis N. Genetic and phenotypic characterization of sylvatic dengue virus type 4 strains. Virology. 2012;423:58–67. doi: 10.1016/j.virol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J. of Med. Entomology. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Sharp TM, Moreira R, Soares MJ, da Costa LM, Mann J, DeLorey M, Hunsperger E, Muñoz-Jordán JL, Colón C, Margolis HS, de Caravalho A, Tomashek KM. Under-recognition of Dengue during 2013 Epidemic in Luanda, Angola. 2015;21(8):1311–1316. doi: 10.3201/eid2108.150368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TM, Hunsperger E, Santiago GA, Munoz-Jordan JL, Santiago LM, Rivera A, et al. Virus specific differences in rates of disease during the 2010 dengue epidemic in Puerto Rico. PLoS Negl Trop Dis. 2013;7:e2159. doi: 10.1371/journal.pntd.0002159. http://dx.doi.org/10.1371/journal.pntd.0002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JS, St John JH, Reynolds FHK. Experimental studies of dengue. Philipp. J. Sci. 1931;44:1–252. [Google Scholar]
- Stoler J, al Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing “malaria”: west Africa as the next front for dengue surveillance and control. Acta Tropica. 2014;134:58–65. doi: 10.1016/j.actatropica.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Stoler J, Delimini RK, Bonney JHK, Oduro AR, Owusu- Agyei S, Fobil JN, Awandare GA. Evidence of recent dengue exposure among malaria parasite-positive children in three urban centers in Ghana. Am J Trop Med Hyg. 2015;92(3):497–500. doi: 10.4269/ajtmh.14-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MG, Siqueira JB, Jr., Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7:e2520. doi: 10.1371/journal.pntd.0002520. http://dx.doi.org/10.1371/journal.pntd.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, O' guinn ML, Dohm DJ, Jones JW. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Vairo F, Nicastri E, Meschi S, Schepisi MS, Paglia MG, Bevilacqua NB, Mangi S, Sciarrone MR, Chiappini R, Mohamed J, Racalbuto V, Di Caro A, Capobianchi MR, Ippolito G. Seroprevalence of dengue infection: a cross-sectional survey in mainland Tanzania and on Pemba Island, Zanzibar. Int. J. of Infect. Dis. 2012;16:44–46. doi: 10.1016/j.ijid.2012.05.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- Villar LA, Rojas DP, Besada-Lombana S, Sarti E. Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS Negl Trop Dis. 2015;9:e0003499. doi: 10.1371/journal.pntd.0003499. http://dx.doi.org/10.1371/journal.pntd.0003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DO, Fenner FJ. Medical Virology. 4th Ed. Academic Press Inc.; San Diego, California: 1994. Togaviridae, Flaviviridae, Bunyaviridae. pp. 418–449. [Google Scholar]
- Woodruff AW, Bowen ETW, Platt GS. Viral-infections in travellers from tropical Africa. BMJ. 1978;1:956–958. doi: 10.1136/bmj.1.6118.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Report of a WHO consultation. World Health Organization; Geneva, Switzerland: Geneva: Dec 3-5, 2001. 2001. Monitoring antimalarial drug resistance. WHO/CDS/CSR/EPH/2002.17 2002. Available at: http://www.who.int/drugresistance /publications/WHO_CDS_CSR_EPH_2002_17/en/index.html. [Google Scholar]
- World Health Organization Dengue guidelines for diagnosis treatment, prevention and control WHO/HTM/NTD/DEN/2009. 2009;1:p1–144. [PubMed] [Google Scholar]
- World Health Organization . Dengue guidelines for diagnosis, treatment, prevention and control. New edition. World Health Organization; Geneva: 2009. Available at: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [PubMed] [Google Scholar]